A Study of Lead Corrosion of Diesel Engine Oil
Xu Jie; Xia Qinghong; Zhang Feng; Wu Zhiqiang
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Abstract: The effect of base oils, sulfur-containing multi-functional additives and dispersants in formulated diesel lubricants on lead corrosion was evaluated by a self-established high temperature corrosion bench test. Test lead coupons were analyzed by XPS to determine the resulting surface chemistry. The results showed a close correlation between the oxidation stability of base oil blend and the lead corrosion of formulated diesel lubricants. The zinc dialkyldithiophosphate(ZDDP) and zinc dialkyldithiocarbamate (ZDDC) have formed different protective films on lead coupon surfaces. A more or less amount of the protective film formed is the main factor affecting the degree of lead corrosion. The glassy zinc phosphates protective film formed by ZDDP is more effective than the zinc sulfides film formed by ZDDC. The interaction between dispersants and ZDDP had a significant impact on lead corrosion.
Key words: diesel engine oil; lead corrosion; base oil; ZDDP; ZDDC; XPS
1 Introduction
In recent years, engine manufactures have continuously improved engine design to meet the requirement of more stringent emissions legislations, which have also promoted the upgrading of oil specification. For diesel engines, increased heat load and operating temperatures,extended oil drain intervals and the use of exhaust gas recirculation (EGR) result in an increased oil deterioration. These causes are more concerned over the corrosion by oil degradation. Higher quality grade diesel lubricants put forward greater demand of oil anti-corrosive performance.
In the API diesel engine oil categories, the anti-corrosive performance of oil is evaluated by bench tests and engine tests. The Cummins Bench Test (CBT) is used in API CG-4/CF-4 and the High Temperature Cummins Bench Test (HTCBT) is used in API CK-4/FA-4/CJ-4/CI-4/CH-4. Copper-lead bearing corrosion is also evaluated in Mack T-9, Mack T-10 and Mack T-12 engine tests as one of the measured oil performance parameters. Lead corrosion is one of the main factors affecting the Mack T-10 and T-12 passing rates upon statistics.
Corrosion of lead and copper by lubricants in diesel engines is mainly detected in bearings, bushings and cam follower pins. Typically, the bearing is a tri-metallic structure with a steel back, a copper-tin-lead lining,and a copper-tin-lead or an indium-lead overlay. The bushing is a bi-metallic structure including a steel back and a copper-tin-lead lining. The cam follower pin is made of phosphor bronze. Recently, the environmentally friendly lead-free sliding bearings have found wide applications. In order to keep the back compatibility of oil performance with previous models of diesel engines, the API diesel engine oil specification still keeps the lead corrosion as one of the necessary measured oil performance parameters. Corrosion of lead and copper containing materials by lubricants has been documented[1-6]. Some researchers attributed the inhibition of sulfur-containing additives on lead corrosion to the formation of protective films of lead sulfides on lead surfaces[3-5]. Jayne D T, et al.[3]studied the copper corrosion in various engine oil formulations, and concluded that sulfide films formed by reactive sulfur on copper were corrosive, but were passive on lead. Cusano C M,et al.[2]proposed that the bearing lead corrosion was resulted from residual chlorine in the lubricant additives.More detailed work is needed to study the mechanisms of lead corrosion inhibition by different types of sulfur-containing corrosion inhibitors.
In this paper, the effect of base oils, sulfur-containing multi-functional additives and dispersants in formulated diesel lubricants on lead corrosion was evaluated by a self-established high temperature corrosion bench test.The mechanisms of lead corrosion inhibition by zinc dialkyldithiophosphate (ZDDP) and zinc dialkyldithiocarbamate (ZDDC) were discussed. Results of this study could provide a support to the development of high performance corrosion protective lubricants.
