Optimum Operating and Regeneration Parameters of ZnI2 Catalyst for Converting Methanol to Triptane: An Ideal Component of Unleaded Aviation Gasoline
Chen Weiwei; Song Yueqin; Zong Rui; Du Changfei; Zhou Xiaolong; Li Chenglie
(1. Department of Petroleum Processing, East China University of Science and Technology, Shanghai 200237;2. Beijing Aviation Oil Technique Developing Center, Beijing 100076)
Abstract: Conversion of methanol (MeOH) to 2,2,3-trimethylbutane (triptane) over zinc iodide(ZnI2)catalyst was investigated in a closed batch reactor. Optimum operating parameters were searched and catalyst deactivation and regeneration behaviors were discussed. The fresh, spent and regenerated catalysts were characterized by XRD, TG and XRF measurements to elucidate the related mechanism. The experimental results showed that the triptane yield reached up to 12.2% under the process conditions, including a reaction temperature of 200 oC, an initial pressure of 0.35 MPa, a reaction time of 2 h, and a MeOH to ZnI2 molar ratio of 2. The catalyst was gradually deactivated after several run cycles. A small amount of iodine was lost and a certain amount of ZnO was formed on the deactivated ZnI2 catalyst. Deactivation of the catalyst could be attributed to the reduction of ZnI2 amount, resulted from iodine loss and formation of ZnO. It was firstly pointed out from our work that the deactivated catalyst could be well regenerated by the hydrogen iodide (HI) and the tert-butanol compensation method, wherein the latter was added as an initiator into the reaction system. The activity recovery of the deactivated(spent) catalyst might be related to the reformation of ZnI2 and acceleration of the initiation step of this reaction.
Key words: triptane; zinc iodide; methanol; catalyst; regeneration; deactivation
1 Introduction
The specification imposed on aviation gasoline is quite complicated and extremely rigorous. This product is, therefore, prepared by blending some possible components to a special formulation that is tailored for use in piston engine powered private planes and most commercial aircraft. Generally, either aviation alkylate from alkylation unit or industrial iso-octane from oligomerization of iso-butylene and hydrogenation is mixed with light hydrocarbons (C4and iso-C5or iso-C5/C6mixture) to prepare a base fuel which is then blended with high octane boosters, including aromatics, MTBE and tetraethyl lead (TEL). The latter has long been considered to be an absolutely necessary constituent in modern high-octane aviation gasoline. However, environmental concerns may require the phase-out of lead in this product, even if it is commonly recognized to be a really difficult task. Althrough the efforts have been pursued in the last two decades, breakthrough has appeared in the patent literature mainly by using more efficient octane boosters, including mesitylene (1,3,5-trimethyl benzene) and/or aromatic amines[1-4]. These boosters have extremely high MON. In addition, they are able to provide an unleaded aviation gasoline formulation with anti-detonation performance better than that of the widely used commercial product Grade 100LL, wherein LL stands for low lead, when it is used on a full scale aircraft engine[5-8]. Some related properties of these pure compounds are listed in Table 1[9-10]. It can be seen that mesitylene and aromatic amines often have rather high boiling point so that more percentage of light components(mainly pentane with a MON of 90.3) must be added to meet the volatility related specifications, including the Reid vapor pressure and the ASTM distillation profiles.Moreover, it is also disclosed that the net heat content of aromatics is always insufficient.
Alkanes are chemically stable. They have sufficiently high net heat content and good blending behaviors.Therefore, the importance to boost up the motor octane number (MON) of the base fuel has also been in the highlight. For this purpose, the industrial iso-octane is used as an alternative to aviation alkylate. The most important component is triptane which has the highest octane number (MON=101) among them. If triptane can be produced in massive scale and blended into the aviation gasoline pool, it is possible to partially substitute for iso-octane and thus further improve the MON of the base fuel. In addition, it is also seen that triptane has lower boiling point (80.9oC) and thus is able to decrease the concentration of iso-pentane (MON= 90.3), which is also advantageous to boosting up the octane number of base fuel. All these factors can reduce the concentration of those boosters and make it possible to produce unleaded aviation gasoline, which is able to comply fully with all the requirements of the specification. Many patent literature has already reported the good results to prepare aviation gasoline by the use of triptane[11-13].

