Evaluation of Left Ventricular Systolic Function after Pulmonary Valve Replacement Using Cardiovascular Magnetic Resonance lmaging
Ali N.Zaidi and W.Aaron Kay, MD
2Division of Pediatric Cardiology, The Heart Center, Nationwide Children’s Hospital, The Ohio State University, Columbus,OH, USA
Abbreviations
ACE-I Angiotensin-converting enzyme inhibitor
CAD Coronary artery disease
CMR Cardiovascular magnetic resonance
LV Left ventricular
LVEDVI Left ventricular end-diastolic volume index
LVEF Left ventricular ejection fraction
LVESVI Left ventricular end-systolic volume index
PR Pulmonary regurgitation
PS Pulmonary stenosis
PVR Pulmonary valve replacement
rTOF Repaired tetralogy of Fallot
RV Right ventricular
RVEF Right ventricular ejection fraction
TOF Tetralogy of Fallot
Background
Tetralogy of Fallot (TOF) accounts for approximately 10% of all cases of congenital heart disease, and is one of the most common cyanotic congenital heart defects [1].It has been more than fi ve decades since the fi rst total TOF repair was performed in 1955 [2].Reparative surgery permits more than 85% of children born with TOF to survive into adulthood [3].With advances in surgical techniques, perioperative support, and imaging modalities, long-term outcomes have also substantially improved in the last 20 years,but resultant abnormalities such as severe pulmonary regurgitation (PR), significant right ventricular (RV) enlargement, dyskinetic interventricular septal motion, and reduced RV systolic function are still present in more than half of these patients [4, 5].It is now commonly accepted that pulmonary valve replacement (PVR) in patients with severe PR and concomitant RV volume overload can result in preservation or recovery of RV function.As such, much of the current literature on congenital heart disease focuses on preservation and recovery of RV function in patients with repaired TOF (rTOF).
In the last two decades, the presence of left ventricular (LV) systolic dysfunction in adults with rTOF has been increasingly recognized, and was reported in multiple recent studies [6–9].Although the left ventricle is not directly involved in the surgical repair of patients with TOF, patients who have undergone total repair appear to be prone to developing LV dysfunction that is out of proportion to the severity of any residual left-toright shunting or residual PR [10].The cause of LV dysfunction in such patients remains unclear.Fibrosis, hypertrophy of the interventricular septum, or the presence of a prosthetic patch could prevent the LV chamber from appropriately changing shape or accommodating an increased preload during diastolic fi lling.Conceivably, such interventricular septal changes could adversely affect LV systolic function, although this remains to be unequivocally proven [11].Studies have also shown that LV dysfunction is independently predictive of adverse clinical events in patients with rTOF [12].Khairy et al.[13] showed that the most important variable associated with the risk of appropriate implantable cardioverterdefibrillator shocks in a multivariate analysis of various predictors in patients with rTOF was an elevated LV end-diastolic pressure rather than PR severity, RV function, or the degree of RV volume loading.Broberg et al.[6] in a multicenter study showed that 21% of 511 patients with rTOF had at least mild LV dysfunction, with 6% of their population having an LV ejection fraction (LVEF)of less than 45%.In their study, predictors of LV dysfunction were RV systolic dysfunction, longer palliative shunt duration before complete repair,and arrhythmia history.
Two recent small cross-sectional studies showed a statistically significant, but modest, increase in LVEF after PVR for volume-overloaded right ventricles.In both studies the degree of improvement in LV systolic function was greater in patients who had depressed LV systolic function before PVR than in those patients who had normal LV systolic function [14].Echocardiographic parameters, including ventricular strain and strain rate, speckle tracking,and vector velocity imaging have also been used to document abnormalities in LV function or to evaluate interventricular dyssynchrony in patients with rTOF [15–17].
