Rice Husk Ash: A New Silicon Source for Preparing SAPO-34 Catalysts Used in the Methanol-to-Olefins Reaction
Ma Shoutao; Wang Yingjun; Ge Dongmei
(1. College of Chemistry & Chemical Engineering, Northeast Petroleum University, Daqing 163318;2. Daqing Petrochemical Research Center of PetroChina, Daqing 163714)
Abstract: SAPO-34 molecular sieves were synthesized directly by hydrothermal method with rice husk ash (RHA) used as the silicon source. The crystal structure, composition, surface morphology and acidity of the synthesized products were characterized by XRD, ICP-OES, SEM and NH3-TPD analyses. The results showed that the synthesized SAPO-34 molecular sieves had a high crystallinity, without any impure phase. Compared with the SAPO-34 prepared by the silica sol, RHASAPO-34 had similar acid properties in strength. The methanol to olefins (MTO) experiments showed that the SAPO-34 molecular sieve synthesized from RHA exhibited both a good catalytic activity and ethylene selectivity.
Key words: SAPO-34; rice husk ash; molecular sieve; MTO
1 Introduction
According to Food and Agriculture Organization (FAO)statistics, the world rice (milled) production in 2016 totaled about 501.14 Mt[1]. Therefore, the total amount of the rice husk byproduct was equal to about 110 Mt. Rice husk has become an agricultural waste in many places,causing pollution to the environment. At present, rice husk recycling is mainly focused on its fuel use to provide energy[2]. Besides, there are also studies on mixing rice husk ash into lime and cement[3-5]. According to the analysis, the rice husk ash (RHA) contains 85%—98%of silica[6]. Therefore, it is important to effectively utilize abundant silica resources contained in the rice husk.
The SAPO-34 molecular sieve was first invented by the Union Carbide Corporation (UCC) in 1984 (SAPO-n,nstands for structural models)[7]. It is connected by PO4,AlO4and SiO4tetrahedron to form a three-dimensional skeleton structure. Hitherto, a lot of research work has reported on the synthesis, physicochemical and catalytic properties of SAPO-34[8-11]. This catalytic material has already been commercially used in the methanol-toolefin (MTO) process, because of its high yield of light olefins[12-15]. Moreover, the technology for synthesizing SAPO-34 with silica sol used as the silicon source is quite mature[16-18]. However, the preparation of SAPO-34 zeolite in the hydrothermal system using rice husk ash as the silicon source has not been reported yet. In this paper,the effects of rice husk ash on the structure and properties of SAPO-34 were investigated by using rice husk ash as silicon sources.
2 Experimental
2.1 Preparation of rice husk ash
Dried rice husk and hydrochloric acid at a mass fraction of 3% were mixed at 1:11, and then boiled for 3.5 h after mixing. After cooling, the solid product was washed with distilled water until the pH value of discharged water was 7 before it was dried at 105 °C to reach a constant weight,and then it was calcined for 3 h at 520 °C in a muffle furnace to yield the rice husk ash[19-20]. The yield of rice husk ash was about 13%.
2.2 Synthesis of SAPO-34
SAPO-34 was prepared by using DEA (diethylamine) as the template, phosphoric acid as the phosphorus source,pseudoboehmite as the aluminum source, silica sol and rice husk ash as the silica sources, respectively. The above materials were mixed to a certain degree prior to being stirred evenly to form gels. The formed gels were transferred into a Teflon lined stainless steel autoclave to be subject to crystallization at specified temperatures.
To stop the crystallization process the autoclave was removed from the oven at a specified duration and quenched with cold water, followed with measurement of the pH value of the reaction products. Products were recovered by emptying the autoclave of its contents in hot water under stirring for several minutes. Then the solid was filtered, washed with hot demineralized water and dried at 110 °C for 6 h. Removal of the template was carried out by air calcination at elevated temperatures. The calcination was performed by heating the samples at 540 °C for 5 h.
2.3 Characterization
The powder XRD patterns were recorded on a Rigaku D/max-IIB diffractometer using CuKα radiation. The scanning speed was 2(°)/min across a scanning range of between 1.5°—70°. The scanning electron microscopy(SEM) images were obtained using a ZEISS SUPRA 55 SAPPHIRE microscope operating at an acceleration voltage of 20 kV. The surface area of samples was calculated using the BET method in ap/p0range of 0.01―0.1. The pore size distribution of samples was calculated from the adsorption branch of the isotherms using the Barret-Joyner-Halenda (BJH) method. The acidic properties of samples were tested by NH3-TPD study using an Autochem II 2920 chemisorption analyzer.Hence, 0.15 g of sample were preheated under N2flow(40 mL/min) from room temperature to 450 °C, kept at that temperature for 2 h, and subsequently after that the temperature was decreased to 150 °C for conducting adsorption of the sample with NH3till saturation at 150 °C for 0.5 h. The ammonia adsorbed on the surface of the sample was purged with helium, while the temperature was raised to 700 °C for the TCD to detect the desorption signal. Chemical analysis of Al, P and Si in the calcined samples was performed by inductively coupled plasma optical emission spectrometry (ICP-OES, Perkin-Elmer 3300DV instrument) after sample dissolution by alkaline fusion.
2.4 Catalytic activity measurements
The MTO reaction was carried out in a fixed-bed microreactor at 450 °C under atmospheric pressure. A total of 2.5 g of catalyst mixed with an equivalent volume of quartz (20―40 mesh) was fed into the center of a stainless steel reactor. Prior to the reaction, the catalyst was activated at 550 °C in air for 1 h. The weight hourly space velocity(WHSV) was 2 h−1. The analysis of the reaction products was performed using an on-line gas chromatograph(Varian3800) equipped with a flame ionization detector and a Varian Porapak Q-HT capillary column.
The conversion of methanol was calculated by applying the molar balance between the feedstock introduced into the reactor and the products leaving the reactor as shown in Eq. (1). The selectivity for the products of interest were expressed as the mass percentage of each product and calculated according to the carbon balance between the feedstock and the reaction products in the reactor(Eq. (2)). Hereby, the superscripts i and o refer to the components identified at the inlet and outlet of the reactor,respectively, while the subscriptxrefers to the number of carbon atoms.

