超高效液相色谱-四级杆飞行时间质谱结合Progenesis QI 分析不同品种橙的指纹图谱及其差异性代谢物
赵希娟,赵无疾,许华超
超高效液相色谱-四级杆飞行时间质谱结合Progenesis QI 分析不同品种橙的指纹图谱及其差异性代谢物
赵希娟,赵无疾,许华超
(西南大学园艺园林学院/南方山地园艺学教育部重点实验室,重庆 400715)
【目的】建立分析不同品种橙之间差异性代谢物的方法,找出各品种橙的特征代谢物,服务于橙的代谢组学研究,为橙果实和橙汁的鉴别提供参考。【方法】选取相同产地8个不同的橙品种,取其果皮的甲醇提取物,使用超高效液相色谱-四级杆飞行时间质谱(UPLC-QToF-MS)得到对应的指纹图谱,结合组学软件Progenesis QI,通过主成分分析、偏最小二乘判别分析和变量变化趋势图等进行综合评价,筛选出各品种间的差异性代谢物。根据精确分子量、二级碎片、标准品以及数据库和相关文献信息,对差异性代谢物进行鉴定。【结果】通过UPLC-QToF-MS指纹图谱得出8个橙品种的代谢物存在明显差异,使用Progenesis QI建立的流程筛选得到各品种正、负离子模式下的差异性代谢物,包含保留时间、分子式、二级碎片等信息。鉴定出17种差异代谢物,其中3-羟基-5,7,3',4'-四甲氧基黄酮和木犀草素-7-O-新橙皮糖苷是血橙区别于其他7种橙的特征代谢物,橙皮苷是S26锦橙的代谢标志物,芸香柚皮苷-4'-O-葡萄糖苷和异橙黄酮可以作为8045甜橙的特征代谢物。【结论】基于UPLC-QToF-MS和Progenesis QI建立的方法可以筛选出8个品种橙果实的差异性代谢物,鉴定了其中的17种物质,分别对应不同品种。该研究方法和技术路线具有通用性,适用于不同水果、不同产地、不同成熟度等因素引起的样品差异分析。
超高效液相色谱-四级杆飞行时间质谱;橙;Progenesis QI;代谢物
0 引言
【研究意义】橙属于代表性的柑橘,富含多种代谢活性物质[1],如类黄酮、香豆素和类胡萝卜素等,具有抗氧化、抗炎等生物活性[2]。因其集药、食两用于一身,成为大众喜爱的水果之一,通常以鲜食或者果汁形式摄入。橙品种众多,每种橙的品质不同,含有的代谢成分也不同。研究表明,橙果实的营养功能成分受品种、产地、成熟度、气候和土壤等多种因素影响[3]。市场上橙的销售容易弄虚作假,将不同品种的橙果实混合一起出售,或者将不同产地品质差异大的同一品种混合,包括用非地理标志产品代替地理标志产品,因外观非常接近,消费者不容易区分而花高价钱买到品质差的橙子;同样,橙汁的掺假也涉及不同品种或者不同产地相同品种但品质差异大的果汁混合,比如在橙汁中添加橘子汁或者其他品种的橙汁[4]。橙果实和橙汁的产地溯源与真实性验证是一项非常复杂的工作,因此,开发一种新的有效的方法来分析判别各品种橙代谢组分的差异以此找到其代谢标志物,对于橙果实和橙汁的真伪鉴别具有重要意义。【前人研究进展】常见的果品真伪鉴别、品质分析和品种区分方法通常是基于其次生代谢产物的代谢轮廓或者定量分析,如有机酸、多酚化合物等[5-7]。实际上,定性定量分析果品中的活性成分不仅对真伪鉴别具有重要作用,而且能够评估果品的品质,对人们的消费提供指导。多种技术可以用于果品次生代谢产物的分析检测,如高效液相色谱(HPLC)[8-9]、液质联用(LC-MS)[10-13]和气质联用(GC-MS)[14]等。随着技术的不断发展,超高效液相色谱(UPLC)和高分辨质谱如四级杆飞行时间质谱(QTof-MS)由于其分离速度快、进样体积小、准确度高等优点得到广泛应用[15-16],尤其在指纹图谱构建和差异性代谢产物的分析鉴定方面得到研究者的关注[17]。其中,基于LC-MS指纹图谱的非靶标代谢组学可以同时分析不同产地或者不同品种的样品中上百种代谢物,并比较其相似点以及差异之处[18-20]。Progenesis QI是新一代的代谢组学数据处理软件,用于代谢物鉴定和生物标志物发现,可以同时分析大样本量的多组分,从色谱数据的对齐到鉴定出具有明显差异的代谢物,处理速度快[21-22]。【本研究切入点】目前Progenesis QI在动物和药物的代谢组学领域应用较多,而国内未见应用于水果代谢组学的研究;此外,与UPLC-QToF-MS结合分析鉴定不同品种橙的差异性代谢物尚未见报道。【拟解决的关键问题】基于UPLC-QTof-MS和Progenesis QI建立不同品种橙果实的指纹图谱并同时分析不同品种间的差异性代谢物,筛选出各品种对应的代谢标志物,并对差异性代谢物进行鉴定。
1 材料与方法
柑橘材料于2015年采自于中国农业科学院柑橘研究所国家果树种质资源(重庆)柑橘圃,试验在西南大学园艺园林学院进行。
1.1 样品信息
为了减少环境因素对试验结果的影响,所有样品(表1)保持产地相同,选取我国特有柑橘资源地方品种以及引进种植的特色品种8个,每个品种均采摘于果实成熟期,从3株长势一致、健康的果树上,于树冠中部外围随机均匀采样混匀。
表1 试验材料具体信息
1.2 试剂
乙腈(色谱纯,美国sigma公司),甲酸(质谱级,美国sigma公司),试验用水为Millipore超纯水,甲醇(分析纯,成都市科龙化工试剂厂)。标准品地奥司明(纯度≥98.0%),橙皮苷(纯度≥98.0%)和5,6,7,8,3′,4′-六甲氧基黄酮(纯度≥98.0%)购买自成都克洛玛生物科技有限公司(成都,中国)。5,6,7,4′-四甲氧基黄酮(纯度≥98.0%),5,7,8,3′,4′-无甲氧基黄酮(纯度≥98.0%)和橘皮素(纯度≥98.0%)购自成都思天德生物科技有限公司(成都,中国)。
1.3 仪器
超声波清洗仪KQ5200DE(昆山市超声仪器有限公司);超纯水系统Milli-Q AdvantageA10(美国Millipore公司);离心机TDL-5A(上海菲恰尔分析仪器有限公司);UPLC-QTof-MS/MS Xevo G2-S超高效液相色谱-四级杆串联飞行时间高分辨质谱仪(美国Waters公司),配备电喷雾离子源,二元溶剂管理器,自动进样系统,柱温箱,PDA紫外检测器;ACQUITY UPLC BEH C18色谱柱(美国Waters公司)。
1.4 样品准备
样品采摘后,果实表面用去离子水洗净、晾干,并分为果皮和果肉两个部分,弃去果肉部分。果皮置于40℃鼓风干燥箱中,待果皮烘干,约48 h后取出,经粉碎机粉碎,过60目筛,粉末密封于密封袋中,储存于玻璃干燥器中备用。
精确称量样品粉末0.5 g,置于10 mL离心管中,加入7 mL甲醇,涡旋摇匀后,室温条件下300 W超声30 min,5 000 r/min下离心15 min,取上清液于25 mL容量瓶中。残渣再加7 mL甲醇重复提取两次,合并上清液,最后用甲醇溶液定容至刻度线。提取液用初始流动相稀释10倍,摇匀后过0.22 µm PTFE针式滤器,前3滴弃去,续滤液置于进样小瓶中以待上机检测。
1.5 液相条件
色谱柱:ACQUITY UPLC BEH C18分析柱(2.1 mm×100 mm,1.7 μm);柱温:40℃;进样量2.0 μL;流速设置为0.4 mL·min-1;流动相的组成包括A相和B相,分别试验了含0.01%甲酸的水和含0.01%甲酸的乙腈、含0.01%甲酸的水和含0.01%甲酸的甲醇、含0.1%甲酸的水和含0.1%甲酸的乙腈、含0.1%甲酸的水和含0.1%甲酸的甲醇,最终优化为A相:0.01%甲酸的水,B相:含0.01%甲酸的乙腈。采用梯度洗脱,保持A相和B相配比总和为10%:0—3.0 min,5%—15% B;1.0—8.0 min,15%—25% B;8.0—9.0 min,25%—35% B;9.0—13.0 min,35%—45% B;13.0—15.0 min,45%—60% B;15.0—16.0 min,60%—90% B;16.0—18.0 min,90%—90% B;18.0—20.0 min,90%—5% B。检测波长为283 nm和330 nm,波长扫描范围200—400 nm。
1.6 质谱条件
UPLC-QTof-MS系统使用ESI离子源,在正负两种离子模式下全扫描采集数据。质谱扫描范围/100—1 000,扫描时间0.2 s,参数设置与文献一致[15]。
1.7 数据分析
使用Unifi软件采集数据并对潜在标志物进行分子式匹配。使用Progenesis QI软件对采集的数据进行代谢组学部分的分析,包括峰对齐、峰提取、去卷积化、归一化、多元统计分析等过程。本研究采用LC-MS技术建立8种橙果实代谢轮廓指纹图谱,并结合主成分分析(PCA)、偏最小二乘法判别分析(PLS-DA)、变量重要性投影(VIP)等化学计量学方法寻找8个品种橙果实之间的代谢差异物,即橙类的潜在分类标志物。
2 结果
2.1 橙的指纹图谱
使用UPLC-QTof-MS/MS系统对8个品种橙果皮的甲醇提取物进行分析,优化了流动相的组成和洗脱梯度。在最优的条件下,得到了8种橙果皮的代谢轮廓指纹图谱。如图1-A所示,负离子模式下,8种橙的代谢成分存在差异,如血橙2—3 min出现的峰对应的物质种类和相对含量与其他橙明显不同;长叶橙可见两个峰分别在12.36 min和11.80 min处,而另外7种橙则没有。同样的,正离子模式下(图1-B),8种橙的代谢指纹图谱差异明显,尤其是北碚447锦橙和血橙。北碚447锦橙在保留时间2—10 min的差异性峰最明显,血橙在5—6 min和8—9 min之间有非常明显区别于其他橙的峰出现。从图1的指纹图谱肉眼可见8种橙的代谢组分存在明显差异,为接下来QI软件分析差异性代谢物奠定了基础。
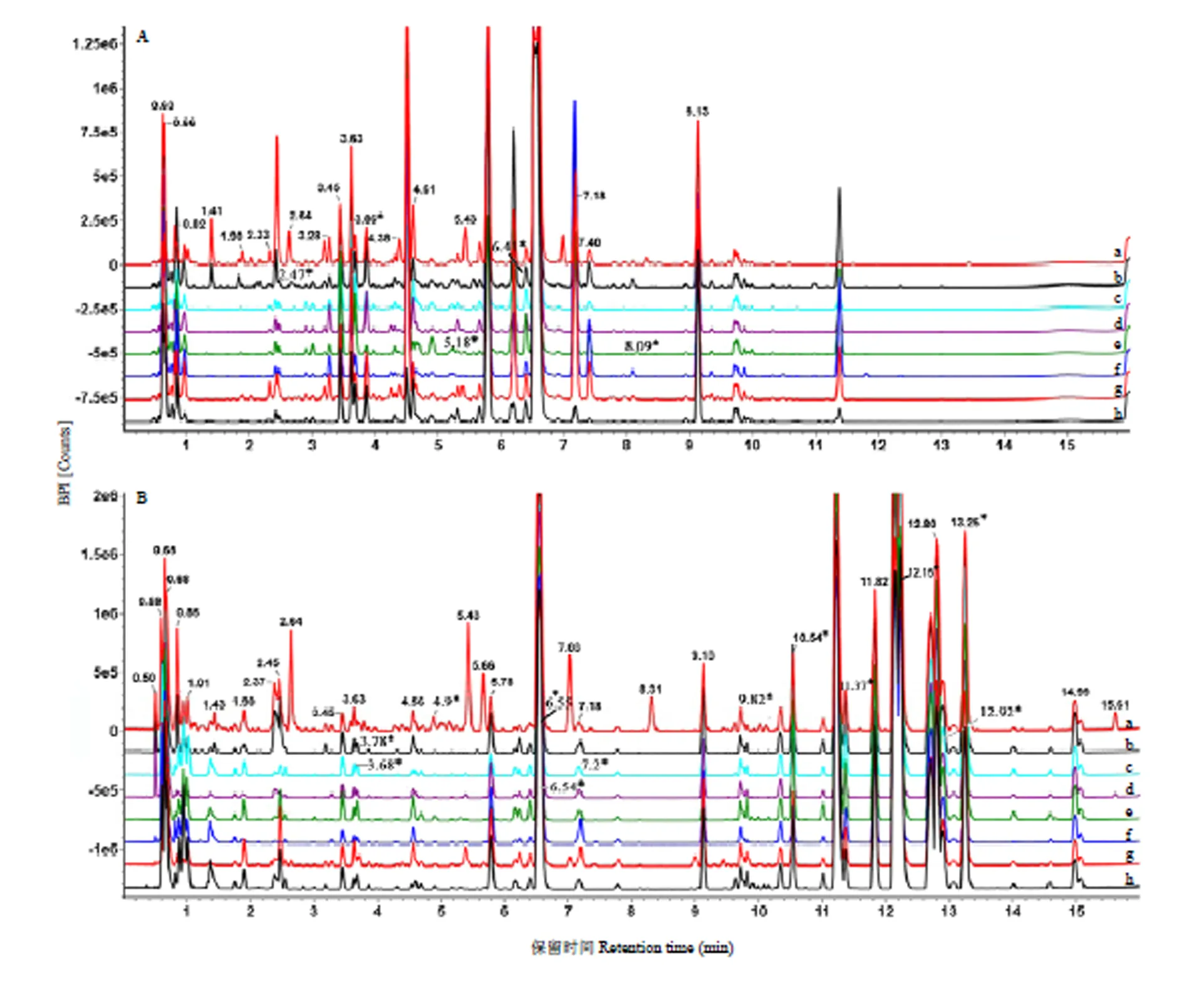
A:负离子模式;B:正离子模式。a:XUEC,血橙;b:XIAC,夏橙;c:XF,先锋橙;d:S26,S26锦橙;e:EG,鹅蛋柑;f:CY,长叶橙;g:BB,北碚447锦橙;h:8045,8045甜橙。*代表已鉴定的差异代谢物。下同
2.2 Progenesis QI分析
在进行QI组学分析之前首先要考察试验数据的可靠性。试验样品一共分为9组,包括一组QC(quality control)样品和8组橙果实样品(分别对应8种橙),每组6个样品。正离子模式下所得数据使用QI软件处理得分析变量为13 926,即所得数据矩阵为54×13926。从图2-A和图2-C可见,QC样本分布集中,且RSD<15%的变量占56.36%,RSD<30%的变量占81.32%。负离子模式下数据矩阵为54× 12015,QC样品聚集程度相对良好,且RSD<15%的化合物占64.80%,RSD<30%的化合物占88.42%(图2-B、2-D)。总体来说,本方法在整个过程中稳定,重复性良好,适合用于橙类的代谢组学分析,所得数据稳定可靠[23-24]。
从图3-A可以看出,正离子模式下各品种各自聚集在一起,其中血橙、长叶橙和8045甜橙距离较远,说明3种橙果实的代谢差异较大,而鹅蛋柑、先锋橙与8045甜橙距离较近,说明这3种橙果实代谢相似。

A:正离子模式下的PCA图;B:负离子模式下的PCA图;C:正离子模式下RSD值分布;D:负离子模式下RSD值分布
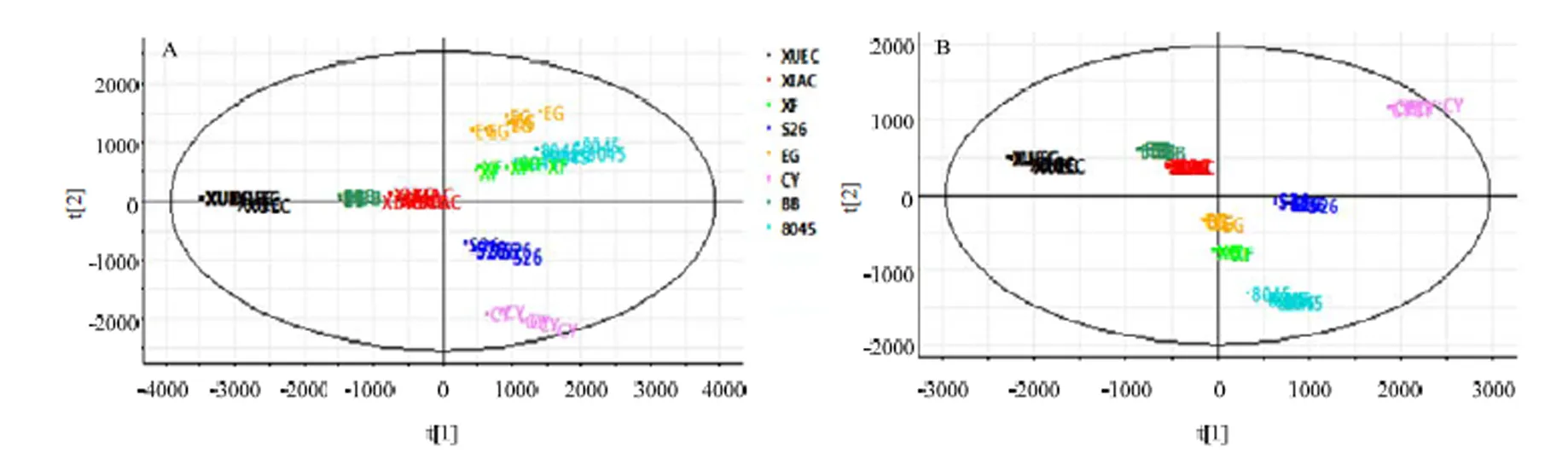
A:正离子模式下PCA得分图,其中第一主成分占46.83%,第二主成分占19.39%;B:负离子模式下PCA得分图,其中第一主成分占45.83%,第二主成分占20.31%
同样,北碚447锦橙与夏橙两种距离相近,说明二者代谢相似。从图3-B可以看出,各品种在负离子模式下更为集中,并且得出的聚类结果与正离子模式下相似:血橙、长叶橙和8045甜橙距离较远,鹅蛋柑、先锋橙与8045甜橙距离较近,北碚447锦橙与夏橙距离相近。
2.3 差异性代谢物的筛选
以鹅蛋柑为例分析其区别于其他7种橙的代谢物。先将UPLC-MS/MS数据导入Progenesis QI软件中,以离子强度图进行图形对齐数据,再进行峰提取,保证数据的零丢失,自动归属同一化合物产生的不同加合信号,再提取MSE数据,软件自动辨别和浏览离子淌度分离结果,提取所有满足条件的组分信号,得到相应的变量信息,数据归一化;再将鹅蛋柑与其他品种分为两组得OPLS-DA散点图(图4-A),鹅蛋柑在第一主成分轴上明显分开,说明鹅蛋柑有明显区别于其他7种橙的代谢成分。模型验证参数2Y=99%,2Q=98%,说明该模型的可靠性及预测性均良好。得到对应的Loadings图,如图4-B所示,正轴表示鹅蛋柑,负轴表示其他品种,一个点代表一个变量,离原点越远表示该变量在该组含量越高。图4-C为S-Plot图,与Loadings图类似,越是在图的两端代表该化合物在该组的含量越高,重复性越好。VIP图,是重要变量性图,通常认为VIP值大于1的变量具有统计学意义。再参照变量变化趋势图来筛选潜在代谢标志物,选择在鹅蛋柑中含量高,在其他品种中含量低或者为零的变量。如图4-D所示,该物质只在鹅蛋柑中存在,在其他橙果实中的含量为零。最后将筛选出的变量借助高分辨质谱的优势用Unifi软件匹配可得较为准确的分子式。使用同样的流程筛选找到其他橙正负离子模式下各自的代谢标志物(附表1—16)。