Table 1 Related properties of some blending components
Up to now, two series of catalysts, including metal halides and zeolites, have been confirmed to have potential use for converting methanol (MeOH) or dimethyl ether(DME) to triptane[14]. It is commonly recognized the zeolite has rather low activity and its stability has rarely been reported[15-16]. On the contrary, the metal halid catalysts,including zinc iodide (ZnI2) or indium iodide (InI3) have been largely reported to have a relatively good catalytic performance in triptane synthesis[17-19]. It has been reported in the earlier literature that MeOH can be converted to hydrocarbons containing a large amount of triptane over ZnI2at 200 °C[20]. The major products obtained from this reaction include triptane, smaller molecular branched alkanes, and hexamethylbenzene. The mechanism about the conversion of MeOH over these catalysts has also been widely reported[21-22].Some published papers have reported in this decade that the triptane yield can be dramatically increased by adding promoters, including hypophosphorous acid or adamantine[23-25]. For the industrial application, two continuous processes have been suggested in a latest patent literature, wherein the hydrogen iodide solution is used to keep the high catalyst activity.However, details have not been given[26].
In research and development of an industrial catalyst, it is important to examine the effect of operating parameters and search for the optimum parameters to achieve high catalytic behaviors. The other work is to maintain the catalytic activity for a long operating duration.Therefore, catalyst deactivation and regeneration must be dealt with in order to find a good way to prolong the life of the catalyst. However, detailed information about this reaction to produce triptane has rarely been published. In this paper, these issues will be addressed. The mechanism of catalyst deactivation and regeneration will also be studied. An effective catalyst regeneration by the hydrogen iodide and tert-butanol compensation method is firstly reported in this paper. Results from our work would pave the way to solve these problems and are advantageous to the industrial production of triptane.This new methanol-to-triptane process can also save the petroleum resources by using methanol obtained from natural gas as the feedstock.
2 Experimental
2.1 Materials and reactor
Starting materials included ZnI2(99%), MeOH (99.9%,anhydrous), tert-butanol (99%), hydroiodic acid (HI) (50%aqueous solution), and nitrogen gas (>99.99%).
Reactions were carried out in an 100-mL stainless steel batch reactor, with its inner lined with glass to solve the corrosion problem.
2.2 Procedure
Definite amounts of MeOH, ZnI2and other liquid reagents were introduced into the reactor. The mixture was stirred to dissolve solid as much as possible. The reactor was closed and then purged and pressurized to a certain initial pressure (P0) with a high-purity nitrogen stream.It was heated with an oil bath to perform this reaction at a specified temperature. After a fixed time duration, the reactor was moved out from the oil bath and cooled down to room temperature until the pressure was kept constant.The products, including those from gas phase, oil phase,and aqueous phase were separated and then collected.They were first qualitatively analyzed on a GC-MS set(Agilent 6890-5973N) and then were analyzed by using a GC-9890A gas chromatograph, which was equipped with a capillary column and a FID detector.
In this work, it has been observed that triptane could hardly be detected in the gaseous products, but existed mainly in oil phase. Moreover, the triptane content in oil phase was the highest among the products. The triptane yield was calculated from the weight of oil phase product and their triptane content, based on moles of carbon. And the total oil yield is calculated from the weight of oil product and weight of MeOH introduced into the reactor:
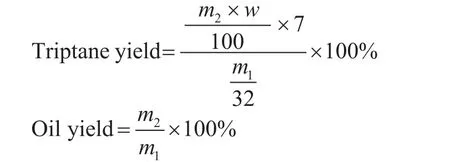
wherem1is the weight of MeOH introduced into the reactor,m2is the weight of obtained oil phase andwis triptane content in the oil phase (%).