Our primary aim was to determine if surgical PVR would correlate with an increase in LVEF measured by cardiovascular magnetic resonance (CMR) analysis in patients with volume-loaded right ventricles secondary to PR.The secondary aims were to determine the prevalence of LV systolic dysfunction in patients with rTOF or critical pulmonary stenosis(PS), a population with similar issues of PR and RV volume loading following surgical intervention, and to evaluate if demographic and clinical variables,including cardiopulmonary bypass duration, heart failure medication use, coexisting hypertension,and concomitant coronary artery disease (CAD) or diabetes, related to LV systolic dysfunction in this cohort.
Methods
Study Population
Following institutional review board approval, a single-center retrospective review of all patients who underwent surgical PVR from January 2006 to September 2011 was performed.Patients aged 16 years or older at the time of surgery with an initial diagnosis of TOF or critical PS were included if they had undergone CMR imaging before and after PVR.All CMR studies were performed 2 years or less before PVR and at least 6 months after PVR.
Patient Data
Relevant medical and surgical history, including date of birth, sex, anatomic diagnosis, surgical history, date of PVR, and date of CMR study,were extracted from medical record review.A history of hypertension, diabetes, or hyperlipidemia and previous or current tobacco use was recorded.Height, weight, and blood pressure at the time of the visit were recorded, if available.New York Heart Association functional class and QRS duration measured from an electrocardiogram (ECG) at the time of CMR imaging were reviewed before and after PVR.
Cardiac Magnetic Resonance lmaging
Studies were performed with a commercially available 1.5 T scanner (Magnetom Avanto, Siemens,Erlangen, Germany).Torso or cardiac phased array coils were chosen according to body size.Ventricular dimensions and function were assessed with an ECG-gated steady-state free precession pulse sequence with the following parameters: echo time 1.27 ms, repetition time 41 ms, fl ip angle 90o,maximum fi eld of view tailored to the patient’s body size to avoid wrap, matrix size 256 × 100,slice thickness 8 mm, interslice gap 20%, and 12–18 views per segment (depending on heart rate).Steady-state free precession cine imaging sequences were acquired in the following planes during breath holds: two-chamber plane, four-chamber plane, and short-axis plane with 12–15 slices fully covering the ventricular mass.Flow measurements were performed in the proximal main pulmonary artery with a prospectively gated velocity-encoded cine MRI pulse sequence with the following parameters: echo time 1.97 ms, repetition time 48.25 ms, maximum fi eld of view based on the patient’s size to avoid wrap of the vessel, matrix size 192 × 99, slice thickness 6 mm, and velocity encoding of 150–400 cm/s during free breathing.End-diastolic volumes, endsystolic volumes, stroke volumes, and ventricular ejection fraction were measured with a commercially available software package (Leonardo, Siemens,Erlangen, Germany).All volumes were indexed to body surface area.An ejection fraction between 45 to 55% was considered mildly depressed; an ejection fraction between 35 and 45% was considered moderately depressed; an ejection fraction of 35%or less was considered severely depressed.The last ECG before PVR and the ECG performed at the time of the post-PVR CMR imaging were measured off-line by a single investigator (WAK).
Statistical Analysis
All continuous variables are presented as the mean ± standard deviation when normally distributed.Pairedttests or the Wilcoxon signed-ranked test were used to compare pre-PVR and post-PVR ventricular volumes and ejection fraction, and a unpairedttest or a Mann-Whitney test was used to compare continuous variables between groups of patients.Statistical significance was inferred when P was less than 0.05.