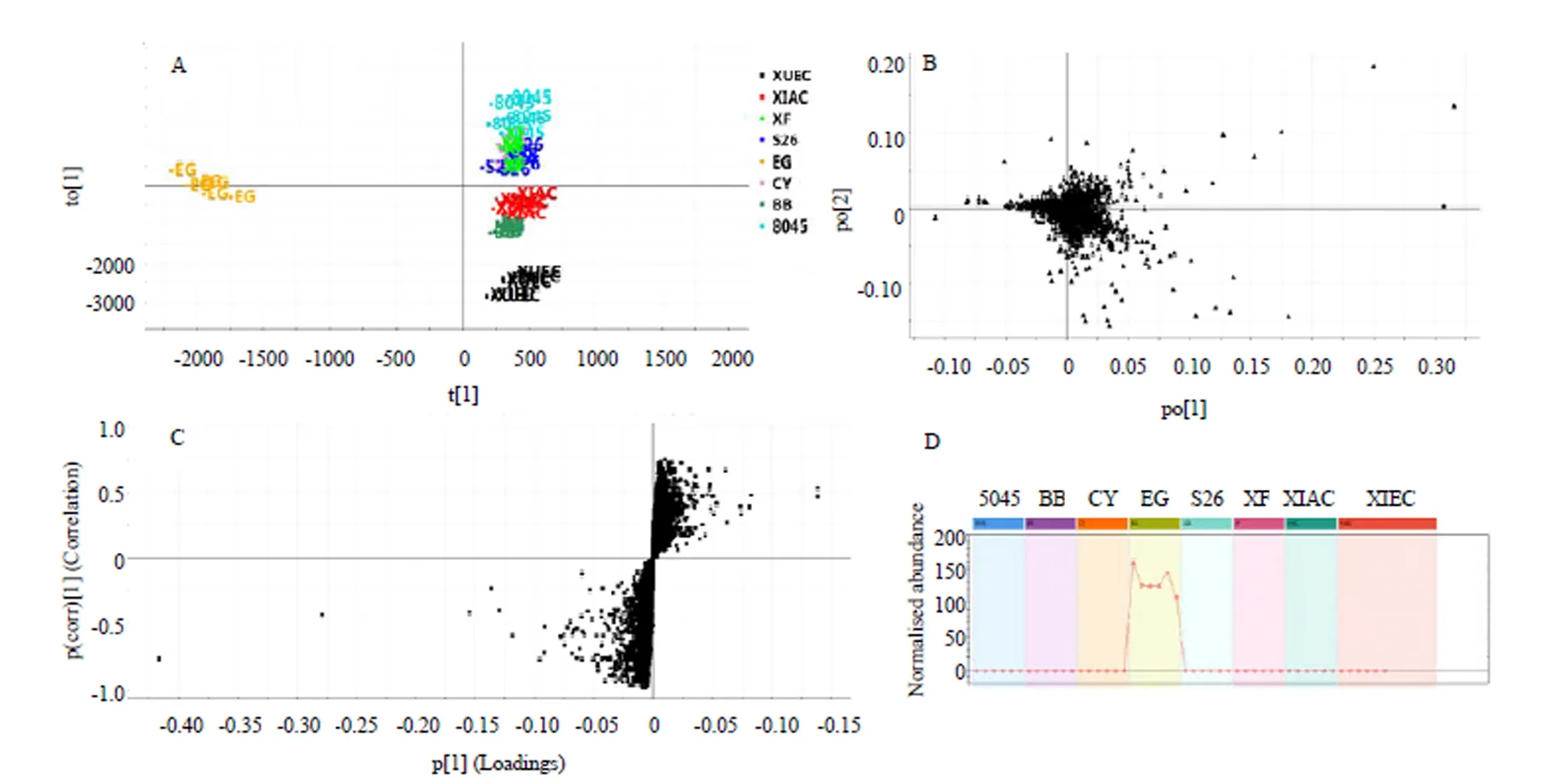
A:EG VS Group的偏最小二乘法判别分析图,R2Y=99%,R2Q=98%;B:载荷图;C:S-Plot图;D:变量变化趋势图
2.4 差异性代谢物的鉴定
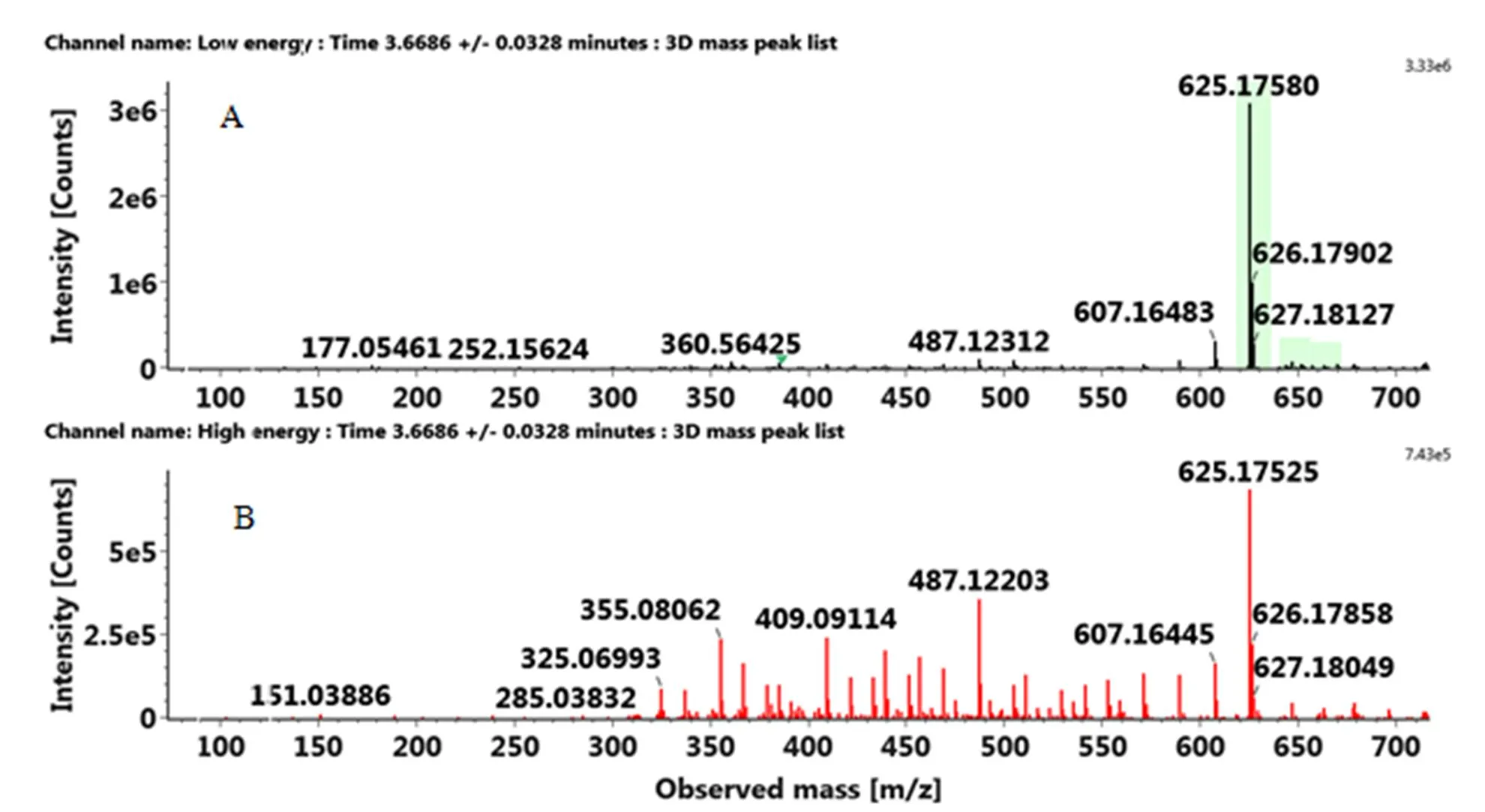
根据保留时间、精确分子量、二级碎片、标准品以及数据库和相关文献信息,对上述筛选出的差异性代谢物进行鉴定。表2所示的是已经鉴定出的部分代谢物分别对应于各个橙品种。化合物5(RT=13.25 min),化合物7(RT=6.41 min),化合物11(RT=6.54 min),化合物14(RT=12.15 min),化合物15(RT=11.37 min)和化合物17(RT=10.54 min)的鉴定结果经过标准品比对确认(附图1—6)。其余11种化合物包括了类黄酮-C-糖苷、类黄酮-O-糖苷、多甲氧基黄酮和其他类型的3种化合物(对应的MS和MS/MS信息见附图7—17)。
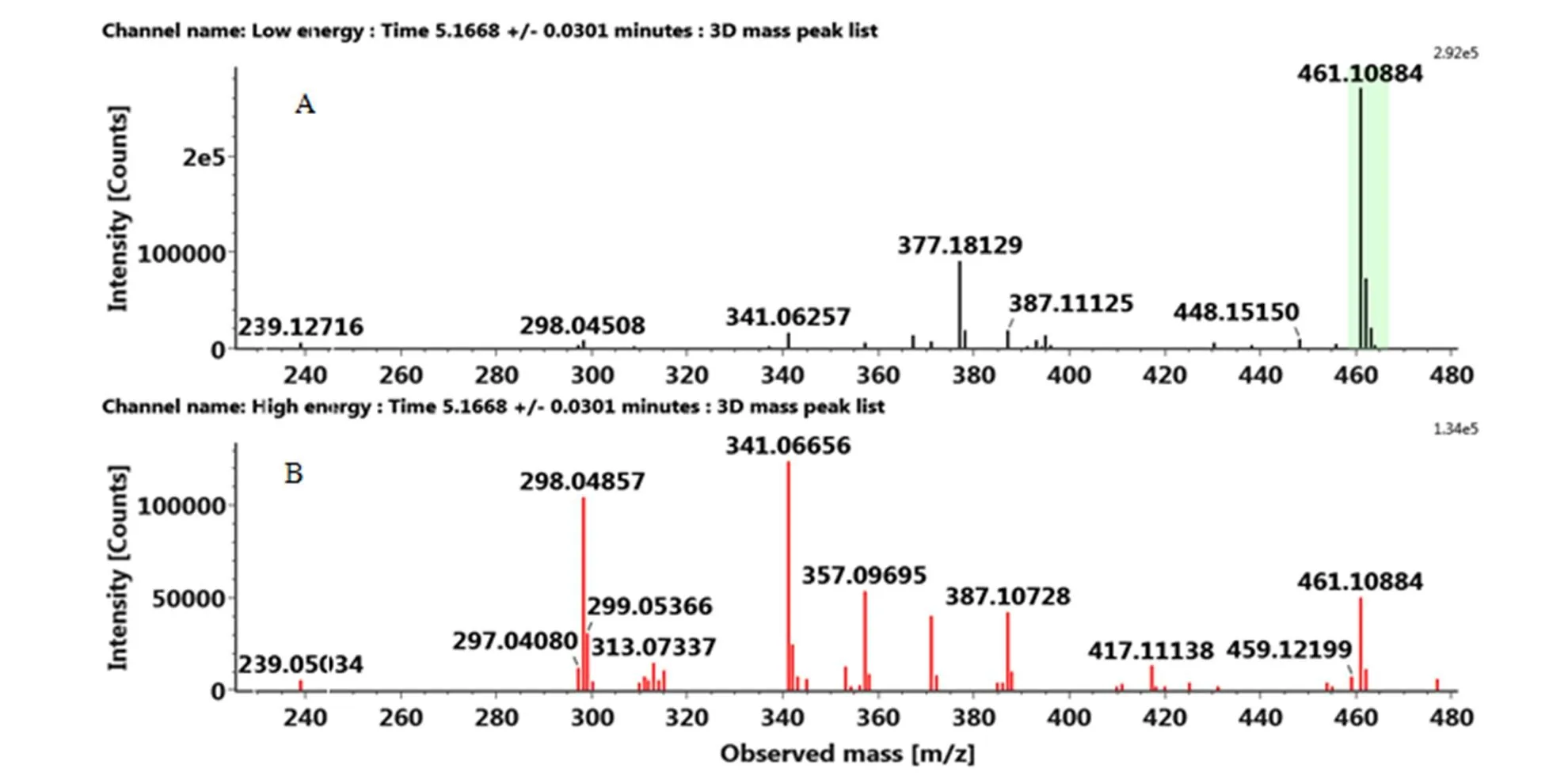
据报道,类黄酮-C-糖苷中C-糖基化仅发生在类黄酮骨架中的C-6和C-8位[25],且黄酮-6-C-糖苷比-8-C-糖苷更容易失水产生碎片[M-H-18]-,该碎片通常可作为鉴定糖基化位点的依据。此外,类黄酮-C-糖苷容易优先发生糖基内部的断裂,产生[M+H–nH2O]+和[M+H–CH2O–2H2O]+等常见的碎片。鹅蛋柑在保留时间为5.18 min处的代谢物[M-H]-为461.10986,特征碎片离子341.0666([(M–H)-C4H8O4]-)说明该化合物为C-糖基-类黄酮,碎片离子299.0537(Y0-)说明该苷元为含有3个羟基和1个甲氧基的黄酮。C环裂解产生的碎片209.0423([0,4A--C2H4O2])和167.0332([0,4A--C3H6O3])证明该黄酮的A环上带有两个羟基,而B+C环上带有一个羟基和一个甲氧基。通过搜索数据库SciFinder和ChemSpider查到两个化合物符合上述裂解规律,分别为金雀花素和荭草素-4-甲基醚,因此该化合物暂定为金雀花素或荭草素-4-甲基醚,但目前的信息不能完全确定其结构,这两种化合物在柑橘中都有报道[26]。同理,将先锋橙在3.68 min出峰的化合物鉴定为香叶木素6,8-二-C-葡萄糖苷。
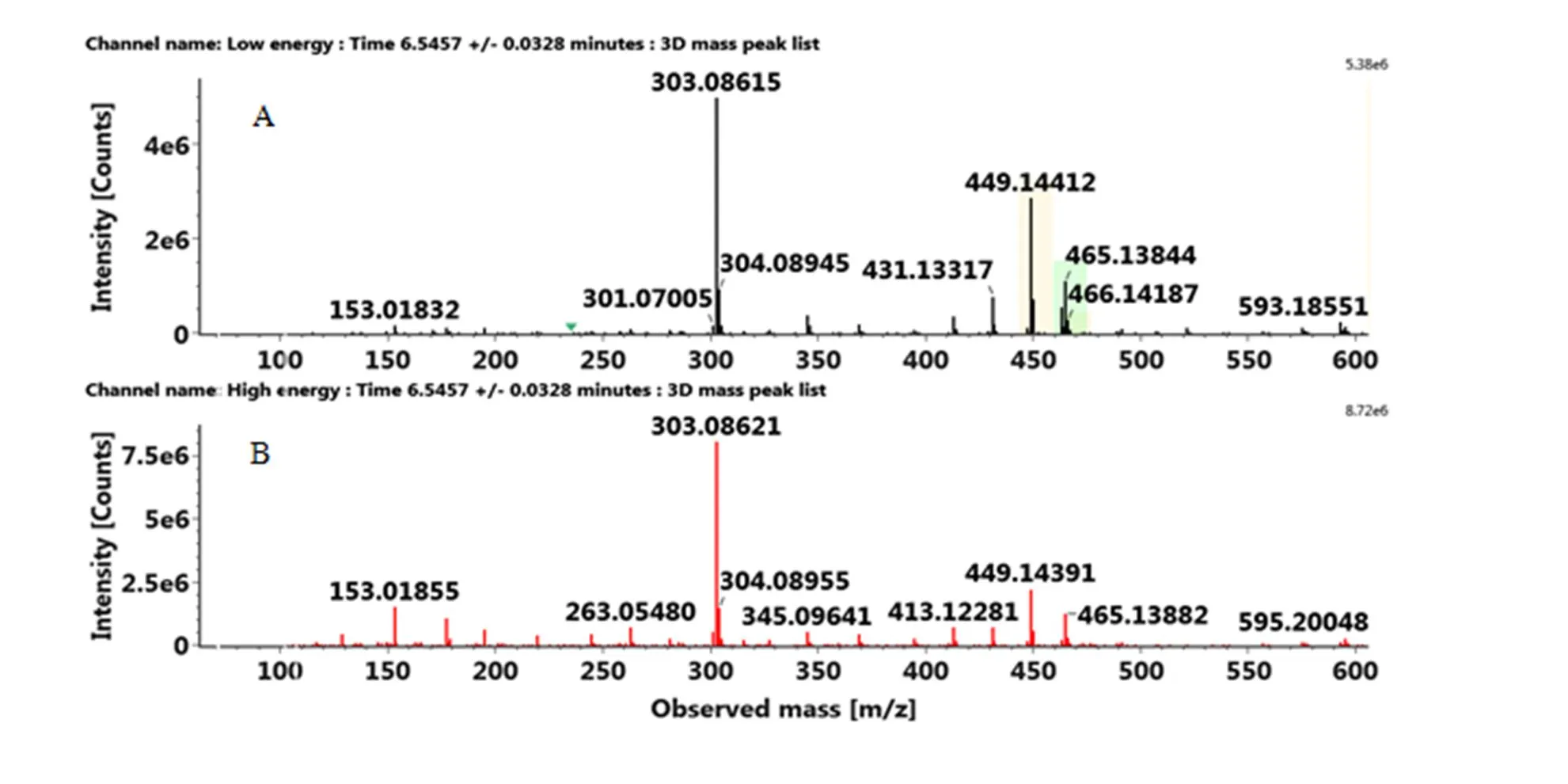
与类黄酮-C-糖苷不同的是,类黄酮-O-糖苷易发生糖基的丢失,据此可判断糖基的类型,如中性丢失308 Da是典型的O-二糖苷,而中性丢失470 Da则说明该化合物是O-三糖苷。以夏橙在6.55 min处出峰的化合物为例介绍类黄酮-O-糖苷的鉴定。该化合物在正离子模式下的准分子离子[M+H]+为465.1384,产生的碎片离子如下:303.0862(Y0+)、153.0186(1,3A0+)、177.0548([0,4B+-H2O])、345.0964(0,2X+)和369.0966([0,4X+-H2O])。其中苷元离子303.0862是丢失一个己糖162 Da产生的,说明该化合物是O-糖基黄烷酮,带有3个羟基和1个甲氧基。C环裂解产生的碎片153.0186和177.0548证明A环上有两个羟基取代,B环上有1个羟基1个甲氧基。据此,该化合物被鉴定为橙皮素-7-O-葡萄糖苷,在Marc.果实染病的果皮中也有检出[27]。同理,化合物2、4、16被鉴定为类黄酮-O-糖苷,如表2所示。
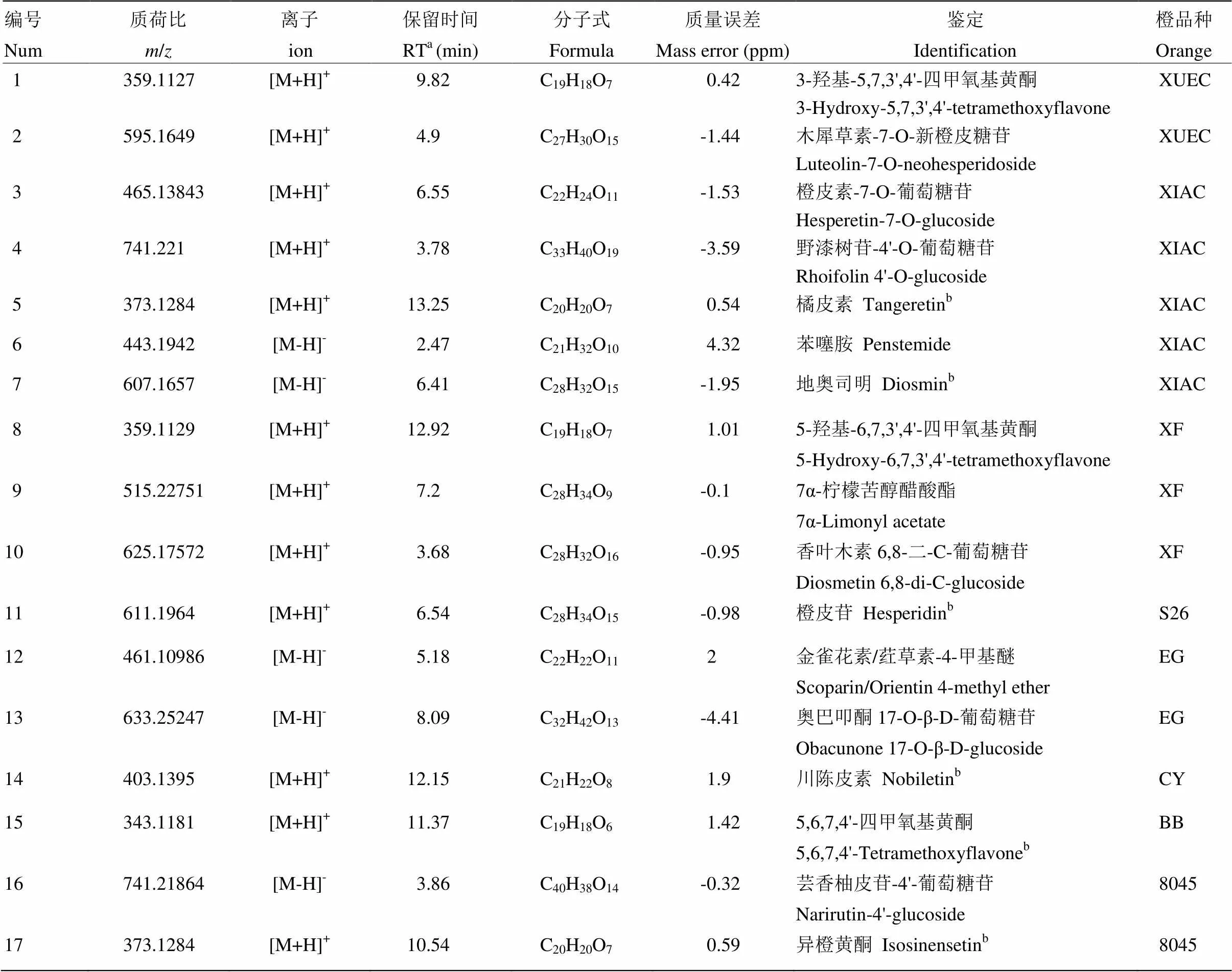
表2 部分差异性代谢物的鉴定信息
a保留时间 Retention time;b经过标准品确认 Confirmed by standard
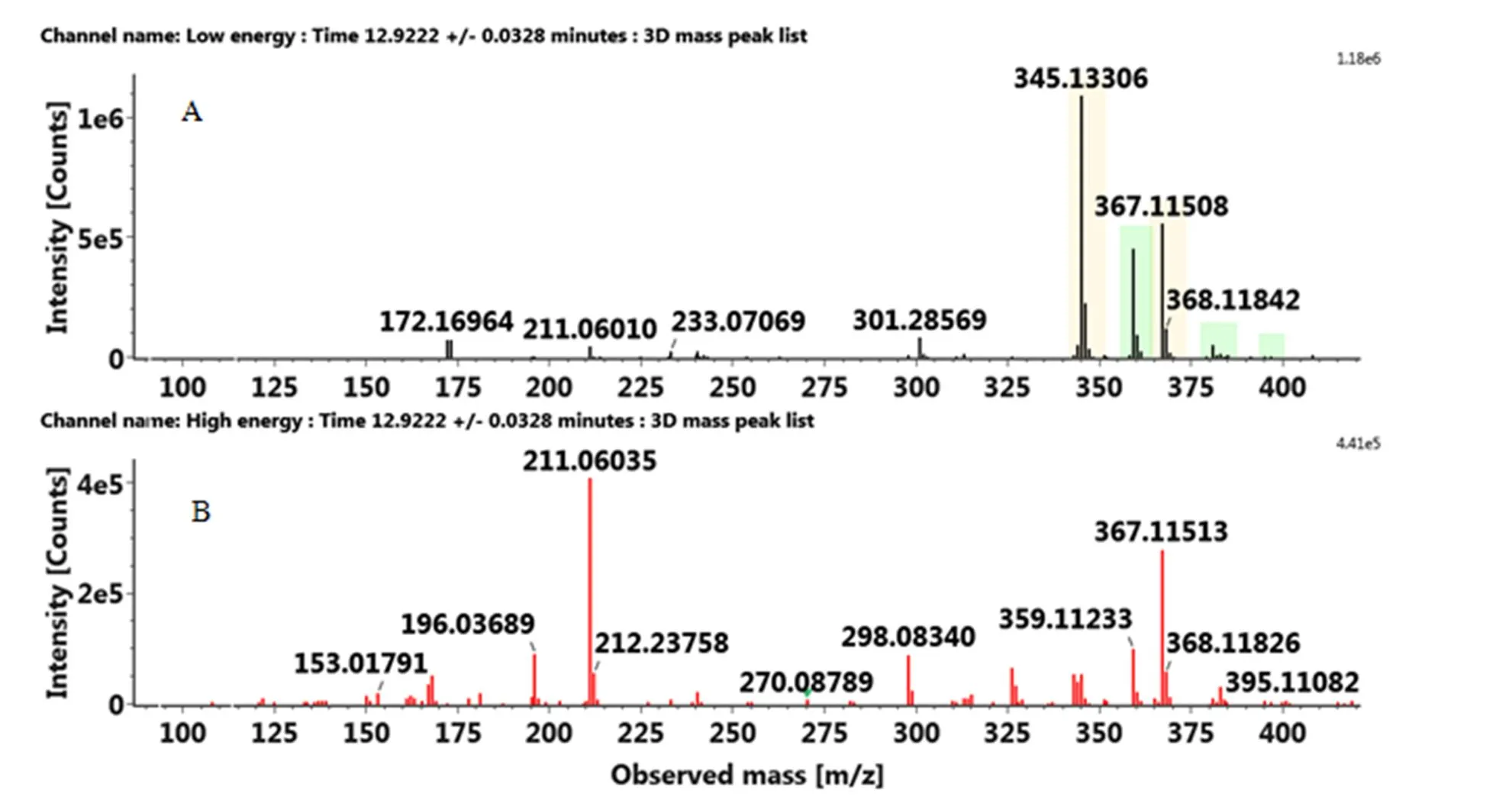
多甲氧基黄酮通常会优先发生中性丢失,如nCH3,然后是C环的断裂,取代基的分布可从C环裂解得到的碎片来判断。正离子模式下,先锋橙中保留时间为12.92 min出现的化合物,准分子离子峰[M+H]+为359.1129,产生一系列的碎片包括326.07742([M+ H-CH3-H2O]+)、344.08696([M+H-CH3]+)、211.05952(1,2A+)和163.07469(1,3B+)。通过UNIFI软件匹配出最接近的分子式为C19H18O7,减去黄酮的基本骨架结构C15H10O2得到差值C4H8O5,说明该化合物为多甲氧基黄酮,其中甲氧基的个数等于差值中C的个数4,而羟基的个数为差值中O的个数减去C的个数,即为1。然后通过C环裂解得到的碎片来判断甲氧基和羟基的取代位点,再通过搜索数据库SciFinder和ChemSpider,将该化合物暂定为5-羟基-6,7,3',4'-四甲氧基黄酮。与之类似,将血橙保留时间为9.82 min处出峰的化合物鉴定为3-羟基-5,7,3',4'-四甲氧基黄酮。
此外,夏橙在2.47 min出峰的化合物被鉴定为苯噻胺,其特征碎片为101.0250;先锋橙在7.2 min出峰的化合物是诺米林的同分异构体,正离子模式下特征碎片为161.0597、303.0857、487.2322和469.2217,鉴定为7α-柠檬苦醇醋酸酯;鹅蛋柑在8.09 min出峰的化合物也是一种类柠檬苦素,易发生中性丢失葡萄糖和CO2,鉴定为奥巴叩酮17-O-β-D-葡萄糖苷,之前在柑橘种子中有检出[28]。
3 讨论
Wang等[29]基于LC-MS的非靶标代谢组学对62份柑橘资源不同组织部位的类黄酮多样性进行了系统的研究,但是该研究只分析检测了类黄酮这一类物质。STANDER等[30]基于一种新的亲水作用液相色谱-质谱联用(LC-MS)方法对南非果汁进行了研究,其中包括了混合果汁和100%橙汁,研究表明柚皮苷和橙皮苷可以作为稳定的标志化合物用于果汁的掺假识别。JANDRIĆ等[3]基于UPLC-QToF-MS和化学计量学对柑橘果实(橙、柚、葡萄柚和宽皮柑橘)、不同来源的橙以及不同类型的果汁进行了区分比较,该研究分析得到的化合物不限于类黄酮,初步鉴定出了对差异性有贡献的8种标志性化合物,例如柠檬苦素-17-β-D-吡喃葡糖苷、诺米林-17-β-D- 吡喃葡糖苷、芸香柚皮苷和橙皮苷等,可以用于柑橘果实/果汁的真伪鉴别;同时,该课题组使用靶向和非靶向代谢组学对印度柑橘(宽皮柑橘、橙、葡萄柚)进行了真伪鉴别,并对特征的标志化合物进行了鉴定,最具影响的标志化合物有香风草甙、野漆树苷、异野漆树苷、新橙皮苷、橙皮苷、柚皮苷、芸香柚皮苷、柠檬苦素葡萄糖苷和维采宁-2[20]。然而目前国内外的文献并没有基于LC-MS的非靶标代谢组学对我国特色橙品种在相同产地条件下进行差异性代谢产物的分析鉴定。橙品种繁多,风味品质和营养价值各不相同。与果肉和种子相比,果皮含有更丰富的次生代谢产物,因此,本文选择了相同产地的8个品种的橙果皮为研究对象。
从本研究结果可以看出,血橙、长叶橙和8045甜橙的代谢物差异性较大,而北碚447锦橙与夏橙的代谢产物接近。利用本文建立的方法筛选出每种橙区别于其他7种橙的特征代谢物共几百种(附表1—16),因柑橘代谢产物数据库尚不完善,差异性代谢物的结构尚不能完全确定。目前,本文已完成了17种差异性代谢物的鉴定。其中通过标准品比对,确定了6种,分别是夏橙在保留时间为6.41 min出峰的地奥司明和13.25 min出峰的橘皮素,S26锦橙在6.54 min出峰的橙皮苷,长叶橙在12.15 min出峰的川陈皮素,北碚447锦橙在11.37 min出峰的5,6,7,4'-四甲氧基黄酮,8045甜橙在10.54 min出峰的异橙黄酮。另外的11种差异性代谢物根据保留时间、精确分子量、二级碎片以及数据库和相关文献信息进行了鉴定。与已有文献对柑橘种间果实/果汁的分析相比,本研究针对橙种内代谢的差异物进行了分析鉴定,对差异性有贡献的特征代谢标志物除了常规的类黄酮、柠檬苦素如橙皮苷、香叶木素6,8-二-C-葡萄糖苷和奥巴叩酮17-O-β-D-葡萄糖苷、7α-柠檬苦醇醋酸酯,还有6种多甲氧基类黄酮,包括3-羟基-5,7,3',4'-四甲氧基黄酮、川陈皮素、异橙黄酮、5-羟基-6,7,3',4'-四甲氧基黄酮、5,6,7,4'-四甲氧基黄酮和橘皮素。本文从代谢水平提供了鉴别橙果实及橙汁的依据。比如,8045甜橙和S26锦橙全果所榨橙汁的鉴别,可通过检测二者的次生代谢产物中有无异橙黄酮和橙皮苷,以及这两种差异性代谢物的相对含量来确定该橙汁是否掺假以及掺假的程度。若检出异橙黄酮,没检出橙皮苷,或者二者相对含量的比值大于1,说明该橙汁是8045甜橙的果汁;反之,则证明该橙汁来自S26锦橙。
4 结论
本文通过超高效液相色谱-四级杆飞行时间质谱(UPLC-QToF-MS)得到8种橙果皮的代谢产物指纹图谱,使用Progenesis QI软件结合主成分分析、偏最小二乘判别分析和变量变化趋势图等手段分析筛选出各品种对应的差异性代谢物,据此得到了区分/鉴别不同品种橙的代谢依据。目前仅鉴定出17种代谢标志物。其中木犀草素-7-O-新橙皮糖苷和3-羟基-5,7,3',4'-四甲氧基黄酮是血橙区别于其他7种橙的特征代谢物,橙皮苷是S26锦橙的代谢标志物,芸香柚皮苷-4'-葡萄糖苷和异橙黄酮可以作为8045甜橙的特征代谢物,而夏橙中鉴定出5种区别于其他橙的化合物,分别为橙皮素-7-O-葡萄糖苷、野漆树苷-4'-O-葡萄糖苷、橘皮素、苯噻胺和地奥司明。该研究方法和技术路线具有通用性,可拓展至不同水果、不同产地、不同成熟度等因素引起的代谢物差异分析,为柑橘及其他水果的代谢组学研究提供一定的借鉴。
附表1 鹅蛋柑正离子模式下区别于其他橙的代谢物
Table S1 The metabolites in Edangan different from those in the other oranges in the positive ion mode
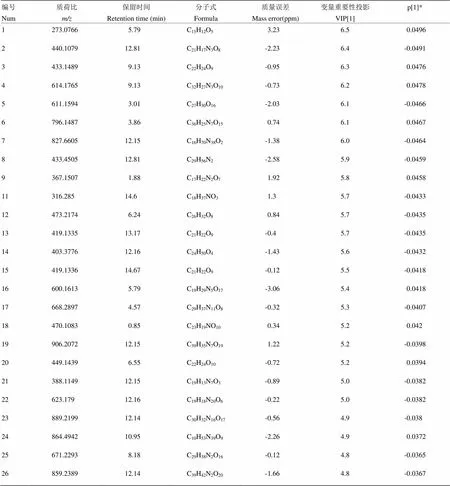
编号Num质荷比m/z保留时间Retention time (min)分子式Formula质量误差Mass error(ppm)变量重要性投影VIP[1]p[1]* 1273.07665.79C15H12O53.236.50.0496 2440.107912.81C21H17N3O8-2.236.4-0.0491 3433.14899.13C22H24O9-0.956.30.0476 4614.17659.13C32H27N3O10-0.736.20.0478 5611.15943.01C27H30O16-2.036.1-0.0466 6796.14873.86C36H25N7O150.746.10.0467 7827.660512.15C16H70N38O2-1.386.0-0.0464 8433.450512.81C29H56N2-2.585.9-0.0459 9367.15071.88C17H22N2O71.925.80.0458 11316.28514.6C18H37NO31.35.7-0.0433 12473.21746.24C26H32O80.845.7-0.0435 13419.133513.17C21H22O9-0.45.7-0.0435 14403.377612.16C24H50O4-1.435.6-0.0432 15419.133614.67C21H22O9-0.125.5-0.0418 16600.16135.79C19H29N5O17-3.065.40.0418 17668.28974.57C29H37N11O8-0.325.3-0.0407 18470.10830.85C23H19NO100.345.20.042 19906.207212.15C39H35N7O191.225.2-0.0398 20449.14396.55C22H24O10-0.725.20.0394 21388.114912.15C19H13N7O3-0.895.0-0.0382 22623.17912.16C19H18N20O6-0.225.0-0.0382 23889.219912.14C30H32N16O17-0.564.9-0.038 24864.494210.95C10H53N39O9-2.264.90.0372 25671.22938.18C29H38N2O16-0.124.8-0.0365 26859.238912.14C39H42N2O20-1.664.8-0.0367