2.3 Catalyst recovery and regeneration
Catalyst was recovered from the aqueous phase (sometimes together with a solid phase) via vacuum distillation. In this research work, the aqueous and solid phase products were transferred to a distillation flask. The whole distillation system was purged with nitrogen gas to replace the air. The distillation was then carried out at 100 °C and a residual pressure of up to 0.08 MPa to remove all the volatile components. Regeneration of catalyst was performed by adding a certain amount of hydrogen iodide (HI) solution into the mixture of the aqueous phase and solid phase before vacuum distillation.
2.4 Catalyst characterization
The TG analyses of the catalysts were performed on a PyrisTM1 thermogravimetric analyzer (TGA). The sample was heated from room temperature to 1 000 °C at a temperature increase rate of 10 °C/min under a nitrogen or air stream with a flow rate of 10 mL/min.
The XRD patterns of the catalysts were recorded on a Rigaku D/Max 2550 X-ray diffractometer using Cu Kα radiation in the 2θangle ranging from 10° to 80°.
The XRF measurement was performed on an ARL Advant’X X-ray fluorescence spectrometer for elemental composition analysis of the catalyst.
3 Results and Discussion
3.1 Effect of reaction condition
3.1.1 Effect of pressure on triptane yield
To examine the effect of initial nitrogen pressure in the batch reactor, tests are performed on 4 g of MeOH and 20 g of ZnI2(at a molar ratio of 2) at 200 °C for 2 hours.It can be seen from Figure 1a that the reaction pressure (PR) was mainly composed of the partial pressure of produced gas phase (PG) besides that of nitrogen pNfrom its initial stateP0. Moreover,PRandPGrose with an increasing initial nitrogen pressure (P0). Figure 1b indicates that both liquid products and their triptane yield rose with an increasing initial nitrogen pressure (P0).These results revealed that the increase inPRduring the reaction was attributed mainly to the formation of gas products, including DME, CH3I and other small molecular hydrocarbon products. Both of the first two products were suggested to be intermediate species for hydrocarbon formation, when they were dissolved into liquid phase to be in contact with ZnI2catalyst[21,27]. At higherPRvalues,more DME and CH3I must be dissolved in liquid phase to speed up the reaction. Afterwards, both the liquid product yield and the triptane yield became almost unchanged after the nitrogen initial pressure reached 0.35 MPa and higher.

Figure 1 Effect of nitrogen initial pressure (P0) on: (a) reaction pressure (PR)=pG +pN; and (b) triptane yield and oil yield
3.1.2 Effect of of MeOH to ZnI2molar ratio on triptane yield
To investigate the effect of MeOH to ZnI2molar ratio on the product yield, similar tests were performed at various loaded ZnI2weight. The other operating parameters remained the same as mentioned above.Results are shown in Figure 2. It was clear that the oil triptane yield decreased with an increasing MeOH to ZnI2molar ratio, but it was not in a linear relationship probably owing to the changes in pressure. When the MeOH/ZnI2molar ratio increased to 6, both the weight of oily product and the triptane yield decreased to a very low level.
It is reported in the literature that the undissolved ZnI2catalyst is responsible for the initiation of reaction, while the dissolved ZnI2can catalyze the chain growth reaction in the course of this reaction[21]. Our results were in line with this conclusion, because ZnI2had been completely dissolved in methanol, when the MeOH/ZnI2molar ratio was greater than 4 and the decrease in both the oil product weight and the triptane yield was related with the entire dissolution of this catalyst that played a negative role in the initiation reaction step.
3.1.3 Effect of reaction time on triptane yield
Tests were also performed to investigate the changes in the content and yield of triptane and the yield of oil products depending upon the reaction time, when other parameters were the same as mentioned above. As shown in Figure 3(a), the triptane content remained almost unchanged, while the patterns of both oil yield and triptane yield were rather alike. Both of them increased rapidly to 17.5% and 12%, respectively, after 1.5 hours of reaction, whilst they slowly reached 22.5% and 16%, respectively, after 6.5 hours. It can be explained by the change of reaction pressure (PR) in Figure 3(b).Increase in this pressure could promote the dissolution of intermediate species in liquid phase that would accelerate the reaction rate and sharply increase the product yield within a certain reaction stage. Then the reagent and intermediates were continuously consumed, while the pressure gradually dropped. Therefore, the reaction rate slowed down and the total yield of triptane only slightly increased.