2 Experimental
2.1 Materials
The base oils were commercial mineral oils that were mainly produced in China. The additives were common functional additives purchased from domestic and international manufacturers. The elemental mass fractions of ZDDP covered the following composition: 8.91% of Zn,7.84% of P, 16.3% of S; while the elemental mass fractions of ZDDC covered the following composition: 11.2%of Zn, and 21.5% of S.
2.2 Test methods
A self-established high temperature corrosion bench test,which was a modified national industrial standard SH/T 0299 test, was employed to identify the lead corrosion by candidate oils. The test conditions included hanging a lead coupon (76 mm×12 mm×0.5 mm) in 85 ml of oil, which was bubbled with 25 mL/min of oxygen gas through the oil. The oil was maintained at 165 °C for ageing in 30 h. The specification of lead coupon and treatment method were described in SH/T 0299. The weight of lead coupon was measured before and after the ageing test to determine the weight loss. The test used a LAWLER Model HT-342-12 oxidation stability bath equipped with oxidation cells. An oxidation cell consisted of a test tube, a condenser, and an oxygen delivery tube, as shown in Figure 1.
The lead contents of new and end-of-test oil were determined by the national standard test method GB/T 17476, which was similar to the ASTM D5185 method.
The X-ray photoelectron spectroscopy (XPS) was used to characterize the surface chemistry of the used lead coupons.

Figure 1 Oxidation cell
The base oil oxidation stability was measured by the national industrial standard method SH/T 0193, which was similar to ASTM D2272 method, requiring the addition of 0.8% of antioxidant T501.
3 Results and Discussion
In the above-mentioned standards for API engine and bench tests, the lead concentration increase in the end-oftest oil has been used to evaluate the bearing corrosion.In the self-established corrosion bench test, the weight loss of lead coupon and the lead concentration increase in the used oil at the end of test were tentatively correlated,as shown in Figure 2. The coupon weight losses showed a linear relationship with the lead levels in the used fluids, and the linear correlation coefficientR2was very high, indicating that most of the corrosion products were in the form of soluble lead salts. The weight loss of lead coupon was a good measure of the degree of lead corrosion. Compared with the API standard tests, the selfestablished corrosion test greatly reduced the test duration by intensifying the test condition. The use of weight loss of lead coupon instead of lead concentration increase in used oil could evaluate lead corrosion capable of further simplifying the experimental procedure.

Figure 2 Pb concentration increase in used oil vs. weight loss of Pb coupon in the self-established test
Table 1 shows the results of some test oils obtained from the standard API engine tests and the self-established corrosion bench test, showing a good correlation between these tests. The Mack T-10 and T-12 tests are more severe engine tests than Mack T-9 used to evaluate the ring liner wear and the bearing lead corrosion of diesel lubricants.The self-established bench test method showed good discrimination between oils with different performance.As the standard API engine tests are very expensive and time-consuming, the self-established corrosion bench test was used as a device for identifying the lead corrosion by candidate oils.
A series of tests were carried out to determine the effect of base oils, the sulfur-containing multi-functional additives ZDDP and ZDDC, as well as the dispersants in diesel engine oil formulations on lead corrosion. The baseline formulation used for testing the typical diesel engine oil components including the base oil, the viscosity index improver, the metal detergent, the ashless dispersant,the antioxidant, the anti-wear agent, and the corrosion inhibitor.