p[1]*小于0.05代表两组之间有统计学差异。下同
p[1]*<0.05 indicated that there are statistical differences between the two groups. The same as below
附表2 鹅蛋柑负离子模式下区别于其他橙的代谢物
Table S2 The metabolites in Edangan different from those in the other oranges in the negative ion mode
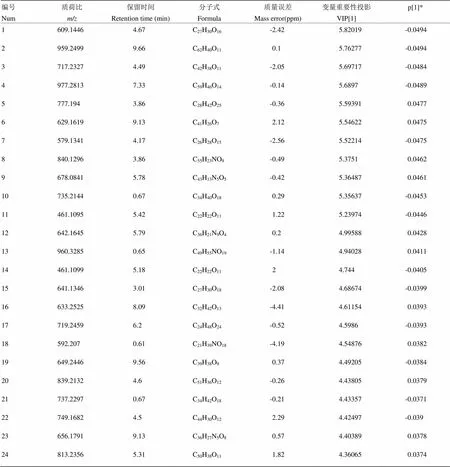
编号Num质荷比m/z保留时间Retention time (min)分子式Formula质量误差Mass error(ppm)变量重要性投影VIP[1]p[1]* 1609.14464.67C27H30O16-2.425.82019-0.0494 2959.24999.66C62H40O110.15.76277-0.0494 3717.23274.49C42H38O11-2.055.69717-0.0484 4977.28137.33C59H46O14-0.145.6897-0.0489 5777.1943.86C28H42O25-0.365.593910.0477 6629.16199.13C41H26O72.125.546220.0475 7579.13414.17C26H28O15-2.565.52214-0.0475 8840.12963.86C55H23NO9-0.495.37510.0462 9678.08415.78C43H13N5O5-0.425.364870.0461 10735.21440.67C34H40O180.295.35637-0.0453 11461.10955.42C22H22O111.225.23974-0.0446 12642.16455.79C36H21N9O40.24.995880.0428 13960.32850.65C49H55NO19-1.144.940280.0411 14461.10995.18C22H22O1124.744-0.0405 15641.13463.01C27H30O18-2.084.68674-0.0399 16633.25258.09C32H42O13-4.414.611540.0393 17719.24596.2C24H48O24-0.524.5986-0.0393 18592.2070.61C21H39NO18-4.194.548760.0382 19649.24469.56C39H38O90.374.49205-0.0384 20839.21324.6C51H36O12-0.264.438050.0379 21737.22970.67C34H42O18-0.214.43357-0.0371 22749.16824.5C44H30O122.294.42497-0.039 23656.17919.13C36H27N5O80.574.403890.0378 24813.23565.31C50H38O111.824.360650.0374
附表3 血橙正离子模式下区别于其他橙的代谢物
Table S3 The metabolites in Xuecheng different from those in the other oranges in the positive ion mode
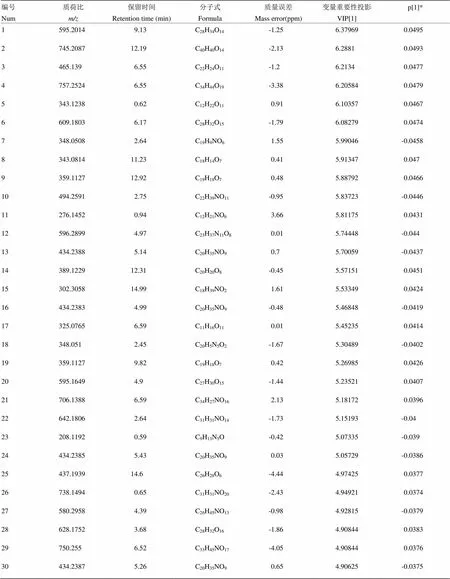
编号Num质荷比m/z保留时间Retention time (min)分子式Formula质量误差Mass error(ppm)变量重要性投影VIP[1]p[1]* 1595.20149.13C28H34O14-1.256.379690.0495 2745.208712.19C40H40O14-2.136.28810.0493 3465.1396.55C22H24O11-1.26.21340.0477 4757.25246.55C34H44O19-3.386.205840.0479 5343.12380.62C12H22O110.916.103570.0467 6609.18036.17C28H32O15-1.796.082790.0474 7348.05082.64C19H9NO61.555.99046-0.0458 8343.081411.23C18H14O70.415.913470.047 9359.112712.92C19H18O70.485.887920.0466 10494.25912.75C22H39NO11-0.955.83723-0.0446 11276.14520.94C12H21NO63.665.811750.0431 12596.28994.97C23H37N11O80.015.74448-0.044 13434.23885.14C20H35NO90.75.70059-0.0437 14389.122912.31C20H20O8-0.455.571510.0451 15302.305814.99C18H39NO21.615.533490.0424 16434.23834.99C20H35NO9-0.485.46848-0.0419 17325.07656.59C11H16O110.015.452350.0414 18348.0512.45C20H5N5O2-1.675.30489-0.0402 19359.11279.82C19H18O70.425.269850.0426 20595.16494.9C27H30O15-1.445.235210.0407 21706.13886.59C34H27NO162.135.181720.0396 22642.18062.64C31H31NO14-1.735.15193-0.04 23208.11920.59C9H13N5O-0.425.07335-0.039 24434.23855.43C20H35NO90.035.05729-0.0386 25437.193914.6C26H28O6-4.444.974250.0377 26738.14940.65C31H31NO20-2.434.949210.0374 27580.29584.39C26H45NO13-0.984.92815-0.0379 28628.17523.68C28H32O16-1.864.908440.0383 29750.2556.52C33H45NO17-4.054.908440.0376 30434.23875.26C20H35NO90.654.90625-0.0375
附表4 血橙负离子模式下区别于其他橙的代谢物
Table S4 The metabolites in Xuecheng different from those in the other oranges in the negative ion mode

编号Num质荷比m/z保留时间Retention time (min)分子式Formula质量误差Mass error(ppm)变量重要性投影VIP[1]p[1]* 1563.14014.54C26H28O14-0.995.772880.0477 2533.17170.66C19H34O17-1.155.770190.0483 3677.16546.51C38H30O12-1.625.764430.0494 4647.15625.79C37H28O110.425.686880.0495 5503.16220.61C18H32O160.955.546560.0465 6251.11791.03C16H16N2O-4.315.45523-0.0467 7615.16629.13C37H28O90.35.303450.0464 8845.26910.62C44H46O173.435.285540.0447 9609.23632.43C33H38O113.555.03086-0.043 10563.144.68C26H28O14-1.084.941390.0409 11593.14946.58C27H30O15-3.044.893650.0424 12377.08930.66C18H18O93.984.835280.0426 13629.20445.23C35H34O112.554.811370.0414 14465.19927.37C21H30N4O80.224.78248-0.0409 15577.1555.86C27H30O14-2.254.759290.0417 16507.20857.97C22H36O130.44.52032-0.0385 17633.337613.44C33H50N2O10-2.624.51162-0.0386 18785.2229.87C45H38O13-2.514.49310.0378 19529.21354.42C21H38O15-0.484.48159-0.0381 20707.14536.6C31H32O19-1.774.475770.0385
附表5 夏橙正离子模式下区别于其他橙的代谢物
Table S5 The metabolites in Xiacheng different from those in the other oranges in the positive ion mode
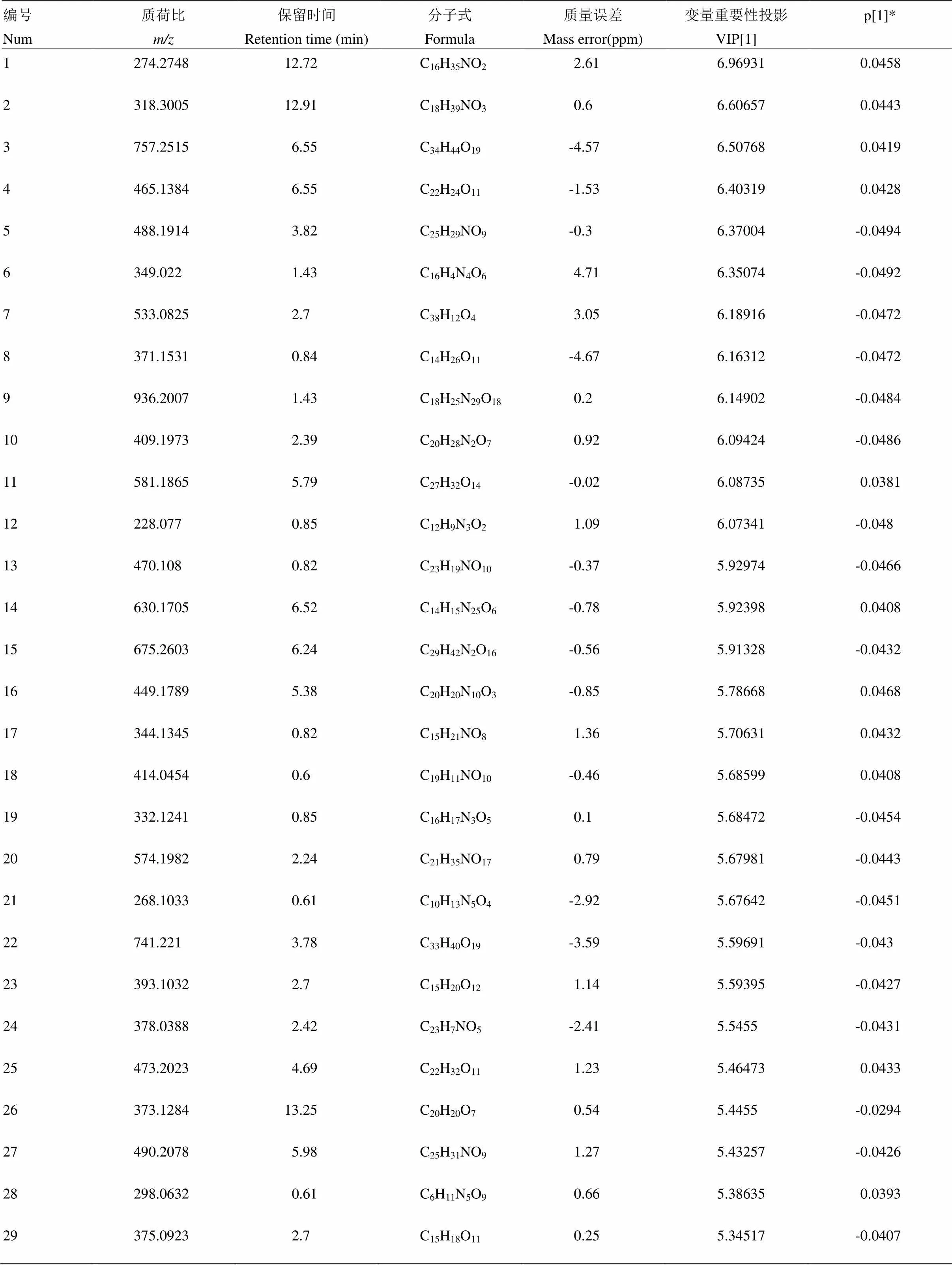
编号Num质荷比m/z保留时间Retention time (min)分子式Formula质量误差Mass error(ppm)变量重要性投影VIP[1]p[1]* 1274.274812.72C16H35NO22.616.969310.0458 2318.300512.91C18H39NO30.66.606570.0443 3757.25156.55C34H44O19-4.576.507680.0419 4465.13846.55C22H24O11-1.536.403190.0428 5488.19143.82C25H29NO9-0.36.37004-0.0494 6349.0221.43C16H4N4O64.716.35074-0.0492 7533.08252.7C38H12O43.056.18916-0.0472 8371.15310.84C14H26O11-4.676.16312-0.0472 9936.20071.43C18H25N29O180.26.14902-0.0484 10409.19732.39C20H28N2O70.926.09424-0.0486 11581.18655.79C27H32O14-0.026.087350.0381 12228.0770.85C12H9N3O21.096.07341-0.048 13470.1080.82C23H19NO10-0.375.92974-0.0466 14630.17056.52C14H15N25O6-0.785.923980.0408 15675.26036.24C29H42N2O16-0.565.91328-0.0432 16449.17895.38C20H20N10O3-0.855.786680.0468 17344.13450.82C15H21NO81.365.706310.0432 18414.04540.6C19H11NO10-0.465.685990.0408 19332.12410.85C16H17N3O50.15.68472-0.0454 20574.19822.24C21H35NO170.795.67981-0.0443 21268.10330.61C10H13N5O4-2.925.67642-0.0451 22741.2213.78C33H40O19-3.595.59691-0.043 23393.10322.7C15H20O121.145.59395-0.0427 24378.03882.42C23H7NO5-2.415.5455-0.0431 25473.20234.69C22H32O111.235.464730.0433 26373.128413.25C20H20O70.545.4455-0.0294 27490.20785.98C25H31NO91.275.43257-0.0426 28298.06320.61C6H11N5O90.665.386350.0393 29375.09232.7C15H18O110.255.34517-0.0407