3.1.4 Effect of reaction temperature on triptane yield Temperature is also an important factor for this reaction.The effect of reaction temperature on the transformation of methanol to triptane is shown in Figure 4, wherein the tests were performed under the same condition as mentioned above except the temperature. In this test, only water and DME were detected at 140 °C and the total oil yield was less than 2.5% and the triptane yield was below 2% when the reaction temperature reached 160 °C. Then they sharply increased up to 18% and 12.2%, respectively,at 200 °C. On the contrary, when the temperature reached 220 °C, both the triptane yield and the triptane content in oil products simultaneously decreased again, whilst the oil yield remained almost unchanged. Based on this test results, it is concluded that the suitable reaction temperature should be 200 °C.

Figure 3 Effect of reaction time on (a) triptane yield; and(b) reaction pressure
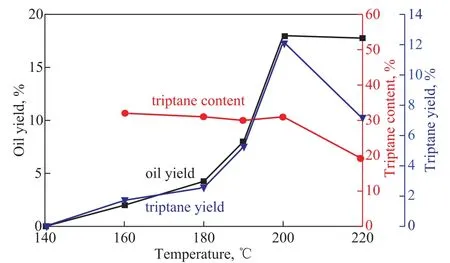
Figure 4 Yield of triptane at various reaction temperatures
3.1.5 Effect of water on triptane yield
During the conversion of methanol to hydrocarbons,water is inevitably formed. The formation of water might inhibit the conversion of MeOH over ZnI2[21]. In order to investigate the effect, water was added into the reaction system, when the reaction proceeded for 1.5 hours. The yield of oil product dropped to 15% and the yield of triptane decreased to 10.5%, when 0.5 g of water was added to the reaction system. In addition, oil products were not detected, when 1.0 g of water was added,although very few gas products, including DME and trace of hydrocarbon, were produced. Obviously, the addition of water could inhibit the reaction. On the other hand, it is reported that the conversion reaction involving DME feed would not happen, if water is absent at the beginning[21].These results demonstrate that a small amount of water is necessary for the reaction, but too much water would play a negative effect and even could terminate this reaction.ZnI2is usually considered to be a Lewis acid and can be easily hydrolyzed to form Brönsted acid sites. Water formed in the reaction can help ZnI2to produce Brönsted acid sites. Therefore, it is postulated that the conversion of methanol to triptane would involve both Lewis acid and Brönsted acid.
3.1.6 Product distribution
Based on the investigation described above, an optimum reaction condition had been selected, which covered 4.0 g of methanol feed, and 20 g of the ZnI2catalyst, so that the MeOH to ZnI2molar ratio was equal to 2. The reaction was performed at 200 °C and an initial nitrogen pressure of 0.35 MPa for 2 hours.
The reaction products were divided into the gas phase and oil phase products. The former was mainly composed of propane, isobutane, isobutene and isopentane. The oil phase products are listed in Table 2, wherein only those products with higher content are given. It can be seen that among the hydrocarbon products, C4—C7branched alkanes and alkenes account for 60% and C7compounds have the highest content, wherein triptane accounts for 31%. In addition to those alkanes and alkenes, hexamethylbenzene also has a relatively high content, reaching 6% in the products. The wide range of product distribution and the high selectivity for triptane are related to the complex reaction processes, including the chain growth by methylation, the chain termination by hydrogen transfer,isomerization, and cracking that can favor the formation of the highly branched C7alkanes, including triptane[28-29].Triptane has an important potential use in aviation gasoline.The other hydrocarbon products listed in Table 2 can also be used as constituents for blending into the gasoline pool.