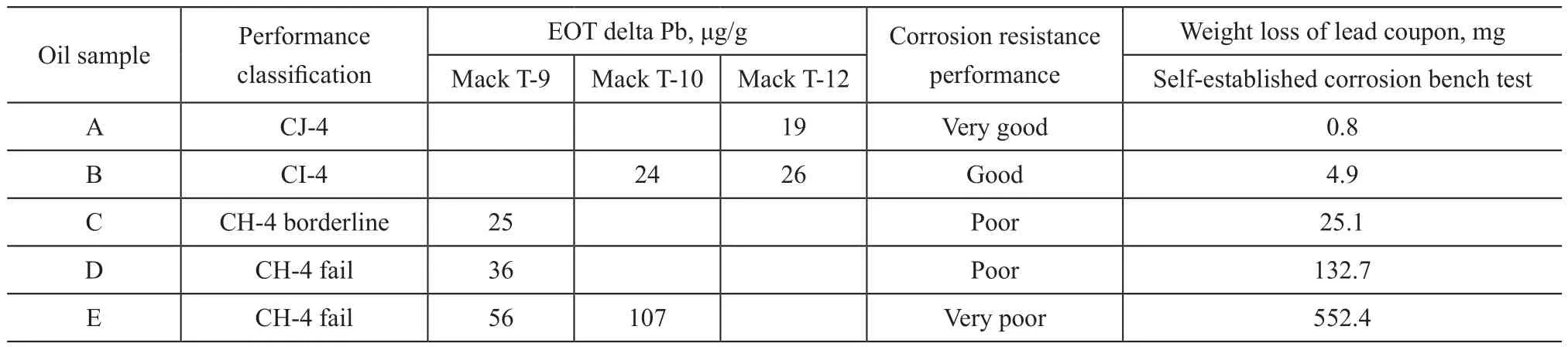
Table 1 Results of testing oils obtained from standard API engine tests and self-established corrosion bench test
3.1 Base oils effects
Different base oils were incorporated into the same baseline formulation to check their effects on lead corrosion.The properties of base oils are shown in Table 2. The base oils #1―#3 are the hydro-treated mineral oils, whereas the base oils #4―#8 are the solvent-refined mineral oils.
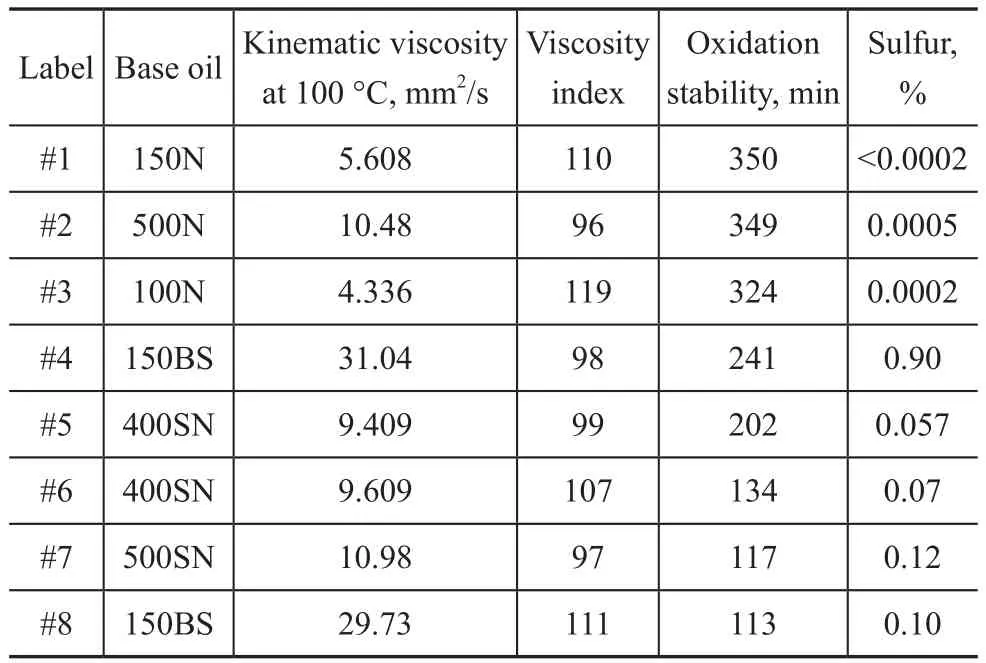
Table 2 Base oils properties
All the base oils were produced in China except the base oils #2 and #4 which were obtained from the overseas market.