3 Results and Discussion
3.1 Charaterization of the RHA
Table 1 shows the amount of minerals in silica obtained via acid digestion of RHA. The content of SiO2is equal to at least 98% as determined by the XRF analysis.The porosity properties of RHA obtained from nitrogen adsorption isotherms are shown in Table 2. The average pore diameter of samples is over 5 nm, which falls into the category of mesopores, while their BET surface area is greater than 200 m2/g. Research results show that RHA consists of nano-SiO2particles (~50 nm) stacked slackly with each other. Grain size particles can be observed from the SEM micrographs, and the size of the particles is in the nanoscale range (0.1—100 nm), which is called the nano-SiO2particles. The nanoscale SiO2particles cause the concentration of a high proportion of the total number of atoms on the surface, which is believed to be very beneficial to the chemical activity of RHA[21-22].

Table 1 Mineral compositions of the extracted silica from RHA determined by XRF analysis w, %

Table 2 Porosity properties of RHA examined by BET analysis

Figure 1 SEM micrographs of RHA
3.2 Crystal structure and morphological analysis
The physicochemical properties of the samples obtained from silica sol and rice husk ash were compared. Figure 2 shows the XRD spectra of the synthesized samples from different silicon sources. The samples exhibit a typical CHA diffraction pattern, showing that no crystalline reflections belonging to other crystalline phases are present. Additionally, it is observed that the peak position of the synthesized products are almost the same as those reported in the literature[23], indicating that the product is the SAPO-34 molecular sieve. Compared with the SAPO-34 sample prepared by using silica sol as the silicon source,the relative crystallinity of RHA-SAPO-34 is relatively high, because the intensity of diffraction peaks in the RHA-SAPO-34 sample is much higher than SAPO-34, as shown in Figure 2.
Polysilicic acid formed by SiO2can usually exist under the highly alkaline condition, but contradictorily, the rate of polysilicic acid hydrolysis into mono-silicic acid is slow, so it can produce a low concentration of silicic acid which cannot fully fill the template space of the micelles and cause the structural defects of the molecular sieve[24].However, because of a large number of SiO2particles in RHA, more silicic acid ions are produced in the solution,and the template space is filled well. Thus, the high crystallinity of the RHA-SAPO-34 molecular sieve was obtained. This phenomenon can also be verified in SEM micrographs.

Figure 2 XRD patterns for samples synthesized using different Si sources
Figure 3 presents the SEM photographs of the synthesized samples. It can be seen that the grains of SAPO-34 sample synthesized from rice husk ash serving as the silicon source are similar to those of the SAPO-34 crystal synthesized by using silica sol as the silicon source.
3.3 Elemental analysis
The chemical composition of samples obtained by ICPOES analysis is presented in Table 3. It can be found that in samples prepared from RHA, the Si/(Si+Al+P)ratio is very close to that of the silicon gels, and this value is slightly lower in the RHA-SAPO-34 sample.Although the amount of silicon incorporated into the network is similar in SAPO-34 and RHA-SAPO-34, the different proportion of phosphorus and aluminum in the solid samples suggests that the silicon distribution in the framework is rather different in both samples.
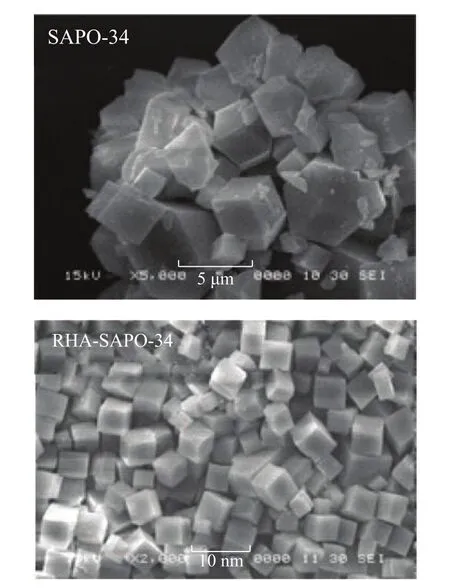
Figure 3 SEM images for samples synthesized using different Si sources