Results
During the study period, 40 patients met the inclusion criteria: 31 had rTOF and 9 had critical PS.The age at the time of PVR was 29 ± 9 years (range 16–53 years).Baseline demographic information is shown in Table 1.Before PVR, LVEF ranged from 35 to 70% as shown in Figure 1.The low-LVEF group consisted of 26 patients (65%) with baseline LVEF of less than 55%; (49 ± 5)%, range 36–54%.Of these, the age at the time of PVR was 31 ± 9 years, with 1.6 ± 0.9 prior open heart surgical procedures.Twenty-one of these patients (81%)had rTOF, and 5 patients had PS.There was no history of CAD in any patient in this group; one patient had diabetes, and four patients used tobacco products.After PVR, in 19% of the patients in thelow-LVEF group, angiotensin-converting enzyme inhibitor (ACE-I) therapy was started, and in 31%of the patients, β-blocker therapy was started.In this low-LVEF group, the use of heart failure medications (either ACE-I or β-blocker) did not correlate with significant postoperative increase in LVEF(P = 0.75).The normal-LVEF group consisted of 14 patients with LVEF of 55% or more before PVR,age of 25 ± 7 years at the time of PVR, and 1.4 ± 0.5 open heart surgical procedures before PVR.Ten patients (71%) in the normal-LVEF group had rTOF,and four patients had PS.No patients in the normal-LVEF group had diabetes or CAD; two patients had a history of tobacco abuse.After PVR, in 29% of patients ACE-itherapy was started, and in 14% of patients β-blocker therapy was stared.In the normal-LVEF group, the use of heart failure medications(either ACE-I or β-blocker) was not associated with a statistically significant difference in postoperative LVEF increase (P = 0.96).Demographics, clinical variables, β-blocker or ACE-I use, prior pregnancies, and cardiopulmonary bypass duration had no effect on post-PVR LVEF (Table 2).
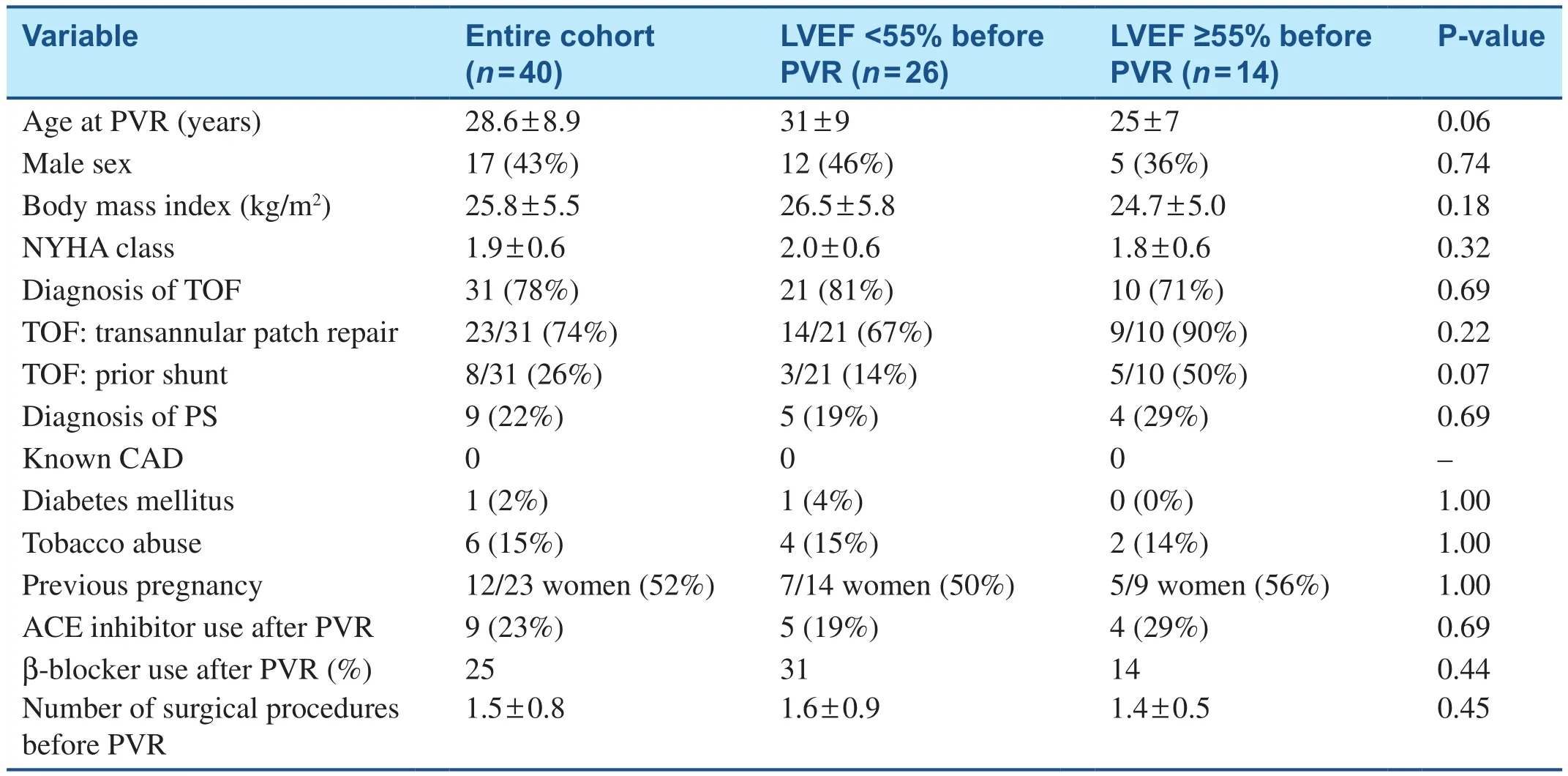
Table 1 Baseline Demographic Data in Patients before Pulmonary Valve Replacement (PVR).

Figure 1 Range of Left Ventricular Ejection Fraction before Pulmonary Valve Replacement in all Patients.