Table 3 shows the results of lead weight loss and property of base oil blends. The base oil blends of oil samples M-6 and M-7 were entirely composed of the hydro-treated base stocks. The lead weight losses of M-6 and M-7 were lower than all other oil samples with base oil blends containing the solvent-refined base stocks. Upon comparing the results of oil samples M-2 and M-3, it was found that the corrosion resistance performance of base oil 150BS(#4) was better than 150BS (#8). Upon comparing the results of M-1, M-3 and M-4, a better corrosion resistance of base oil 400SN (#5) over that of 400SN (#6) was found, while the performance of the base oil 500SN was shown to be even worse than 400SN (#6). It was concluded that the base oil corrosion resistance performance can be related to its oxidation stability. Good oxidation stability of the base oil was beneficial to lead corrosion resistance. There was no verified relationship between the sulfur content in base oil and the lead corrosion. The base oil blends listed in Table 3 were also analyzed by SH/T 0193 method for studying their oxidation stability. The results showed a close correlation between the oxidation stability of base oil blend and the lubricating oil corrosion resistance. The better the oxidation stability of the base oil blend, the lower the lead corrosion by the finished oil.

Table 3 Lead weight loss results and property of base oil blends
3.2 Effects of ZDDP and ZDDC
The sulfur-containing multi-functional additives ZDDP and ZDDC were added separately into the same baseline formulation comprising identical base oil blend, viscosity index improver and detergent, dispersant, etc., to determine their effects on lead corrosion. The results in Table 4 show a decrease of lead weight loss with the increase of ZDDP dosage. This fact indicates that ZDDP was effective to inhibit lead corrosion. However, the improvement in lead corrosion was not significant when the ZDDP dosage was greater than 2.0%. Too high a dosage of ZDDP in fully formulated lubricants would have an adverse effect on the wear protection performance[7]. Therefore, the ZDDP treat level in engine oil should be controlled to be no more than adequate to protect the engines. Table 4 shows the effect of ZDDC on lead corrosion. Similarly,lead weight loss decreased with the increase of ZDDC dosage. However, the lead corrosion inhibition effect of ZDDC was less significant than ZDDP at the same or similar treat level. This indicates that the ZDDP is a more effective lead corrosion inhibitor than ZDDC.

Table 4 Effects of ZDDP and ZDDC on lead corrosion
3.3 Effect of dispersants
Different types of succinimide dispersants were added separately into the same baseline formulation at a constant concentration to determine their effects on lead corrosion. ZDDP was used as a corrosion inhibitor in the baseline formulation. The results in Table 5 show the borated dispersant had the lowest lead weight loss.In Figure 3, the dispersant TBN was plotted against the lead weight loss. There was a positive correlation between the lead corrosion and the dispersant TBN.Higher concentration of dispersant TBN produced more severe lead corrosion. This was due to the antagonistic interactions between ZDDP and the dispersant. It has been suggested that dispersants can reduce the activity of ZDDP through complexation. Some researchers have stated that the complexation is between ZDDP and the dispersant through P-N bonding[8-9], whereas others suggested the Zn-N bonding[10-11]. Ramakumar, et al.[12]also found that the low-N-content dispersants could form weak complexes in solution, whereas the higher-N-content dispersants could form more tight bonds. Since the dispersant TBN is proportional to the N-content, it could affect the complexation and ZDDP activity. The result of the borated dispersant was not plotted in Figure 3, as the structure of the dispersant was different from others. Although the TBN of borated dispersant was relatively high (TBN=40 mgKOH/g), the borating could significantly reduce the interaction between the ZDDP and the dispersant, resulting in a very low level of lead corrosion.