Table 3 Content of main components in test samples
3.4 Acidity
As a useful strategy to characterize the acidic properties of samples, the NH3-TPD technique was applied herein to compare the acidic concentration and strength of the two zeolite samples prepared from different Si sources, with the results shown in Figure 4.
Both of the samples show two desorption peaks with maxima at 180 °C and 420 °C, which are assigned to NH3desorption from weak and strong acid sites,respectively. Obviously, both of the samples synthesized from different Si sources have similar acidic properties in the strength, albeit different in the amount. The two peak positions can represent the weak Brønsted acid sites or Lewis acid sites and the stronger Brønsted acid sites[25], respectively. It can be seen from Figure 4 that the weak acidity of RHA-SAPO-34 is weaker than that of the traditional SAPO-34.
The silicon atom in the skeleton is the main source of the acidity in SAPO-34 molecular sieve. The Si (4Al) structure in the skeleton represents an acidic site. When the “silicon island” Si (4Si) is formed in the skeleton, the silicon atom in the center will not produce the Brønsted acid, which can reduce the acidity of the molecular sieve[26]. The nanoscale SiO2in RHA can produce more silicic acid in a short time when hydrolysis happens. Some silicon atoms can form the“silicon island”. Therefore, the RHA-SAPO-34 molecular sieve with weaker acidity is obtained.
3.5 Catalytic activity measurements
The catalytic activity of the zeolite samples was then tested during the MTO reaction. The MTO reactivity of the synthesized RHA-SAPO-34 molecular sieve was similar to that of SAPO-34 zeolite synthesized from silica sol, and the conversion of methanol reached 100%, which decreased rapidly only at the end of MTO reaction. It can be seen from Figure 5 that under the same experimental conditions, the initial ethylene selectivity of the two samples was very close, which was equal to around 38%. The initial selectivity of propylene and the trend of selectivity were similar. The maximum selectivity of ethylene and propylene was about 88%.The RHA-SAPO-34 sample had similar catalytic activity and selectivity as those of the SAPO-34 sample prepared from silica sol used as the silicon source. The run length of RHA-SAPO-34 was comparable to that of the contrast sample, which could reach 400 min coupled with a methanol conversion exceeding 98%.
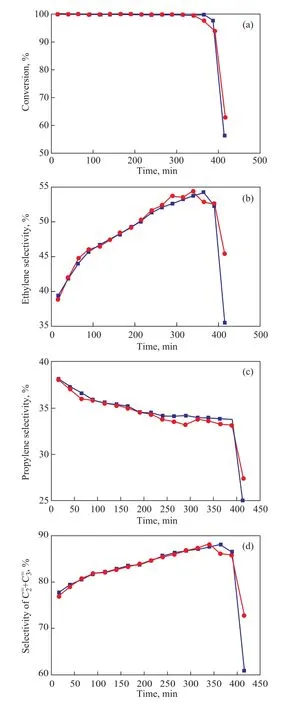
Figure 5 Conversion and selectivity for samples synthesized using different Si sources
4 Conclusions
The molecular sieve labeled as RHA-SAPO-34 was synthesized from the agricultural waste rice husk used as the silicon source. The RHA-SAPO-34 sample was characterized by XRD and SEM techniques, showing that the said molecular sieve featured high crystallinity, fine grain size, and weak acidity. The performance test results have revealed that the RHA-SAPO-34 molecular sieve has catalytic activity and selectivity comparable to those of SAPO-34 molecular sieve prepared by using silica sol as the silicon source.
Acknowledgments:This work was supported by the Cultivation Foundation of Northeast Petroleum University (2017PYYL-03).
- 中国炼油与石油化工的其它文章
- Denitrification of Coal Tar Diesel Fraction by Phosphate Imidazolium Based Polymeric Ionic Liquids
- Development and Application of Hydrocracking Catalysts RHC-1 /RHC-5 for Maximizing High Quality Chemical Raw Materials Yield
- Study of Isothermal Equilibrium, Kinetics and Thermodynamics of Adsorptive Desulfurization on Synthesized CuIYIIIY Zeolite
- Direct Conversion of Glucose to 5-Hydroxymethylfurfural over Zirconium Phosphate Catalyst in a Biphasic System
- Mass Transfer Characteristics of H2 and CO in Mimicked F-T Slurry Bubble Column Reactor
- An Approach for Preparation of Excellent Antiwear PTFE Nanocomposites by Filling As-prepared Carbon Nanotubes/Nanorods (CNT/CNR) Mixed Nano-Carbon Material