Table 2 Typical reaction product distribution
3.2 Catalyst deactivation and regeneration
3.2.1 Catalyst deactivation
For the successful application of this catalyst, the activity of recycled catalyst was investigated with the approach for regeneration of deactivated catalyst being discussed in this section. After each reaction, a brown muddy liquid phase was obtained. ZnI2catalyst after each batch reaction in repeated runs was recovered by using vacuum distillation.The catalytic activity of recycled ZnI2catalyst is shown in Figure 5. It can be clearly seen that the yield of oil product and triptane yield gradually decreased with the increase in run cycles (cycle index). After six reaction cycles, the yield of oil products sharply dropped from 18% to 7.5%, and the triptane yield decreased from 12.2% to 5%, although the triptane selectivity was still almost unchanged. After the reaction was repeated for several cycles, this deactivated catalyst sample, which was designated as the spent catalyst,was collected and used to examine the deactivation behaviors as shown in the following graph.
3.2.2 Regeneration of spent ZnI2catalyst
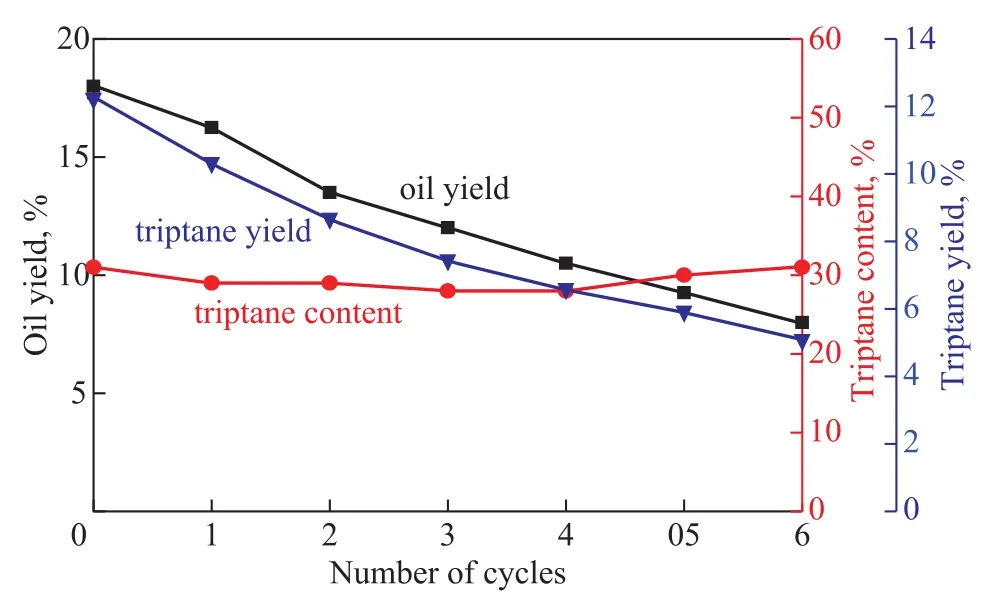
Figure 5 Changes in triptane yield with the number of cycles (T=200 °C, time=2 h, P=0.35 MPa, MeOH=4 g, ZnI2=20 g, MeOH to ZnI2 molar ratio=2:1 in mole)
In the first study, HI was selected for regeneration of spent catalyst. For the sake of comparison, a definite amount of fresh ZnI2catalyst was treated with a certain amount of HI and it was designated as the compared catalyst. Catalytic behaviors of these four catalyst samples are listed in Figure 6, wherein tests were performed atT=200 °C,reaction time=2 h, andP=0.35 MPa. It can be seen from Figure 6 that the triptane yield over both the fresh and compared catalysts was very close between each other,demonstrating that the addition of HI had no effect on the catalytic activity of the fresh catalyst. On the other hand,the triptane yield over the HI regenerated catalyst was still much lower than that of the fresh catalyst. These results demonstrated that the addition of only HI was not able to recover the activity of deactivated catalyst.

Figure 6 Triptane yield over (a) fresh catalyst, (b) spent catalyst, (c) HI regenerated catalyst, and (d) compared catalyst
In our work, it is important to have observed that HI is able to regenerate the above spent catalyst, if tert-butanol is blended to methanol feedstock as an initiator, with the result shown in Figure 6. Under this condition, the triptane yield over the regenerated catalyst increased from 6.7% achieved by the spent catalyst to 16.3%, which was even greater than 15.5% achieved by the fresh catalyst.This yield also reached the same level as that of the compared catalyst. It has been indicated in the published papers that some higher alcohols, olefins or aromatics can be able to play the role of initiators in the conversion of methanol to triptane[19-20]. In our work, it was for the first time confirmed that the deactivated catalyst could be thoroughly regenerated by HI solution, if tert-butanol was blended to methanol feed as an initiator.