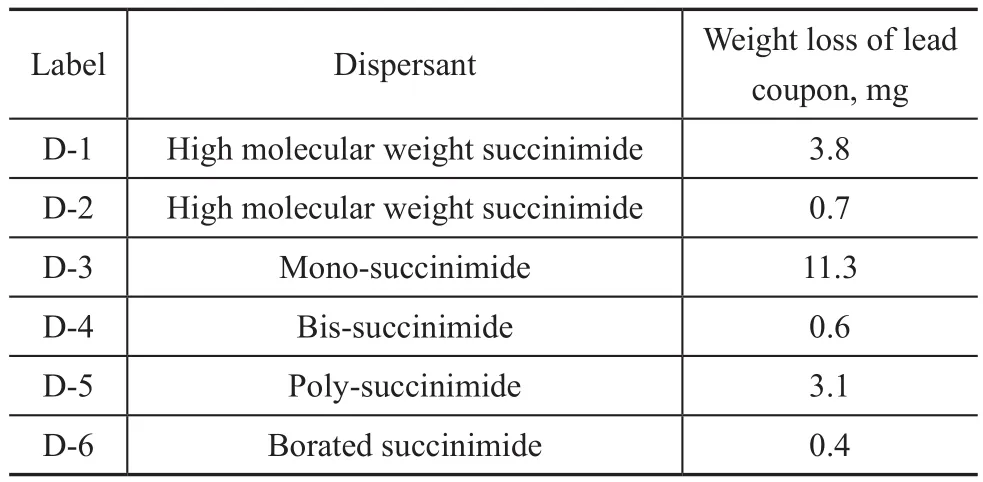
Table 5 Effects of dispersants on lead corrosion

Figure 3 Weight loss of Pb coupon vs. dispersant TBN
3.3 Surface analysis of lead coupons
In order to investigate the mechanism of lead corrosion inhibition by ZDDP and ZDDC, we selected five used test coupons listed in Table 4 for further evaluation of the surface chemistry by XPS. The surface elemental composition and chemical state were analyzed.

Table 6 Used lead coupon elemental composition (atom %)
Table 6 shows the XPS results on the used lead coupons. The oil samples M-12, M-14 and M-15 treated with ZDDC had higher concentrations of S and Pb on the surface than the oil samples treated with ZDDP. Among the oil samples treated with ZDDP, M-8 with better lead corrosion resistance had higher concentration of Zn and P on the surface than M-11.Among the oil samples treated with ZDDC, M-12 with the least corrosion had the highest level of Zn and S on the surface, likewise, the lead surface for M-15 with the greatest corrosion had the lowest level of Zn and S.

Figure 4 XPS spectra of typical elements at the surface on lead coupons run with ZDDP
Figure 4 shows the XPS spectra of typical elements on lead coupons of the ZDDP treated oil samples. In Figure 4(a), the spectrum of Pb4f7 of M-8 illustrates the existence of peaks at 138.78 eV and 137.27 eV,respectively, which can be attributed to PbO, PbSO3and PbS, the Pb4f7 peak of M-11 at a binding energy of 139.04 eV is consistent with PbSO4. Upon taking into account the spectrum of S2p3 in Figure 4(b), the S2p3 peak of M-8 at a binding energy of 161.34 eV corresponds to sulfides, which is also indicative of PbS formed on the surface, the S2p3 peak of M-8 at a binding energy of 168.40 eV corresponds to sulfates. The S2p3 spectrum of M-11 shows a relatively strong peak for sulfates at 168.29 eV and a weak peak for sulfides, indicating that the high valence state of sulfur in sulfates dominates the sulfur species on the M-11 test coupon surface. This is consistent with the single Pb4f7 peak for PbSO4found on the M-11 test coupon. Figure 4(c) shows the Zn2p peak of M-8 at 1 022.41 eV and Figure 4(a) shows the P2p peak of M-8 at 133.39 eV,corresponding to glassy zinc phosphates[13-14]. The Zn2p peak of M-8 at 1 022.41 eV also can be attributed to ZnO,and in combination with the analysis of the spectrum of ZnLMM, the Auger kinetic energy of Zn was determined at 987.00 eV, indicating further the existence of ZnO. The concentrations of Zn2p and P2p on the M-8 coupon surface are much higher than those on the M-11 coupon, as shown in Table 6, which indicates that a greater amount of glassy zinc phosphates has been formed on the M-8 coupon surface. This is the main factor accounting for the better lead corrosion resistance of oil sample M-8.