附表6 夏橙负离子模式下区别于其他橙的代谢物
Table S6 The metabolites in Xiacheng different from those in the other oranges in the negative ion mode
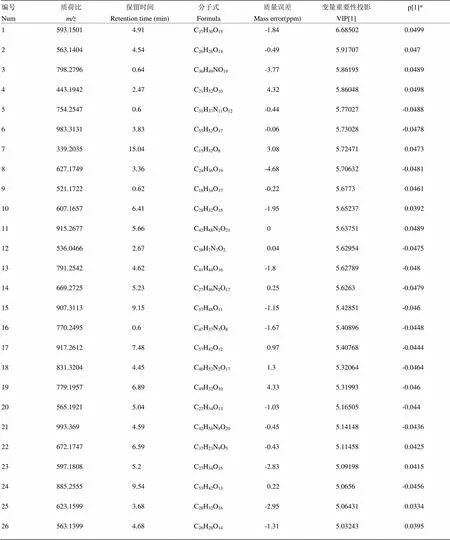
编号Num质荷比m/z保留时间Retention time (min)分子式Formula质量误差Mass error(ppm)变量重要性投影VIP[1]p[1]* 1593.15014.91C27H30O15-1.846.685020.0499 2563.14044.54C26H28O14-0.495.917070.047 3798.27960.64C36H49NO19-3.775.861950.0489 4443.19422.47C21H32O104.325.860480.0498 5754.25470.6C31H37N11O12-0.445.77027-0.0488 6983.31313.83C55H52O17-0.065.73028-0.0478 7339.203515.04C15H32O83.085.724710.0473 8627.17493.36C24H36O19-4.685.70632-0.0481 9521.17220.62C18H34O17-0.225.67730.0461 10607.16576.41C28H32O15-1.955.652370.0392 11915.26775.66C42H48N2O2105.637510.0489 12536.04662.67C38H7N3O20.045.62954-0.0475 13791.25424.62C41H44O16-1.85.62789-0.048 14669.27255.23C27H46N2O170.255.6263-0.0479 15907.31139.15C57H48O11-1.155.42851-0.046 16770.24950.6C47H37N3O8-1.675.40896-0.0448 17917.26127.48C57H42O120.975.40768-0.0444 18831.32044.45C40H52N2O171.35.32064-0.0464 19779.19576.89C49H32O104.335.31993-0.046 20565.19215.04C27H34O13-1.035.16505-0.044 21993.3694.59C42H58N8O20-0.455.14148-0.0436 22672.17476.59C37H23N9O5-0.435.114580.0425 23597.18085.2C27H34O15-2.835.091980.0415 24885.25559.54C53H42O130.225.0656-0.0456 25623.15993.68C28H32O16-2.955.064310.0334 26563.13994.68C26H28O14-1.315.032430.0395
附表7 先锋橙正离子模式下区别于其他橙的代谢物
Table S7 The metabolites in Xianfengcheng different from those in the other oranges in the positive ion mode
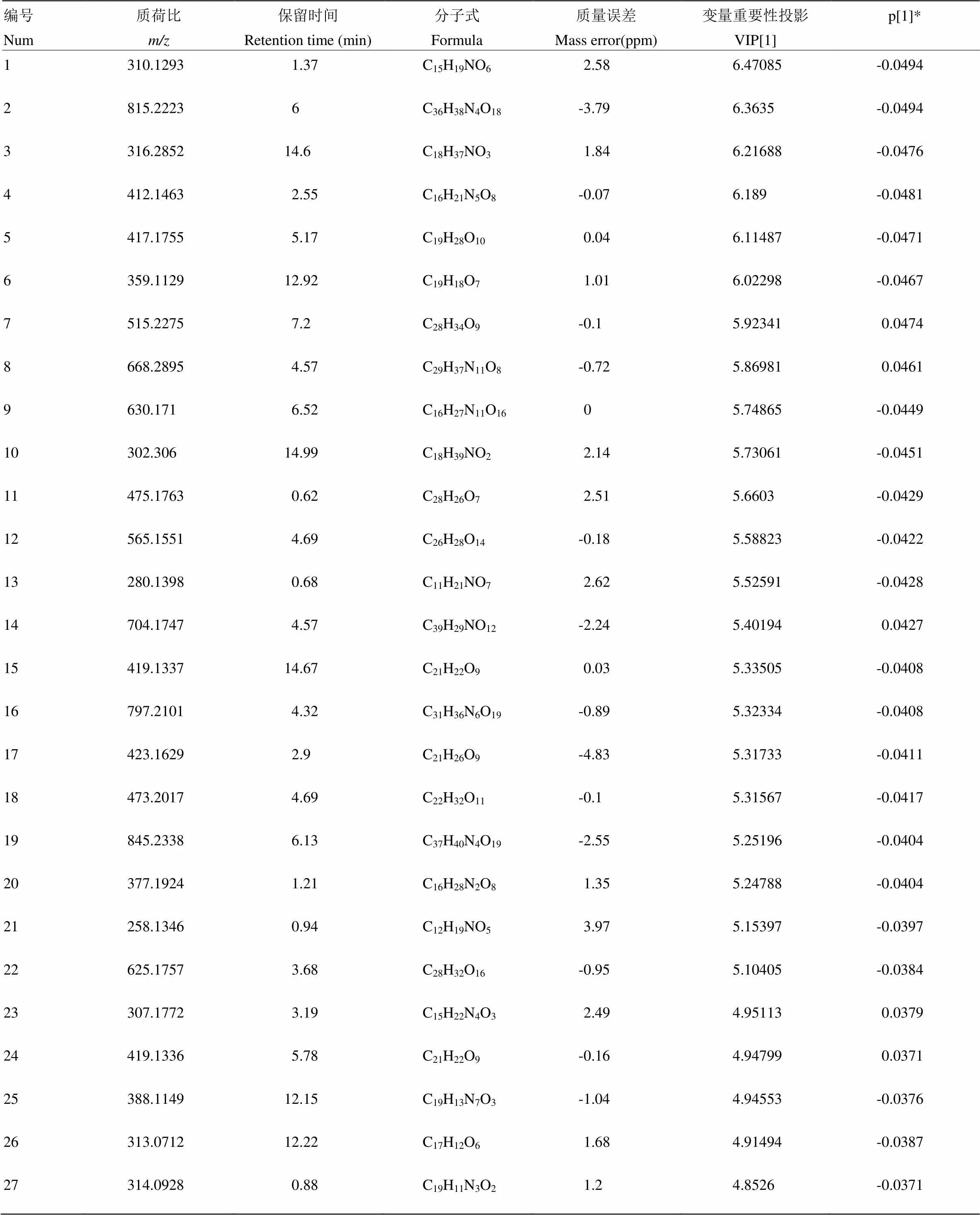
编号Num质荷比m/z保留时间Retention time (min)分子式Formula质量误差Mass error(ppm)变量重要性投影VIP[1]p[1]* 1310.12931.37C15H19NO62.586.47085-0.0494 2815.22236C36H38N4O18-3.796.3635-0.0494 3316.285214.6C18H37NO31.846.21688-0.0476 4412.14632.55C16H21N5O8-0.076.189-0.0481 5417.17555.17C19H28O100.046.11487-0.0471 6359.112912.92C19H18O71.016.02298-0.0467 7515.22757.2C28H34O9-0.15.923410.0474 8668.28954.57C29H37N11O8-0.725.869810.0461 9630.1716.52C16H27N11O1605.74865-0.0449 10302.30614.99C18H39NO22.145.73061-0.0451 11475.17630.62C28H26O72.515.6603-0.0429 12565.15514.69C26H28O14-0.185.58823-0.0422 13280.13980.68C11H21NO72.625.52591-0.0428 14704.17474.57C39H29NO12-2.245.401940.0427 15419.133714.67C21H22O90.035.33505-0.0408 16797.21014.32C31H36N6O19-0.895.32334-0.0408 17423.16292.9C21H26O9-4.835.31733-0.0411 18473.20174.69C22H32O11-0.15.31567-0.0417 19845.23386.13C37H40N4O19-2.555.25196-0.0404 20377.19241.21C16H28N2O81.355.24788-0.0404 21258.13460.94C12H19NO53.975.15397-0.0397 22625.17573.68C28H32O16-0.955.10405-0.0384 23307.17723.19C15H22N4O32.494.951130.0379 24419.13365.78C21H22O9-0.164.947990.0371 25388.114912.15C19H13N7O3-1.044.94553-0.0376 26313.071212.22C17H12O61.684.91494-0.0387 27314.09280.88C19H11N3O21.24.8526-0.0371
附表8 先锋橙负离子模式下区别于其他橙的代谢物
Table S8 The metabolites in Xianfengcheng different from those in the other oranges in the negative ion mode
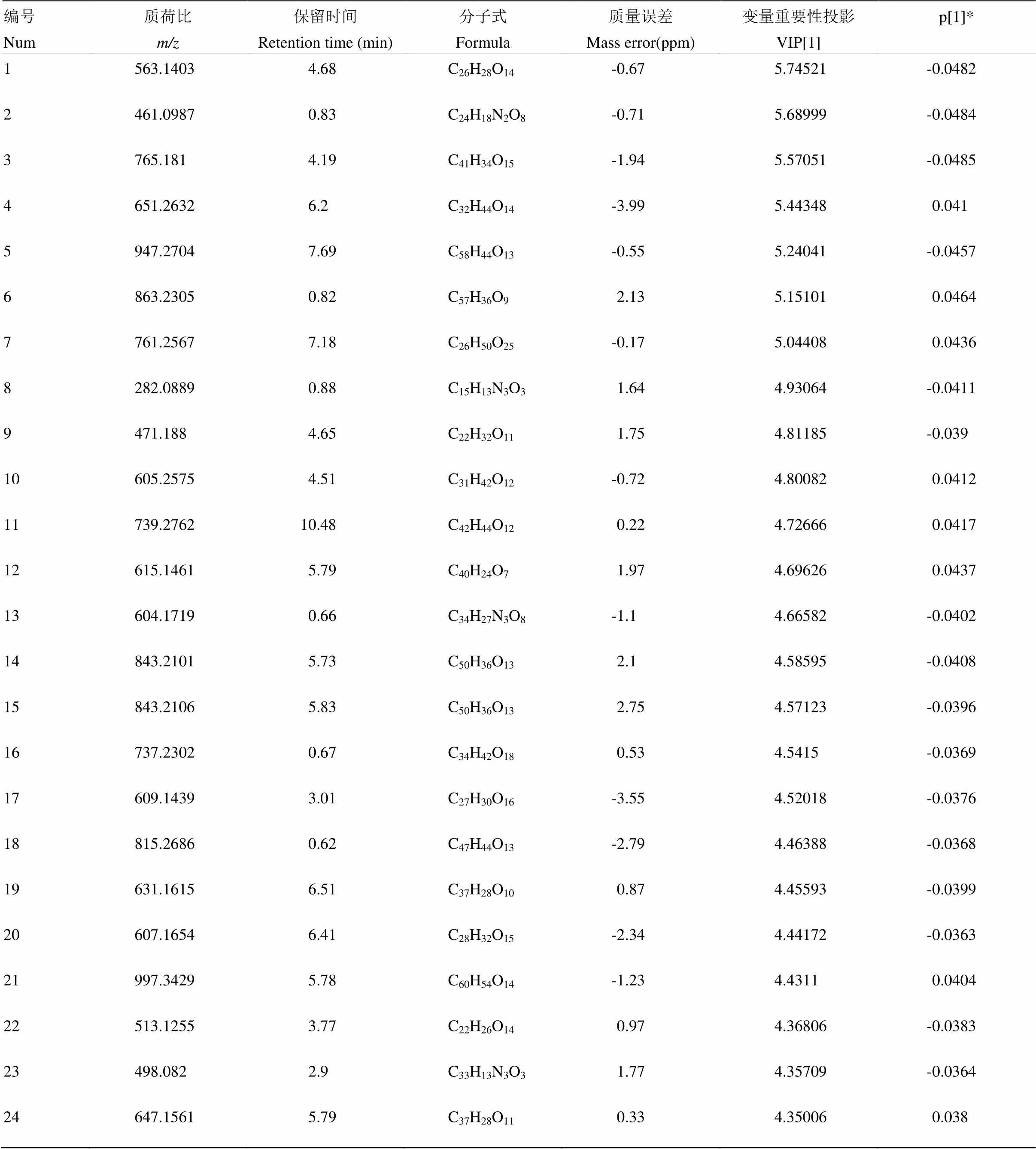
编号Num质荷比m/z保留时间Retention time (min)分子式Formula质量误差Mass error(ppm)变量重要性投影VIP[1]p[1]* 1563.14034.68C26H28O14-0.675.74521-0.0482 2461.09870.83C24H18N2O8-0.715.68999-0.0484 3765.1814.19C41H34O15-1.945.57051-0.0485 4651.26326.2C32H44O14-3.995.443480.041 5947.27047.69C58H44O13-0.555.24041-0.0457 6863.23050.82C57H36O92.135.151010.0464 7761.25677.18C26H50O25-0.175.044080.0436 8282.08890.88C15H13N3O31.644.93064-0.0411 9471.1884.65C22H32O111.754.81185-0.039 10605.25754.51C31H42O12-0.724.800820.0412 11739.276210.48C42H44O120.224.726660.0417 12615.14615.79C40H24O71.974.696260.0437 13604.17190.66C34H27N3O8-1.14.66582-0.0402 14843.21015.73C50H36O132.14.58595-0.0408 15843.21065.83C50H36O132.754.57123-0.0396 16737.23020.67C34H42O180.534.5415-0.0369 17609.14393.01C27H30O16-3.554.52018-0.0376 18815.26860.62C47H44O13-2.794.46388-0.0368 19631.16156.51C37H28O100.874.45593-0.0399 20607.16546.41C28H32O15-2.344.44172-0.0363 21997.34295.78C60H54O14-1.234.43110.0404 22513.12553.77C22H26O140.974.36806-0.0383 23498.0822.9C33H13N3O31.774.35709-0.0364 24647.15615.79C37H28O110.334.350060.038
附表9 S26锦橙正离子模式下区别于其他橙的代谢物
Table S9 The metabolites in S26 Jincheng different from those in the other oranges in the positive ion mode
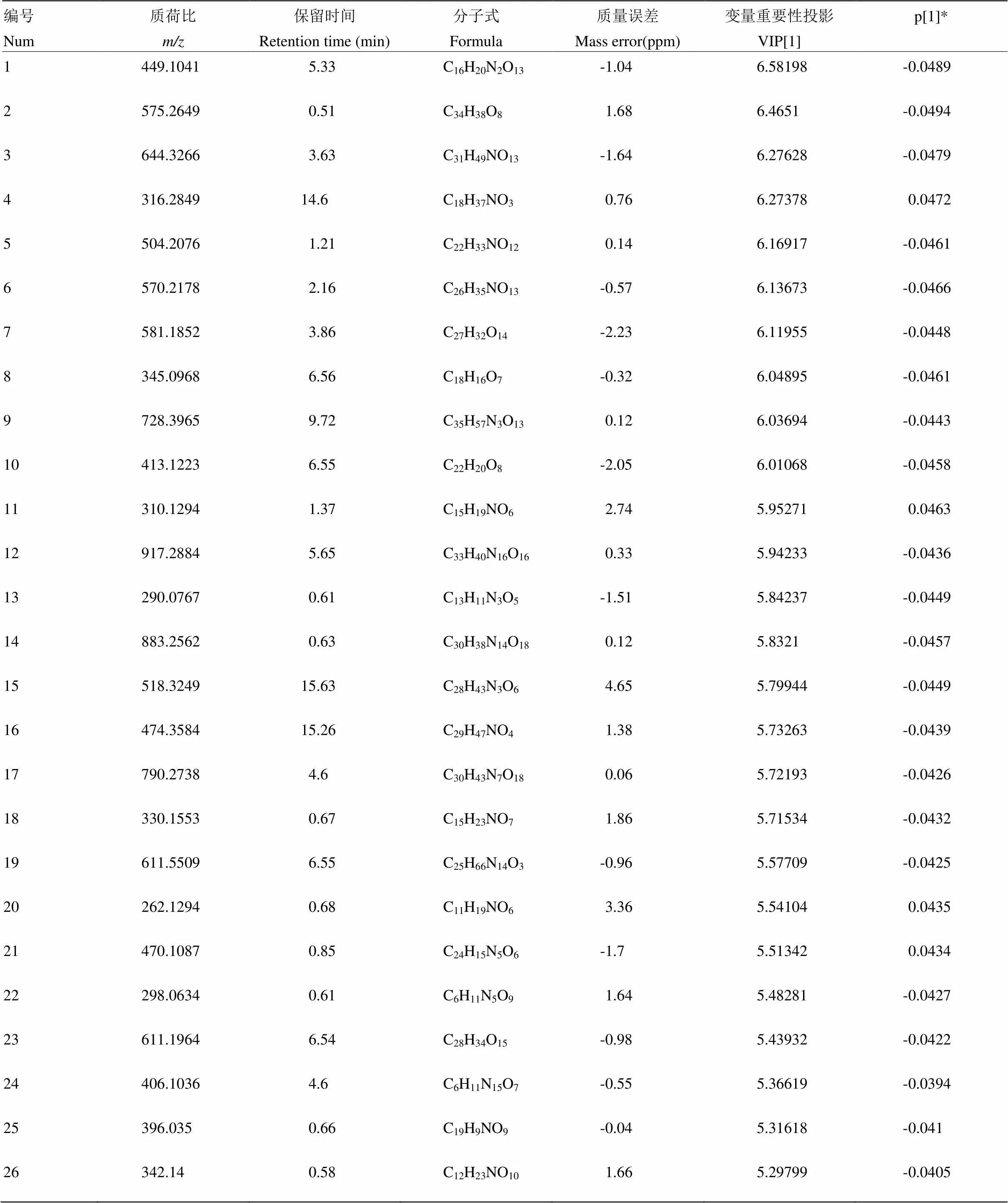
编号Num质荷比m/z保留时间Retention time (min)分子式Formula质量误差Mass error(ppm)变量重要性投影VIP[1]p[1]* 1449.10415.33C16H20N2O13-1.046.58198-0.0489 2575.26490.51C34H38O81.686.4651-0.0494 3644.32663.63C31H49NO13-1.646.27628-0.0479 4316.284914.6C18H37NO30.766.273780.0472 5504.20761.21C22H33NO120.146.16917-0.0461 6570.21782.16C26H35NO13-0.576.13673-0.0466 7581.18523.86C27H32O14-2.236.11955-0.0448 8345.09686.56C18H16O7-0.326.04895-0.0461 9728.39659.72C35H57N3O130.126.03694-0.0443 10413.12236.55C22H20O8-2.056.01068-0.0458 11310.12941.37C15H19NO62.745.952710.0463 12917.28845.65C33H40N16O160.335.94233-0.0436 13290.07670.61C13H11N3O5-1.515.84237-0.0449 14883.25620.63C30H38N14O180.125.8321-0.0457 15518.324915.63C28H43N3O64.655.79944-0.0449 16474.358415.26C29H47NO41.385.73263-0.0439 17790.27384.6C30H43N7O180.065.72193-0.0426 18330.15530.67C15H23NO71.865.71534-0.0432 19611.55096.55C25H66N14O3-0.965.57709-0.0425 20262.12940.68C11H19NO63.365.541040.0435 21470.10870.85C24H15N5O6-1.75.513420.0434 22298.06340.61C6H11N5O91.645.48281-0.0427 23611.19646.54C28H34O15-0.985.43932-0.0422 24406.10364.6C6H11N15O7-0.555.36619-0.0394 25396.0350.66C19H9NO9-0.045.31618-0.041 26342.140.58C12H23NO101.665.29799-0.0405