3.3 Catalyst characterization in deactivation and regeneration steps
At first, the crystalline structure of these catalysts was characterized by XRD measurements, as shown in Figure 7. It can be seen that compared to the fresh ZnI2catalyst,there was no any new diffraction peak appearing on both the deactivated (spent) catalyst and the HI regenerated catalyst. However, the diffraction peaks on these two catalyst samples become weakened and broadened,indicating to the decrease in ZnI2crystal size by partial dissolution. As described in Section 3.1.2, it could probably lead to catalyst deactivation because of the negative effect on the initiation step of this reaction.

Figure 7 X-ray diffraction patterns of fresh catalyst, spent catalyst, and HI-regenerated catalyst
The TG measurements were performed on various catalysts, with the results shown in Figure 8. It can be seen from profile (a) in Figure 8 for the fresh ZnI2catalyst that the thermal weight loss under N2stream began at 350 °C and reached 100% at 490 °C, denoting the good volatility of ZnI2. On the other hand, the weight-loss percentage of the deactivated (spent) catalyst still remained at 96%when it was heated to 1 000 °C as exhibited by the profile(b) in Figure 8. The profile of HI regenerated catalyst,exhibited by the curve (c), showed a similar pattern with the graph (a), but the thermal weight loss was obtained at lower temperature. This lowering in temperature might be in line with the smaller ZnI2crystal size formed by partial dissolution which had been observed by the XRD analysis. In short, the above results revealed that the spent catalyst contained 4% of thermo-stable substance, which remained in a solid state at 1 000 °C. Furthermore, the said 4% of thermo-stable solid was able to react with HI,as evidenced by the curve (c) in TG measurement for the HI regenerated catalyst. To identify the thermo-stable substance, another TG test for fresh ZnI2was also performed with air serving as the carrier gas. The weight loss was 95%, while about 5% of residue was not volatile at a temperature of over 1 000 °C. The similar residue was also observed on the TG pattern of the spent catalyst. It is known that ZnI2would be quickly decomposed into ZnO and I2in air stream even at room temperature. In this reaction involving methanol, the formation of the 5% of stable substance from ZnI2were probably related to the formation of ZnO in the presence of methanol. The product ZnO was very thermo-stable at high temperature, but it could easily react with HI. Thus, it may be suggested that the 4% of residue in TG curve emanating from the spent catalyst were attributed to ZnO species. Combined with the catalytic reaction results, it could be concluded that the deactivation of ZnI2was related to the formation of ZnO species and the decrease in the amount of ZnI2catalyst. This test also indicated that the catalyst activity had already dropped to a very low level, notwithstanding 96% of ZnI2were still present during the recycle reaction.This result must be attributed to enrichment of highly dispersed ZnO particles on ZnI2catalyst surface. In addition to the very low content, the ZnO related spectra did not appear on the XRD profiles.In order to confirm the above postulation, elemental analyses of the fresh, spent and regenerated catalysts were carried out by XRF measurement, as shown in Table 3. It is observed that the iodine to zinc molar ratio of fresh ZnI2catalyst sample was about 1.98, which was close to the theoretic value. This ratio for the spent catalyst after six reaction cycles had dropped to 1.73, which was resulted from the iodine loss. When the spent ZnI2catalyst was regenerated by using HI, this ratio increased once again to 2.18. It has been proved that ZnO can be soluble in HI acid. In other words, HI was able to react with ZnO and could convert it into ZnI2. Therefore, it is suggested that a certain amount of ZnO was formed and a part of iodine species was lost during the catalytic reaction and there would be two causes leading to deactivation.