Figure 5 shows the XPS spectra of typical elements on lead coupons of the ZDDC treated oil samples. In Figure 5(a), the spectrum of Pb4f7 of M-12 and M-15 shows peaks at 138.11 eV and 138.35 eV, respectively, which can be attributed to PbO, PbSO3or PbS, while the weak peak at 136.11 eV of M-12 can be attributed to elemental Pb. In Figure 5(b), the S2p3 peaks of M-12 and M-15 at close to 161.30 eV correspond to sulfides, the S2p3 peaks at close to 168.10 eV correspond to sulfates. The S2p3 spectrum of M-12 shows a distinctly strong peak for sulfides and a weak peak for sulfates, indicating that the low valence state of sulfur in sulfides dominates the sulfur species on the low corrosion coupon surface. Figure 5(c)shows the Zn2p peak of M-12 at 1 021.63 eV, which could be attributed to ZnS, in combination with the analysis of the spectrum of ZnLMM, while the Auger kinetic energy of Zn was determined at 989.1 eV, further indicating to the existence of ZnS. The concentrations of S2p3 and Zn2p on the M-12 coupon surface were obviously higher than those on the M-15 coupon, as shown in Table 6, indicating that a greater amount of ZnS was formed on the M-12 coupon surface. This is the main factor contributing to the better lead corrosion resistance of M-12, although there might be PbS formation on the coupon surfaces.
It should be noted that chloride was detected on the lead surface immersed in oil samples M-12 and M-14,as shown in Table 6. However, no confirmed effect of chloride on lead corrosion was found, and the residual chlorine from the dispersant manufacture process is not a factor in this case.
3.4 Discussion
The copper-lead bearing corrosion is caused by chemical attack on metal surfaces by reactive agents, which could be acidic oil oxidation products or decomposition products of certain oil additives. The acidic oxidation products and contaminants usually attack the lead in the alloy matrix, resulting in lead leaching that weakens the copper structure. The lead reacts relatively rapidly with the organic acids and peroxides that are present in oil oxidation products. Peroxides can first oxidize the lead surface, and the lead surface oxides are then converted by the organic acids to lead soaps. These soaps may dissolve in the oil or become dispersed in the form of sludge. The findings in 3.1 agree with the mechanism of lead corrosion by lubricants.The oxidation stability of base oil or base oil blend can be taken as a valuable assessment parameter for screening of the highly corrosion-resistant formulations.
There are two ways to inhibit lead corrosion by lubricants:one is to reduce the formation of peroxides and organic acids by inhibiting the oxidation of lubricating oil, and the another one is to form protective films on the surface of lead metals or lead alloys. The multifunctional additive ZDDP and ZDDC can take effect by both ways. On one hand, ZDDP and ZDDC can act as peroxide decomposers to retard lubricant oxidation, and on the other hand, both of them can form passive forms on the lead surface,which not only can prevent further corrosion on the lead surface, but also can prevent lead metal from releasing pro-oxidant ions to the lubricating oil.