附表10 S26锦橙负离子模式下区别于其他橙的代谢物
Table S10 The metabolites in S26 Jincheng different from those in the other oranges in the negative ion mode
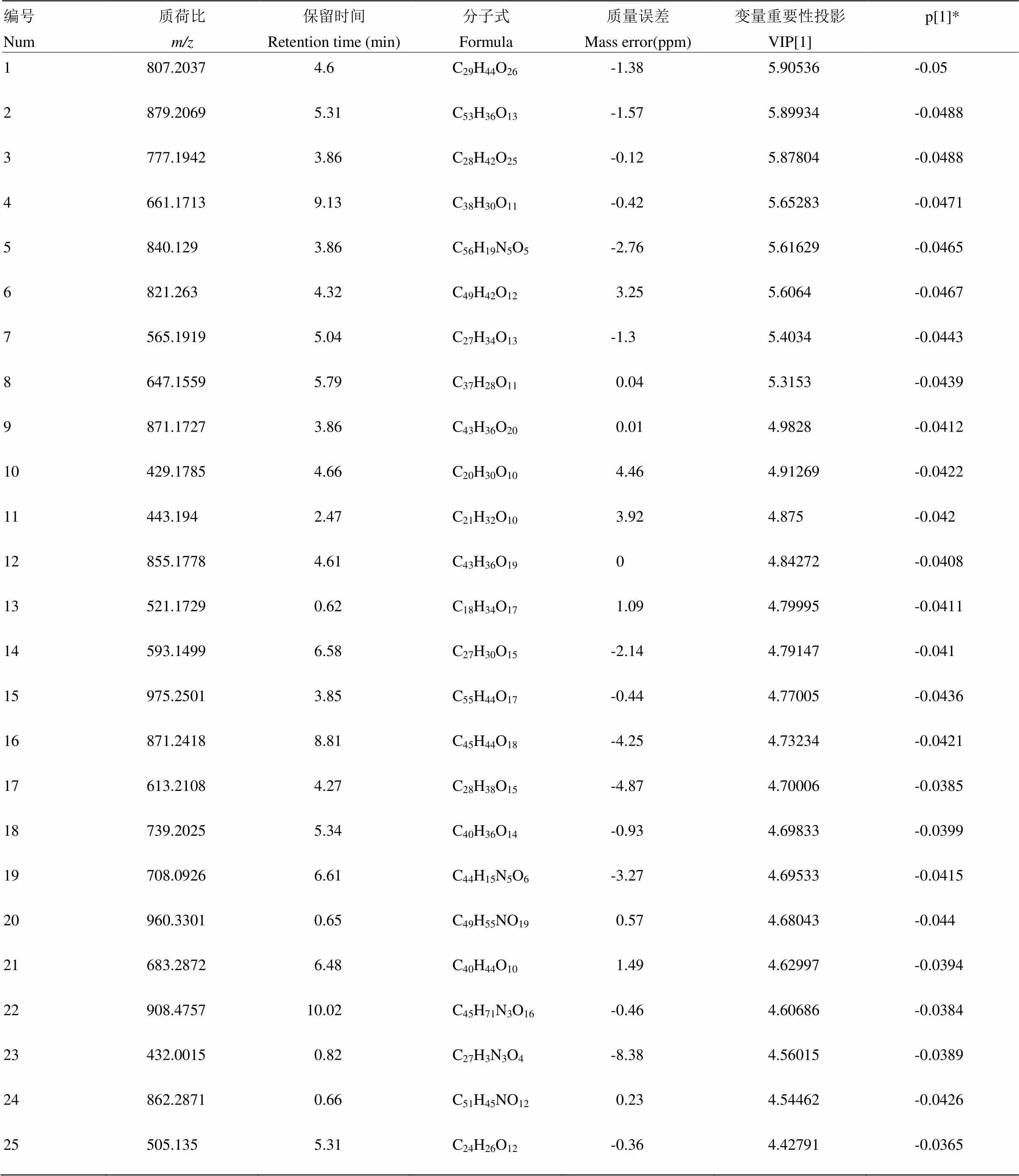
编号Num质荷比m/z保留时间Retention time (min)分子式Formula质量误差Mass error(ppm)变量重要性投影VIP[1]p[1]* 1807.20374.6C29H44O26-1.385.90536-0.05 2879.20695.31C53H36O13-1.575.89934-0.0488 3777.19423.86C28H42O25-0.125.87804-0.0488 4661.17139.13C38H30O11-0.425.65283-0.0471 5840.1293.86C56H19N5O5-2.765.61629-0.0465 6821.2634.32C49H42O123.255.6064-0.0467 7565.19195.04C27H34O13-1.35.4034-0.0443 8647.15595.79C37H28O110.045.3153-0.0439 9871.17273.86C43H36O200.014.9828-0.0412 10429.17854.66C20H30O104.464.91269-0.0422 11443.1942.47C21H32O103.924.875-0.042 12855.17784.61C43H36O1904.84272-0.0408 13521.17290.62C18H34O171.094.79995-0.0411 14593.14996.58C27H30O15-2.144.79147-0.041 15975.25013.85C55H44O17-0.444.77005-0.0436 16871.24188.81C45H44O18-4.254.73234-0.0421 17613.21084.27C28H38O15-4.874.70006-0.0385 18739.20255.34C40H36O14-0.934.69833-0.0399 19708.09266.61C44H15N5O6-3.274.69533-0.0415 20960.33010.65C49H55NO190.574.68043-0.044 21683.28726.48C40H44O101.494.62997-0.0394 22908.475710.02C45H71N3O16-0.464.60686-0.0384 23432.00150.82C27H3N3O4-8.384.56015-0.0389 24862.28710.66C51H45NO120.234.54462-0.0426 25505.1355.31C24H26O12-0.364.42791-0.0365
附表11 长叶橙正离子模式下区别于其他橙的代谢物
Table S11 The metabolites in Changyecheng different from those in the other oranges in the positive ion mode
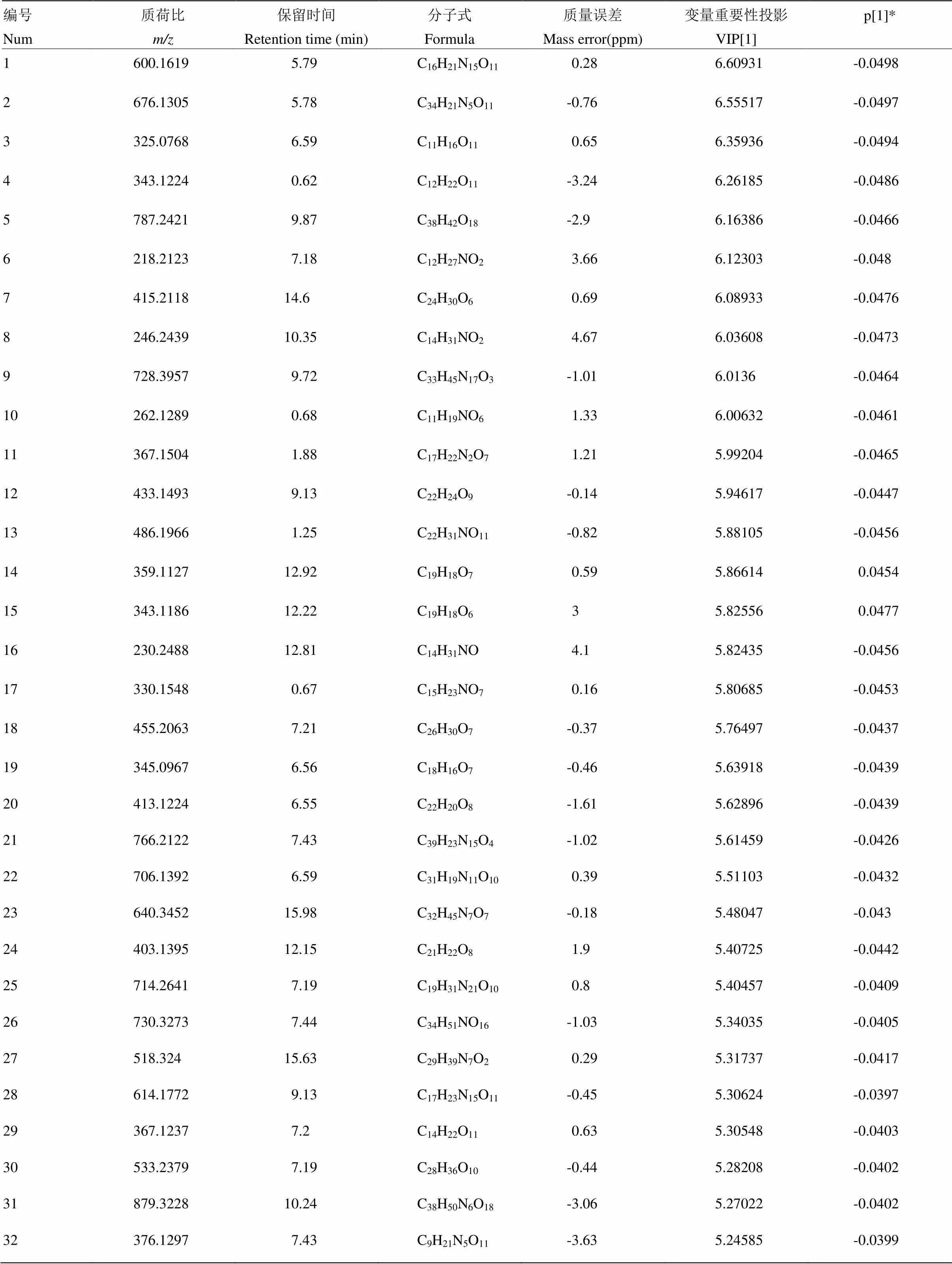
编号Num质荷比m/z保留时间Retention time (min)分子式Formula质量误差Mass error(ppm)变量重要性投影VIP[1]p[1]* 1600.16195.79C16H21N15O110.286.60931-0.0498 2676.13055.78C34H21N5O11-0.766.55517-0.0497 3325.07686.59C11H16O110.656.35936-0.0494 4343.12240.62C12H22O11-3.246.26185-0.0486 5787.24219.87C38H42O18-2.96.16386-0.0466 6218.21237.18C12H27NO23.666.12303-0.048 7415.211814.6C24H30O60.696.08933-0.0476 8246.243910.35C14H31NO24.676.03608-0.0473 9728.39579.72C33H45N17O3-1.016.0136-0.0464 10262.12890.68C11H19NO61.336.00632-0.0461 11367.15041.88C17H22N2O71.215.99204-0.0465 12433.14939.13C22H24O9-0.145.94617-0.0447 13486.19661.25C22H31NO11-0.825.88105-0.0456 14359.112712.92C19H18O70.595.866140.0454 15343.118612.22C19H18O635.825560.0477 16230.248812.81C14H31NO4.15.82435-0.0456 17330.15480.67C15H23NO70.165.80685-0.0453 18455.20637.21C26H30O7-0.375.76497-0.0437 19345.09676.56C18H16O7-0.465.63918-0.0439 20413.12246.55C22H20O8-1.615.62896-0.0439 21766.21227.43C39H23N15O4-1.025.61459-0.0426 22706.13926.59C31H19N11O100.395.51103-0.0432 23640.345215.98C32H45N7O7-0.185.48047-0.043 24403.139512.15C21H22O81.95.40725-0.0442 25714.26417.19C19H31N21O100.85.40457-0.0409 26730.32737.44C34H51NO16-1.035.34035-0.0405 27518.32415.63C29H39N7O20.295.31737-0.0417 28614.17729.13C17H23N15O11-0.455.30624-0.0397 29367.12377.2C14H22O110.635.30548-0.0403 30533.23797.19C28H36O10-0.445.28208-0.0402 31879.322810.24C38H50N6O18-3.065.27022-0.0402 32376.12977.43C9H21N5O11-3.635.24585-0.0399
附表12 长叶橙负离子模式下区别于其他橙的代谢物
Table S12 The metabolites in Changyecheng different from those in the other oranges in the negative ion mode
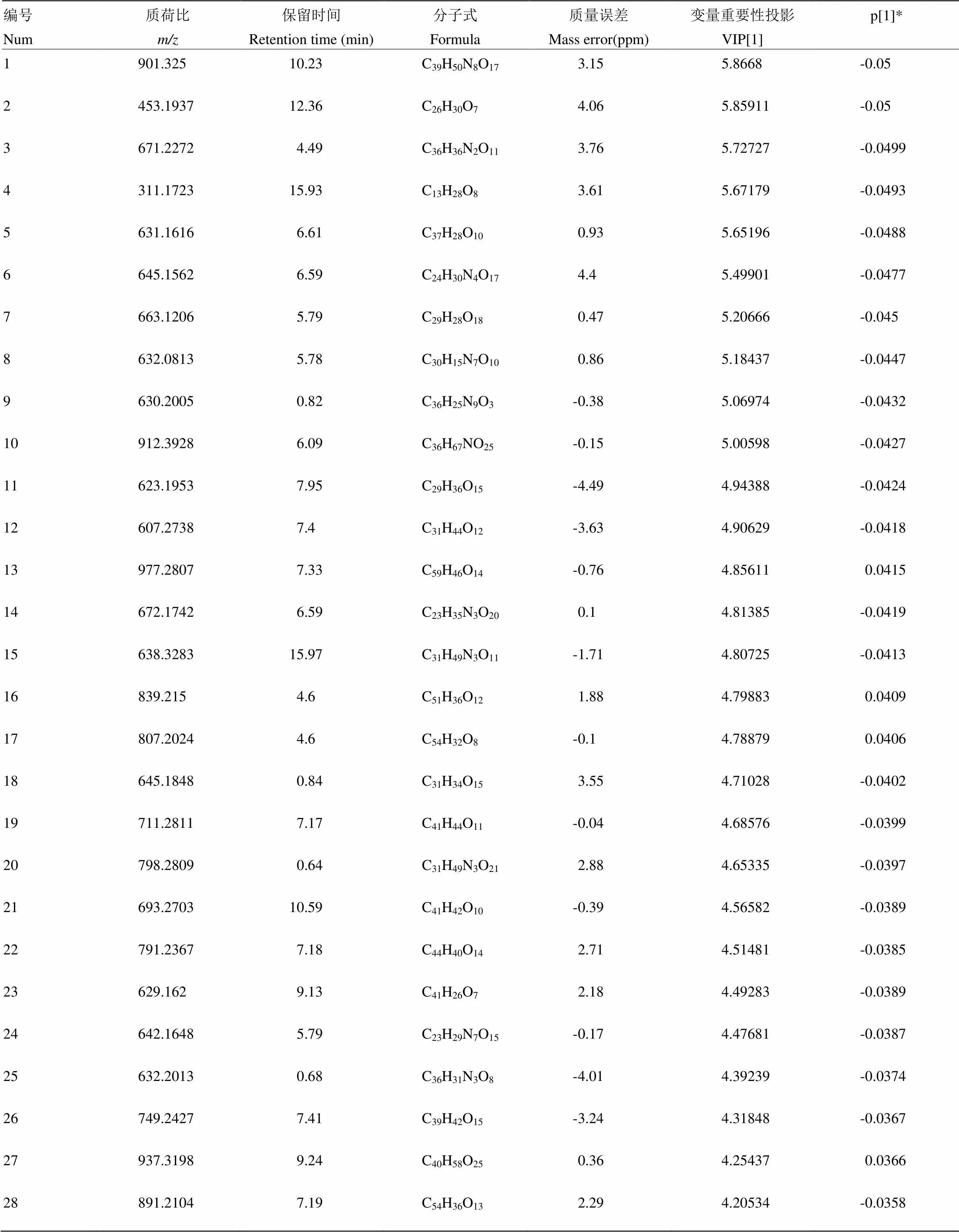
编号Num质荷比m/z保留时间Retention time (min)分子式Formula质量误差Mass error(ppm)变量重要性投影VIP[1]p[1]* 1901.32510.23C39H50N8O173.155.8668-0.05 2453.193712.36C26H30O74.065.85911-0.05 3671.22724.49C36H36N2O113.765.72727-0.0499 4311.172315.93C13H28O83.615.67179-0.0493 5631.16166.61C37H28O100.935.65196-0.0488 6645.15626.59C24H30N4O174.45.49901-0.0477 7663.12065.79C29H28O180.475.20666-0.045 8632.08135.78C30H15N7O100.865.18437-0.0447 9630.20050.82C36H25N9O3-0.385.06974-0.0432 10912.39286.09C36H67NO25-0.155.00598-0.0427 11623.19537.95C29H36O15-4.494.94388-0.0424 12607.27387.4C31H44O12-3.634.90629-0.0418 13977.28077.33C59H46O14-0.764.856110.0415 14672.17426.59C23H35N3O200.14.81385-0.0419 15638.328315.97C31H49N3O11-1.714.80725-0.0413 16839.2154.6C51H36O121.884.798830.0409 17807.20244.6C54H32O8-0.14.788790.0406 18645.18480.84C31H34O153.554.71028-0.0402 19711.28117.17C41H44O11-0.044.68576-0.0399 20798.28090.64C31H49N3O212.884.65335-0.0397 21693.270310.59C41H42O10-0.394.56582-0.0389 22791.23677.18C44H40O142.714.51481-0.0385 23629.1629.13C41H26O72.184.49283-0.0389 24642.16485.79C23H29N7O15-0.174.47681-0.0387 25632.20130.68C36H31N3O8-4.014.39239-0.0374 26749.24277.41C39H42O15-3.244.31848-0.0367 27937.31989.24C40H58O250.364.254370.0366 28891.21047.19C54H36O132.294.20534-0.0358
附表13 北碚447正离子模式下区别于其他橙的代谢物
Table S13 The metabolites in Beibei 447 different from those in the other oranges in the positive ion mode