Figure 8 TG patterns of: (a) fresh catalyst, (b) spent catalyst, and (c) HI-regenerated catalyst

Table 3 Elemental analysis of fresh, spent and HI-regenerated catalyst samples
The iodine to zinc molar ratio can also be calculated to be 1.74 from TG result using nitrogen as the carrier gas, wherein the spent catalyst contained 96% of ZnI2and 4% of ZnO.Clearly, this reault was very consistent with that obtained via the XRF analyses and could provide another proof of iodine loss. The formation of brown aqueous phase was related to the dissolution of iodine and CH3I was also detected in the gas products. These phenomena were all indicative of the iodine loss from the catalyst.
During the conversion of methanol over ZnI2, iodine might play an important role in the reaction. Firstly, after a small amount of iodine was lost along with the formation of ZnO,the catalyst was deactivated. In addition, CH3I was detected in products. These results revealed that ZnO had no catalytic activity for the conversion of methanol to triptane and the presence of iodine was essential for this reaction. In the literature it is also indicated that the conversion of MeOH to triptane over zinc or zinc oxide is observed, when CH3I also exists in the system[27]. This fact also suggests that the coexistence of both zinc and iodine is indispensable for the conversion of methanol to triptane. CH3I might be the intermediate species formed in the reaction.
Moreover, the catalyst regeneration experiment results also indicated that the deactivated catalyst was not fully regenerated only by the addition of HI, but the catalytic activity could be effectively recovered by HI in the presence of tert-butanol. As given in Section 3.1.2 of this paper along with the results reported in the literature[21-22]on the conversion of methanol to triptane, the first step is the formation of C=C bonds on undissolved ZnI2catalyst and the second stage is the chain growth reaction to form higher hydrocarbons on the dissolved ZnI2catalyst. In our HI and tet-butanol compensation method to regenerate the deactivated catalyst, the formation of C=C bonds from tert-butanol is easier than that from methanol,because dehydration of tert-butanol is one of the methods to produce isobutene[30]. In other words, the addition of tert-butanol can accelerate the first step, which has been slowed down by the dissolution of ZnI2catalyst.
4 Conclusions
The present work has investigated the process for the conversion of MeOH to triptane over ZnI2catalyst. The effect of reaction conditions on the triptane yield was studied. The triptane yield increased with the increase in initial nitrogen pressure but decreased with the decrease in MeOH/ZnI2molar ratio. It increased with an increasing reaction temperature from 140 °C to 200 °C and then decreased at higher temperature. In a reaction time of two hours, a sharp increase in the triptane yield was observed and then it slowed down. The optimum conditions covered: a reaction temperature of 200 °C, an initial nitrogen pressure of 0.35 MPa, a reaction time of 2 hours, and a MeOH to ZnI2molar ratio of 2. Under these conditions,12.2% of triptane yield could be obtained. The catalyst activity decreased with the number of cycles. After 6 cycles of run, the triptane yield decreased to 5%. During the recycle reaction, a part of iodine in ZnI2catalyst was clearly lost and consequently ZnO was formed, which played a main role in catalyst deactivation. Judging from our work referred to in this paper, it is concluded that the deactivated catalyst can be entirely regenerated by using the HI solution and tert-butanol compensation method.
- 中国炼油与石油化工的其它文章
- Denitrification of Coal Tar Diesel Fraction by Phosphate Imidazolium Based Polymeric Ionic Liquids
- Development and Application of Hydrocracking Catalysts RHC-1 /RHC-5 for Maximizing High Quality Chemical Raw Materials Yield
- Study of Isothermal Equilibrium, Kinetics and Thermodynamics of Adsorptive Desulfurization on Synthesized CuIYIIIY Zeolite
- Direct Conversion of Glucose to 5-Hydroxymethylfurfural over Zirconium Phosphate Catalyst in a Biphasic System
- Mass Transfer Characteristics of H2 and CO in Mimicked F-T Slurry Bubble Column Reactor
- An Approach for Preparation of Excellent Antiwear PTFE Nanocomposites by Filling As-prepared Carbon Nanotubes/Nanorods (CNT/CNR) Mixed Nano-Carbon Material