Figure 5 XPS spectra of typical elements at the surface on lead coupons run with ZDDC
The formation of protective films by reactive sulfur on lead surface was considered to be the major mechanism of corrosion inhibition[3-5]. In this work, the XPS surface analysis results of the ZDDP treated oil samples show the existence of sulfur species on both the high and low lead corrosion coupons. Quite a part of the sulfur species on the low corrosion-affected coupon are sulfides with low valence state of sulfur, i.e. PbS; the primary part of the sulfur species on the high corrosion-affected coupon are sulfates with high valence state of sulfur, i.e. PbSO4.There is little difference of the sulfur concentration between the low and high corrosion-affected coupon surfaces. This indicates that the protective films of sulfur species formed by ZDDP on the lead coupons are not the major factor affecting the degree of lead corrosion.The XPS results also show the formation of glassy zinc phosphates by ZDDP on the lead coupons surfaces, which is similar to the composition of the thermal reaction films formed by ZDDP on the iron surface[15]. ZDDP can adsorb and form thermal reaction films on metal surfaces. These“thermal films” have similar composition as tribofilms,consisting mainly of a thin outer layer of polyphosphate grading to pyro- or orthophosphate in the bulk[16-17].Unlike tribofilms, the thermal films of ZDDP on iron show little evidence of iron in the film itself, with the main cation being zinc[15]. In this work, the concentrations of P and Zn on the low corrosion-affected coupon are significantly higher than those on the high corrosionaffected coupon. This indicates that greater amount of thermal reaction films containing glassy zinc phosphates was formed on the low corrosion-affected coupon surface.The lead coupon surface was phosphated by ZDDP contained in oil, which became resistant to corrosion. A more or less amount of glassy zinc phosphates protective films formed on lead coupons is the main factor affecting the degree of lead corrosion.
ZDDC contains no phosphorus, and cannot form the phosphate protective films on lead coupon surfaces. The XPS results show the formation of ZnS on lead coupons of the ZDDC treated oil samples. The concentrations of S and Zn on low corrosion coupon are significantly higher than those on high corrosion coupon. This indicates that greater amount of ZnS protective films was formed on the low corrosion-affected coupon surface immersed in the ZDDC treated oil samples. A more or less amount of ZnS films formed on lead coupons is the main factor affecting the degree of lead corrosion by the ZDDC treated samples.
Judging from the results of Table 4 and XPS surface analysis, it is concluded that the glassy zinc phosphates protective film formed by ZDDP is more effective than the zinc sulfides film formed by ZDDC.
4 Conclusions
The oxidation stability of base oil blend was shown to have a close relationship with the lead corrosion performance of formulated lubricants, which can be taken as a valuable assessment parameter for screening of the highly corrosion-resistant formulations.
For ZDDP treated oil samples, different dosages of ZDDP have formed different amounts of thermal films by zinc polyphosphate glasses on the surface of lead coupons,which is the main factor linked to the lead corrosion difference. The formation of protective films composed of sulfur species by ZDDP on the lead coupons is not the major factor that could affect the degree of lead corrosion.The interaction between dispersants and ZDDP had a significant impact on lead corrosion.
For the ZDDC treated oil samples, different dosages of ZDDC have formed different amounts of protective films composed of zinc sulfides on the surface of lead coupons,which is the main factor for the lead corrosion difference.The glassy zinc phosphates protective film formed by ZDDP is more effective than the zinc sulfides film formed by ZDDC.
Acknowledgement:The work was financilly supported by the Research Project of China Petroleum & Chemical Corporation(112066).
- 中国炼油与石油化工的其它文章
- Denitrification of Coal Tar Diesel Fraction by Phosphate Imidazolium Based Polymeric Ionic Liquids
- Development and Application of Hydrocracking Catalysts RHC-1 /RHC-5 for Maximizing High Quality Chemical Raw Materials Yield
- Study of Isothermal Equilibrium, Kinetics and Thermodynamics of Adsorptive Desulfurization on Synthesized CuIYIIIY Zeolite
- Direct Conversion of Glucose to 5-Hydroxymethylfurfural over Zirconium Phosphate Catalyst in a Biphasic System
- Mass Transfer Characteristics of H2 and CO in Mimicked F-T Slurry Bubble Column Reactor
- An Approach for Preparation of Excellent Antiwear PTFE Nanocomposites by Filling As-prepared Carbon Nanotubes/Nanorods (CNT/CNR) Mixed Nano-Carbon Material