编号Num质荷比m/z保留时间Retention time (min)分子式Formula质量误差Mass error(ppm)变量重要性投影VIP[1]p[1]* 1295.11390.61C10H18N2O81.016.691560.0481 2397.19722.57C19H28N2O70.596.49125-0.0494 3376.038514.47C11H5N9O70.036.39979-0.0486 4344.13410.82C15H21NO80.46.379440.0481 5467.17575.23C19H30O13-0.516.24184-0.0472 6314.160.67C15H23NO60.666.082650.0449 7630.17046.52C16H27N11O16-0.915.957910.0442 8259.0930.61C10H14N2O62.085.904460.0418 9280.13960.68C11H21NO71.735.865630.046 10250.09260.61C9H15NO71.975.673160.0436 11343.118111.37C19H18O61.425.657190.049 12343.12350.62C12H22O110.045.633470.0412 13625.17563.68C28H32O16-1.145.562010.0442 14277.10320.61C10H16N2O70.525.527670.0393 15246.243910.35C14H31NO24.635.507680.0437 16467.18882.33C19H26N6O80.735.4848-0.0412 17415.211614.6C24H30O60.115.476730.0388 18511.20184.22C21H34O14-0.615.44497-0.041 19326.12360.82C15H19NO70.445.432130.0406 20503.21019.35C17H18N201.045.42297-0.0412 21395.20386.69C18H34O90.145.34499-0.0405 22247.13365.39C15H18O33.125.34028-0.0413 23511.20123.97C21H34O14-1.855.22583-0.0391 24362.327213.07C20H43NO42.065.223640.0415 25698.17093.72C33H31NO16-0.995.20769-0.0396 26359.18181.5C16H26N2O71.515.179750.0388 27419.13385.8C21H22O90.395.12756-0.0389 28481.22928.09C21H36O122.495.06653-0.0385 29368.19197.8C15H29NO91.185.03442-0.0381
附表14 北碚447负离子模式下区别于其他橙的代谢物
Table S14 The metabolites in Beibei 447 different from those in the other oranges in the negative ion mode
编号Num质荷比m/z保留时间Retention time (min)分子式Formula质量误差Mass error(ppm)变量重要性投影VIP[1]p[1]* 1515.193511.37C27H32O102.325.971780.0423 2403.16224.25C18H28O103.045.80393-0.0493 3672.17426.59C21H23N17O100.115.76580.05 4369.15627.57C18H26O81.785.4549-0.0465 5635.21220.61C34H36O12-1.935.302970.0486 6439.226.69C19H36O113.515.29236-0.0446 7805.34077.72C39H54N2O160.765.28646-0.045 8423.18943.38C18H32O111.215.24883-0.0435 9777.2240.84C36H42O19-0.975.22820.038 10677.16546.51C38H30O12-1.525.189540.0454 11447.15213.41C19H28O122.795.179-0.0441 12461.09860.83C24H18N2O8-0.865.132050.0446 13592.20760.61C21H39NO18-3.085.121330.045 14423.18673.16C18H32O11-1.145.1065-0.0423 15411.18824.83C17H32O112.465.09322-0.0432 16726.3789.72C30H57N5O150.225.089570.0445 17293.1030.6C15H18O6-0.285.080030.046 18912.39326.09C38H59N9O17-2.615.009130.0435 19435.18846.93C19H32O112.674.96371-0.0419 20707.14476.6C31H32O19-2.594.915340.0432 21439.21997.02C20H32N4O70.134.84335-0.0409 22737.23040.67C34H42O180.714.695190.0386 23721.28417.74C39H46O13-3.444.59857-0.0391 24607.16526.17C28H32O15-2.654.542790.0356 25591.19133.63C25H36O16-3.024.49702-0.0388 26604.17160.66C34H27N3O8-1.624.460730.0384
附表15 8045甜橙正离子模式下区别于其他橙的代谢物
Table S15 The metabolites in 8045 Tiancheng different from those in the other oranges in the positive ion mode

编号Num质荷比m/z保留时间Retention time (min)分子式Formula质量误差Mass error(ppm)变量重要性投影VIP[1]p[1]* 1403.13911.01C21H22O80.616.73686-0.0492 2373.306911.23C20H40N2O42.166.30116-0.048 3274.27512.72C16H35NO23.356.28815-0.0487 4303.08656.55C16H14O60.556.27881-0.0469 5767.228812.19C37H38N2O16-0.786.25069-0.0487 6470.10790.85C23H19NO10-0.676.101870.0474 7230.13960.94C11H19NO43.866.04968-0.0466 8675.26066.24C29H42N2O16-0.226.009490.0455 9316.285214.6C18H37NO31.685.93551-0.0453 10379.20761.26C16H30N2O80.345.92258-0.0456 11389.123612.31C20H20O81.235.65466-0.0416 12413.21290.5C16H32N2O10-0.255.647760.0452 13409.18190.92C16H28N2O100.615.56839-0.0429 14478.337214.18C24H47NO8-0.495.41434-0.0414 15318.300912.91C18H39NO31.865.40105-0.0414 16393.22332.33C17H32N2O80.335.27559-0.0404 17409.19663.28C20H28N2O7-0.885.253640.0409 18925.25728.07C38H40N10O18-2.475.24354-0.0406 19412.14642.55C16H21N5O80.295.19921-0.0411 20351.17641.21C14H26N2O80.455.18822-0.0401 21328.15479.05C19H21NO41.095.1572-0.0395 22373.128410.54C20H20O70.595.11595-0.0389 23212.02099.63C9HN5O22.895.03473-0.0389 24339.086511.23C19H14O60.614.99968-0.0384 25704.17534.57C39H29NO12-1.314.99680.0399 26381.18660.82C15H28N2O9-0.394.96277-0.0382 27373.092511.83C19H16O81.924.95627-0.0371 28704.395310.59C33H57N3O13-1.614.929360.0363 29668.29044.57C32H45NO14-1.384.922440.0392 30609.18096.17C28H32O15-0.854.80608-0.0368
附表16 8045甜橙负离子模式下区别于其他橙的代谢物
Table S16 The metabolites in 8045 Tiancheng different from those in the other oranges in the negative ion mode
编号Num质荷比m/z保留时间Retention time (min)分子式Formula质量误差Mass error(ppm)变量重要性投影VIP[1]p[1]* 1741.21863.86C40H38O14-0.326.29005-0.0473 2633.25258.09C32H42O13-4.365.714530.0465 3719.24646.2C24H48O240.125.687420.0488 4845.26850.62C44H46O170.025.62835-0.0495 5403.16254.25C18H28O103.765.433790.0489 6638.19610.93C25H37NO183.675.33017-0.0473 7813.2375.31C39H38N6O14-0.425.29398-0.0407 8585.2860.93C36H42O70.415.26986-0.0465 9485.15260.85C18H30O152.855.23309-0.0481 10429.17745.85C20H30O101.95.008690.0435 11631.22035.41C35H36O112.94.95879-0.0426 12807.20414.6C54H32O83.444.93239-0.0402 13839.21414.6C36H32N12O130.234.87234-0.0396 14205.040.68C13H6N2O44.84622-0.0419 15733.19854.52C34H38O18-0.054.842430.0432 16795.19098.58C42H36O16-2.674.77309-0.0408 17425.18375.39C21H30O94.684.754460.0431 18563.17658.94C27H32O13-0.914.7076-0.0393 19311.172115.93C13H28O83.074.68871-0.0408 20645.15666.59C29H30N2O15-1.154.55511-0.039 21777.22297.18C36H42O19-2.334.477170.0373
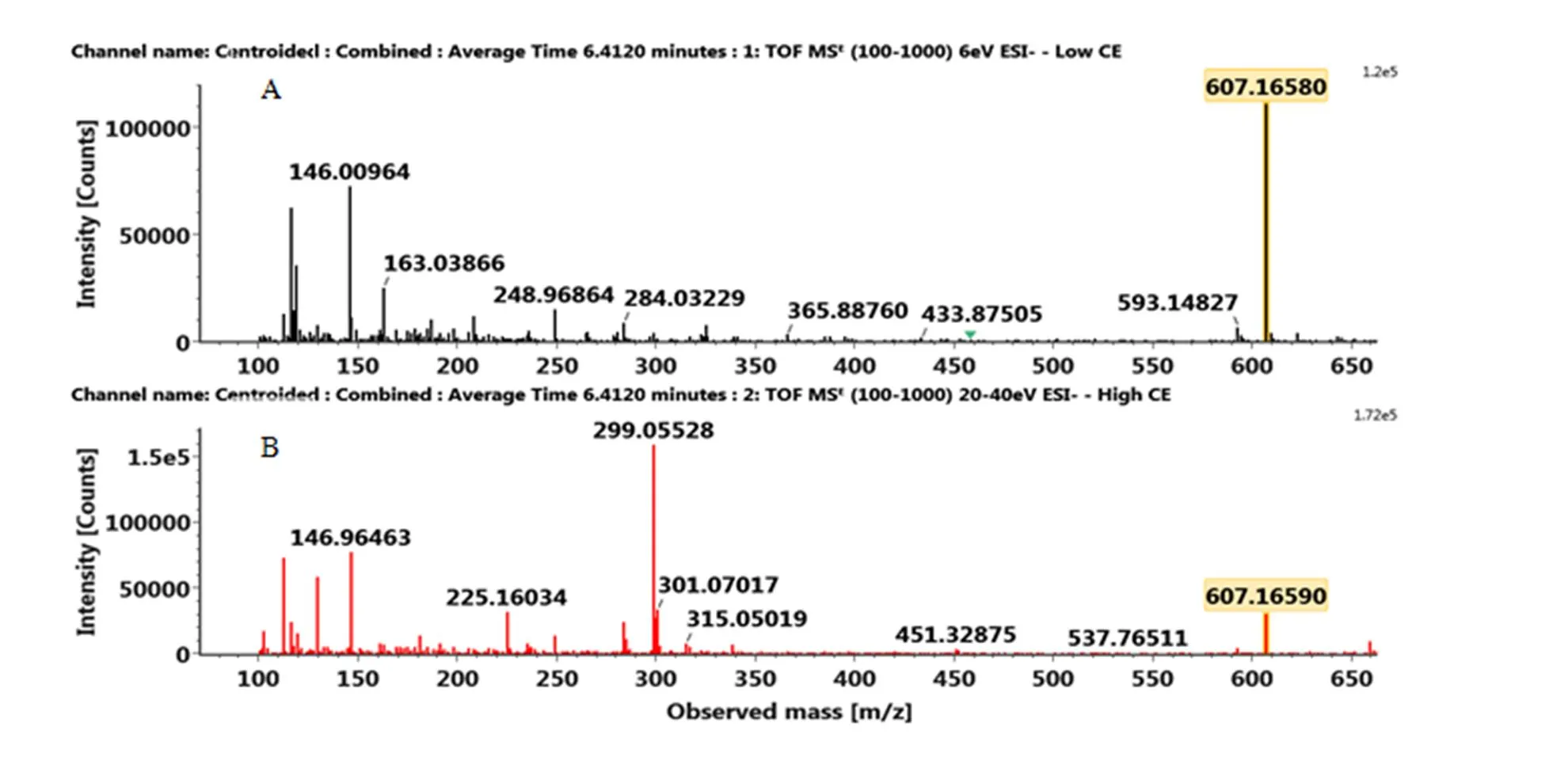
附图1 正离子模式下橘皮素标准品的MSE图:(A)MS(B)MS/MS
Fig. S1 The MSEspectra of tangeretin standard in the postitive ion mode: (A) MS, (B) MS/MS
附图2 负离子模式下地奥司明标准品的MSE图:(A)MS(B)MS/MS
Fig. S2 The MSEspectra of diosmin standard in the negative ion mode: (A) MS, (B) MS/MS
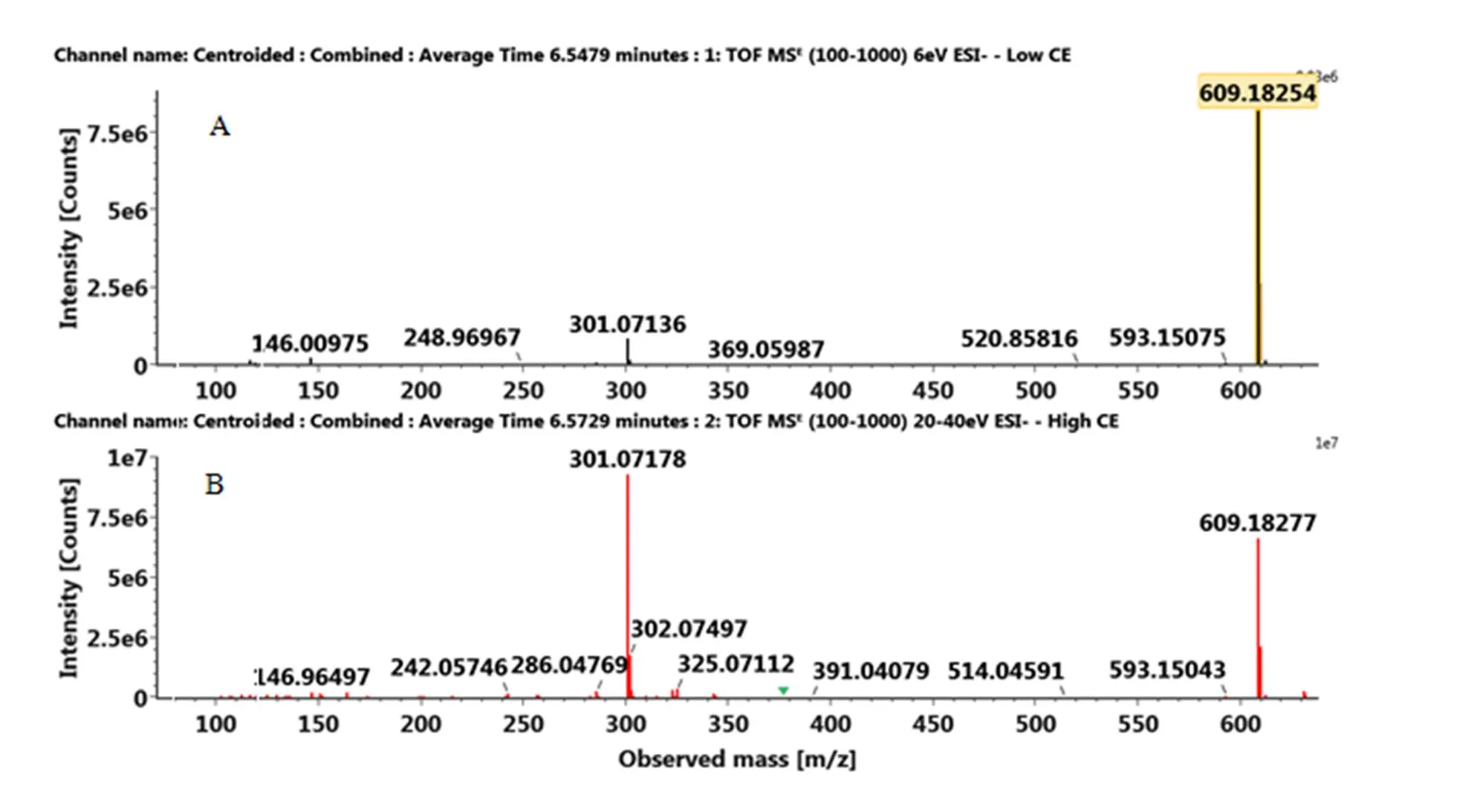
附图3 负离子模式下橙皮苷标准品的MSE图:(A)MS(B)MS/MS
Fig. S3 The MSEspectra of hesperidin standard in the negative ion mode: (A) MS, (B) MS/MS

附图4 正离子模式下川陈皮素标准品的MSE图:(A)MS(B)MS/MS
Fig. S4 The MSEspectra of nobiletin standard in the positive ion mode: (A) MS, (B) MS/MS
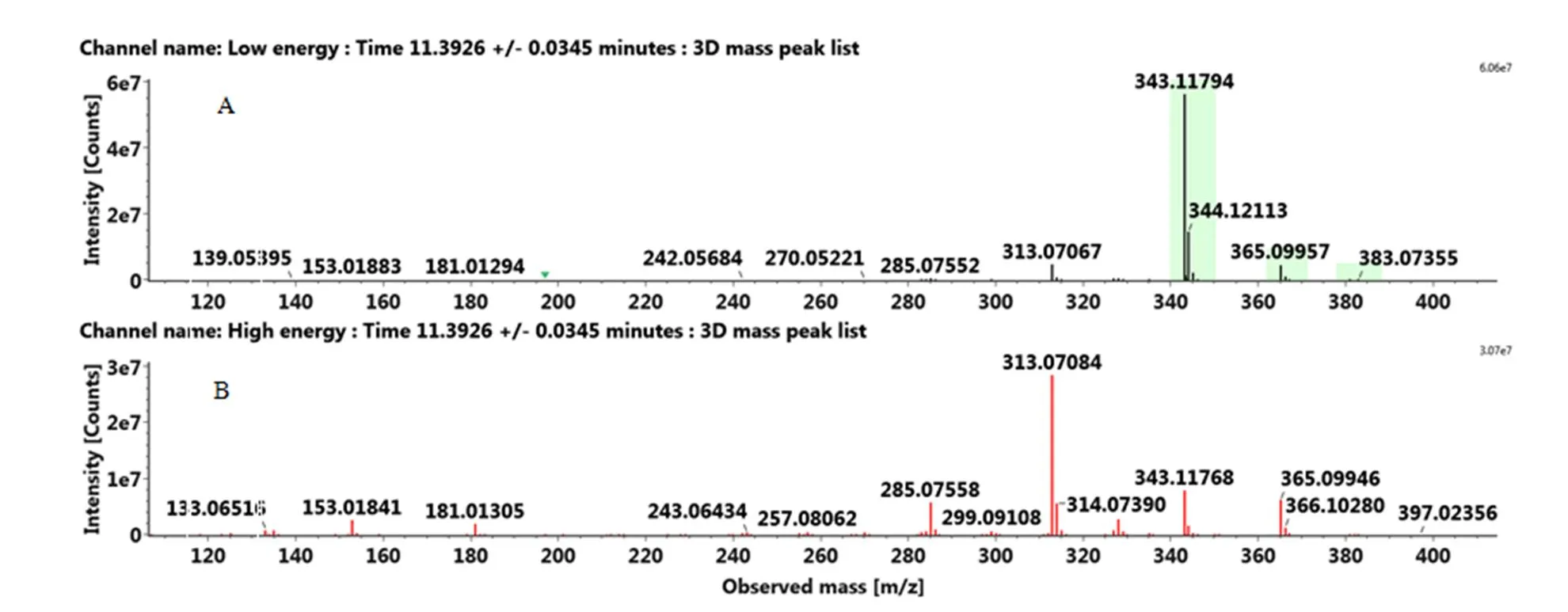
附图5 正离子模式下5,6,7,4'-四甲氧基黄酮标准品的MSE图:(A)MS(B)MS/MS
Fig. S5 The MSEspectra of 5,6,7,4'-tetramethoxyflavone standard in the positive ion mode: (A) MS, (B) MS/MS

附图6 正离子模式下异橙黄酮标准品的MSE图:(A)MS(B)MS/MS
Fig. S6 The MSEspectra of isosinensetin standard in the positive ion mode: (A) MS, (B) MS/MS
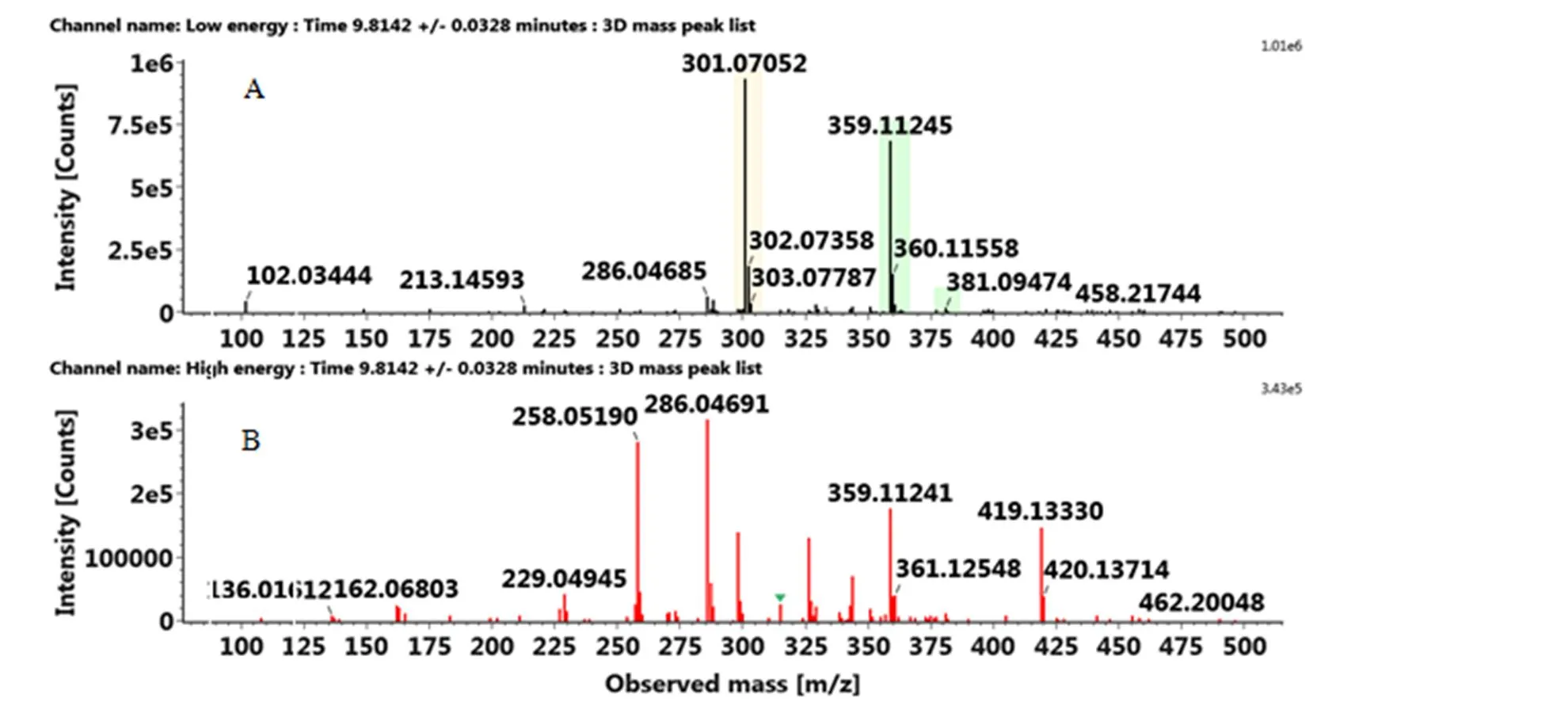
附图7 正离子模式下3-羟基-5,7,3',4'-四甲氧基黄酮的MSE图:(A)MS(B)MS/MS
Fig. S7 The MSEspectra of 3-hydroxy-5,7,3',4'-tetramethoxyflavone in the positive ion mode: (A) MS, (B) MS/MS
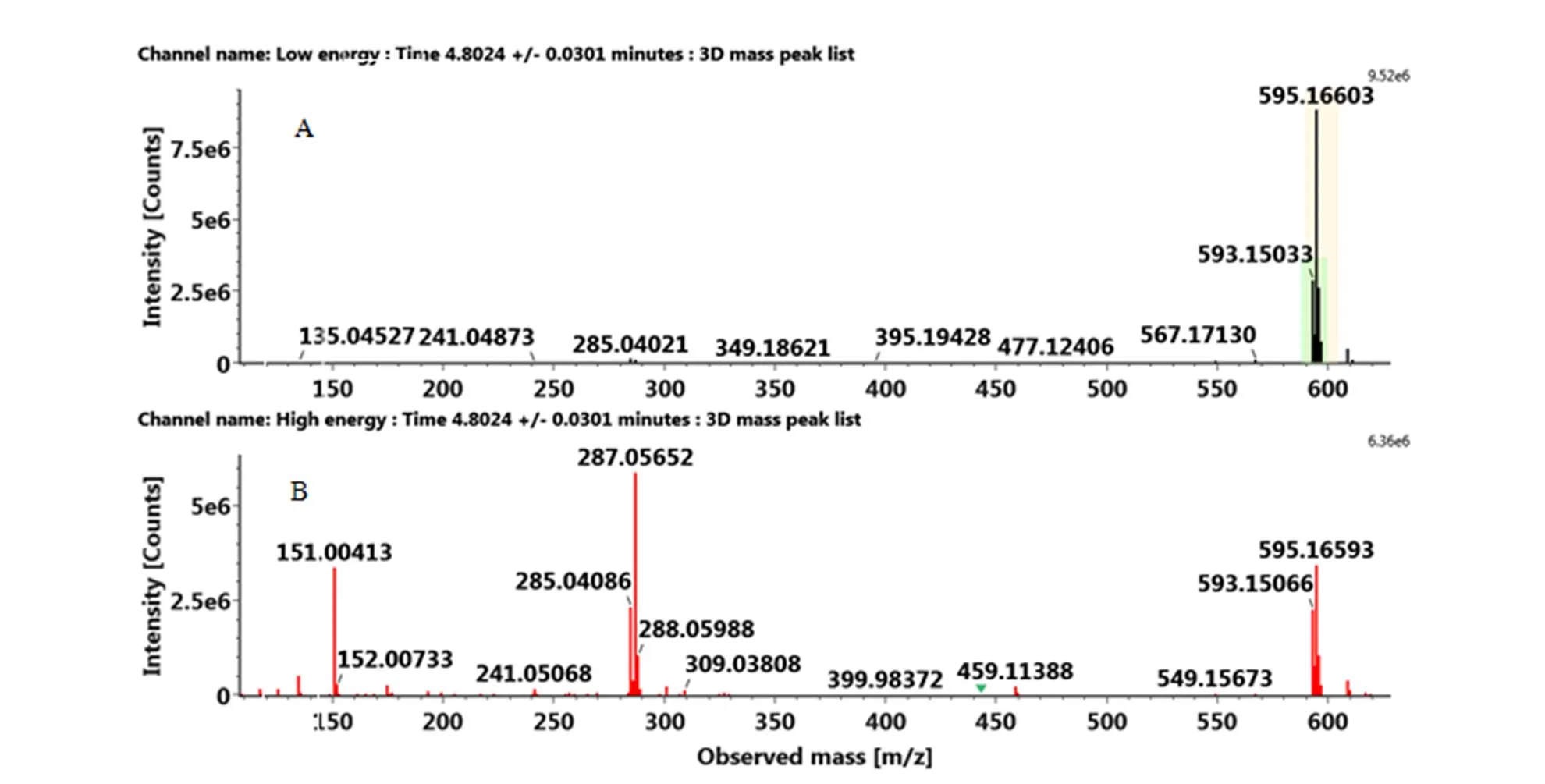
附图8 负离子模式下木犀草素-7-O-新橙皮糖苷的MSE图:(A)MS(B)MS/MS
Fig. S8 The MSEspectra of luteolin-7-O-neohesperidoside in the negative ion mode: (A) MS, (B) MS/MS
附图9 正离子模式下橙皮素-7-O-葡萄糖苷的MSE图:(A)MS(B)MS/MS
Fig. S9 The MSEspectra of hesperetin-7-O-glucoside in the positive ion mode: (A) MS, (B) MS/MS

附图10 正离子模式下野漆树苷-4'-O-葡萄糖苷的MSE图:(A)MS(B)MS/MS
Fig. S10 The MSEspectra of rhoifolin 4'-O-glucoside in the positive ion mode: (A) MS, (B) MS/MS

附图11 负离子模式下苯噻胺的MSE图:(A)MS(B)MS/MS
Fig. S11 The MSEspectra of penstemide in the negative ion mode: (A) MS, (B) MS/MS
附图12 正离子模式下5-羟基-6,7,3',4'-四甲氧基黄酮的MSE图:(A)MS(B)MS/MS
Fig. S12 The MSEspectra of 5-hydroxy-6,7,3',4'-tetramethoxyflavone in the positive ion mode: (A) MS, (B) MS/MS

附图13 正离子模式下7α-柠檬苦醇醋酸酯的MSE图:(A)MS(B)MS/MS
Fig. S13 The MSEspectra of 7α-limonyl acetate in the positive ion mode: (A) MS, (B) MS/MS
附图14 正离子模式下香叶木素6,8-di二-C-葡萄糖苷的MSE图:(A)MS(B)MS/MS
Fig. S14 The MSEspectra of diosmetin 6,8-di-C-glucosiden the positive ion mode: (A) MS, (B) MS/MS
附图15 正离子模式下金雀花素的MSE图:(A)MS(B)MS/MS
Fig. S15 The MSEspectra of scoparin in the positive ion mode: (A) MS, (B) MS/MS

附图16 负离子模式下奥巴叩酮17-O-β-D-葡萄糖苷的MSE图:(A)MS(B)MS/MS
Fig. S16 The MSEspectra of obacunone 17-O-β-D-glucoside in the negative ion mode: (A) MS, (B) MS/MS
附图17 负离子模式下芸香柚皮苷-4'-葡萄糖苷的MSE图:(A)MS(B)MS/MS
Fig. S17 The MSEspectra of narirutin-4'-glucoside in the negative ion mode: (A) MS, (B) MS/MS
[1] 刘贤青, 张红艳. HPLC-Q-TOF/MS分析脐橙果实中的类黄酮. 植物科学学报, 2014, 32(6): 638-644.
LIU X Q, ZHANG H Y. Determination of flavonoids from Navel Orange () fruits by HPLC-Q-TOF/MS., 2014, 32(6): 638-644. (in Chinese)
[2] 从彦丽, 彭梦雪, 刘冬, 孙海燕, 唐旭蔚. 柑橘在体外模拟胃肠消化过程中总多酚、总黄酮及总抗氧化活性的变化规律. 食品科学, 2016, 37(17): 96-103.
CONG Y L, PENG M X, LIU D, SUN H Y, TANG X W. Changes in total polyphenols, total flavonoids and antioxidant activity of Citrus duringgastrointestinal digestion process., 2016, 37(17): 96-103. (in Chinese)
[3] JANDRIĆ Z, CANNAVAN A. An investigative study on differentiation of citrus fruit/fruit juices by UPLC-QToF MS and chemometrics., 2017, 72: 173-180.
[4] Muntean E. Simultaneous carbohydrate chromatography and unsuppressed ion chromatography in detecting fruit juices adulteration., 2010, 71(1): 69-74.
[5] PROTTI M, VALLE F, POLI F, RAGGIA M A, MERCOLINI L. Bioactive molecules as authenticity markers of Italian Chinotto (×) fruits and beverages., 2015, 104: 75-80.
[6] LIM S, LEE J G, LEE E J. Comparison of fruit quality and GC–MS-based metabolite profiling of kiwifruit ‘Jecy green’: Natural and exogenous ethylene-induced ripening., 2017, 234: 81-92.
[7] BORGES G, CROZIER A. HPLC–PDA–MS fingerprinting to assess the authenticity of pomegranate beverages., 2012, 135: 1863-1867.
[8] 陈永刚, 林励. 柑橘属常用中药黄酮类成分HPLC指纹图谱研究与比较. 中国中药杂志, 2011, 36(19): 2660-2665.
CHEN Y G, LIN L. Study and comparison on HPLC fingerprints of flavonoids of frequently used Chinese materia medica in citrus., 2011, 36(19): 2660-2665. (in Chinese)
[9] 郑洁, 赵其阳, 张耀海, 焦必宁. 超高效液相色谱法同时测定柑橘中主要酚酸和类黄酮物质. 中国农业科学, 2014, 47(23): 4706-4717.
ZHENG J, ZHAO Q Y, ZHANG Y H, JIAO B N. Simultaneous determination of main flavonoids and phenolic acids in citrus fruit by ultra performance liquid chromatography., 2014, 47(23): 4706-4717. (in Chinese)
[10] 刘贤青, 涂虹, 王守创, 张红艳, 罗杰, 徐娟. 不同类型柑橘果实汁胞中类黄酮的液相色谱质谱联用分析. 植物生理学报, 2016, 52(5): 762-770.
LIU X Q, TU H, WANG S C, ZHANG H Y, LUO J, XU J. Flavonoid composition of citrus juice sacs determined by high-performance liquid chromatography coupled with tandem electrospray ionization mass spectrometry., 2016, 52(5): 762-770. (in Chinese)
[11] SANDÍN-ESPAÑ P, MATEO-MIRANDA M, LÓPEZ-GOTI C, DE CAL A, ALONSO-PRADOS J L. Development of a rapid and direct method for the determination of organic acids in peach fruit using LC-ESI-MS., 2016, 192(Sul C): 268-273.
[12] AVULA B, SAGI S, WANG Y H, WANG M, GAFNER S, MANTHEY J A, KHAN I A. Liquid chromatography-electrospray ionization mass spectrometry method analysis of limonoid aglycones, limonoid acids, limonoid glucosides and flavonoids from grapefruit seeds, other citrus species and dietary supplements., 2016, 82: 1058-1069.
[13] LÓPEZ-COBO A, GÓMEZ-CARAVACA A M, PASINI F, CABONI M F, SEGURA-CARRETERO A, FERNÁNDEZ-GUTIÉRREZ A. HPLC-DAD-ESI-QTOF-MS and HPLC-FLD-MS as valuable tools for the determination of phenolic and other polar compounds in the edible part and by-products of avocado., 2016, 73: 505-513.
[14] 陈林林, 米强, 辛嘉英. 柑橘皮精油成分分析及抑菌活性研究. 食品科学, 2010, 31(17): 25-28.
CHEN L L, MI Q, XIN J Y. Composition analysis and antibacterial activity of the essential oil from citrus peel., 2010, 31(17): 25-28. (in Chinese)
[15] XING T T, ZHAO X J, ZHANG Y D, LI Y F. Fast separation and sensitive quantitation of polymethoxylated flavonoids in the peels of Citrus using UPLC-Q-TOF-MS., 2017, 65(12): 2615-2627.
[16] YANG Y, ZHAO X J, PAN Y, ZHOU Z. Identification of the chemical compositions of Ponkan peel by ultra performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry., 2016, 8(4): 893-903.
[17] JANDRIĆ Z, ROBERTS D, RATHOR M N, ABRAHIM A, IALAM M, CANNAVAN A. Assessment of fruit juice authenticity using UPLC–QToF MS: A metabolomics approach., 2014, 148: 7-17.
[18] 常玉玮, 王国栋. LC-MS在植物代谢组学分析中的应用. 生命科学, 2015, 27(8): 978-985.
CHANG Y W, WANG G D. Application of LC-MS in plant metabolomics., 2015, 27(8): 978-985. (in Chinese)
[19] GUO J, YUE T, YUAN Y, WANG Y. Chemometric classification of apple juices according to variety and geographical origin based on polyphenolic profiles., 2013, 61(28): 6949-6963.
[20] JANDRIĆ Z, ISLAM M, SINGH D K, CANNAVAN A. Authentication of Indian citrus fruit/fruit juices by untargeted and targeted metabolomics., 2017, 72: 181-188.
[21] ZHANG J, YANG W, LI S, YAO S, QI P, YANG Z, FENG Z, HOU J, CAI L, YANG M, WU W, GUO D-A. An intelligentized strategy for endogenous small molecules characterization and quality evaluation of earthworm from two geographic origins by ultra-high performance HILIC/QTOF MSEand Progenesis QI.
, 2016, 408(14): 3881-3890.
[22] YAO C, YANG W, ZHANG J, QIU S, CHEN M, SHI X, PAN H, WU W, GUO D. UHPLC-Q-TOF-MS-based metabolomics approach to compare the saponin compositions of Xueshuantong injection and Xuesaitong injection., 2017, 40(4): 834-841.
[23] GIKA H G, THEODORIDIS G A, WINGATE J E, WILSON I D. Within-Day reproducibility of an HPLC-MS-based method for metabonomic analysis: application to human urine., 2007, 6(8): 3291-3303.
[24] CHEN S, KONG H, LU X, LI Y, YIN P, ZENG Z, XU G. Pseudotargeted metabolomics method and its application in serum biomarker discovery for hepatocellular carcinoma based on ultra high-performance liquid chromatography/triple quadrupole mass spectrometry., 2013, 85(17): 8326-8333.
[25] CUYCKENS F, CLAEYS M. Mass spectrometry in the structural analysis of flavonoids., 2004, 39(1): 1-15.
[26] YE X, CAO D, ZHAO X, SONG F, HUANG Q, FAN G, WU F. Chemical fingerprint and metabolic profile analysis of Citrus reticulate ‘Chachi’ decoction by HPLC-PDA-IT-MSn and HPLC- Quadrupole-Orbitrap-MS method., 2014, 970(Sul C): 108-120.
[27] KIM H G, KIM G-S, LEE J H, PARK S, JEONG W Y, KIM Y-H, KIM J H, KIM S T, CHO Y A, LEE W S, LEE S J, JIN J S, SHIN S C. Determination of the change of flavonoid components as the defence materials ofMarc. fruit peel againstby liquid chromatography coupled with tandem mass spectrometry., 2011, 128(1): 49-54.
[28] JAYAPRAKASHA G K, DANDEKAR D V, TICHY S E, PATIL B S. Simultaneous separation and identification of limonoids from citrus using liquid chromatography-collision-induced dissociation mass spectra., 2011, 34(1): 2-10.
[29] WANG S, YANG C, TU H, ZHOU J, LIU X, CHENG Y, LUO J, DENG X, ZHANG H, XU J. Characterization and metabolic diversity of flavonoids in Citrus species., 2017, 7(1): 10549.
[30] STANDER M A, KÜHN W, HITEN N F. Survey of South African fruit juices using a fast screening HILIC-MS method., 2013, 30(9): 1473-1484.
(责任编辑 赵伶俐)
Analysis of the Fingerprints of Different Orange Varieties and Their Differential Metabolites Based on Ultra-Performance Liquid Chromatography Coupled with Quadrupole Time-of-Flight Mass Spectrometry and Progenesis QI
ZHAO XiJuan, ZHAO WuJi, XU HuaChao
(Key Laboratory of Horticulture Science for Southern Mountainous Regions, Ministry of Education/College of Horticulture and Landscape Architecture, Southwest University, Chongqing 400715)
【Objective】The objective of this study is to establish an efficient method to investigate the differences between different varieties of oranges, and to find their differential metabolites, which will serve the citrus metabolomics and provide reference for the identification of oranges and orange juice. 【Method】8 orange cultivars from the same area were chosen to obtain the methanol extracts of their peels, and then based on ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (UPLC-QToF-MS), the LC-MS fingerprints of the 8 oranges were obtained with obvious differences. Combined with the software Progenesis QI, the differential metabolites were screened in positive and negative modes through principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) as well as loadings plots and VIP (variable importance for projection) values. Furthermore, the differential metabolites were identified based on retention times, accurate mass, MS/MS fragments, reference standards and public databases.【Result】Based on the established method, differential metabolites of the 8 orange cultivars were screened. 17 compounds had been identified and among them, and 6 compounds were confirmed by comparison with their commercial standards. 3-Hydroxy-5,7,3',4'-tetramethoxyflavone and luteolin-7-O-neohesperidoside were the characteristic metabolites of Xuecheng. Hesperidin was the marker compound of S26 Jincheng. Narirutin-4'-glucoside and Isosinensetin could act as the characteristic metabolites of 8045 Tiancheng. 【Conclusion】The method was versatile and suitable for the analysis of sample differences caused by varieties, geographical origins and maturity.
ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry; orange; Progenesis QI; metabolites
2017-12-14;
2018-03-26
中央高校基本科研业务费(XDJK2016B014,XDJK2017D093和SWU115065)
通信作者赵希娟,E-mail:xijuanzh@swu.edu.cn
10.3864/j.issn.0578-1752.2018.13.010