Twelve women had successful pregnancies; seven of these had baseline LVEF of less than 55%.Two women had one pregnancy, six women had two pregnancies, three women had three pregnancies,and one woman had four pregnancies.There were no differences in pre-PVR or post-PVR LVEF or volumetric indices when CMR data for women with and without pregnancies were compared (Table 1).There were no patients with known CAD; one patient had diabetes mellitus, one patient had a history of hypertension, and six patients (15%) were known smokers.There was no statistically signif icant correlation between these risk factors for CADand reduced LVEF before or after PVR.There were also no differences in any of the CMR variables (PR,ejection fraction, or volumetric indices) between patients with a pre-PVR BMI of less than 25 kg/m2and those with a pre-PVR BMI of 25 kg/m2or more(P = 0.32) (Table 1).

Table 2 Demographic Data in Patients After Pulmonary Valve Replacement (PVR).
All patients with moderately reduced LVEF (LVEF 35–45%) also had moderately to severely reduced RV ejection fraction (RVEF) before PVR (Figure 2).There was no significant correlation between LVEF and RVEF before PVR.There was no statistically significant difference in RVEF before PVR between the rTOF group and the PS group; (40 ± 9)% and(36 ± 13)%, respectively (P = 0.21) (Table 3).The pre-PVR and post-PVR RV end-diastolic volume indices were comparable in both groups.There was also no statistically significant difference in pulmonary regurgitant fraction between the rTOF group and the PS group (P = 0.195) (Table 3).
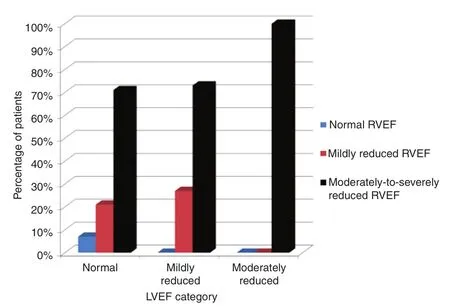
Figure 2 Correlation of Right Ventricular Ejection Fraction (RVEF) and Left Ventricular Ejection Fraction(LVEF) before Pulmonary Valve Replacement (PVR).
Pre-PVR CMR studies were done at 173 ± 103 days(range 33–459 days) before surgery, and the post-PVR CMR studies were done at 412 ± 196 days(range 188–730 days, median 375 days) after surgery.In the entire cohort, LVEF increased from(54 ± 8) to (57 ± 6)% (P = 0.023).Before PVR, there were 26 patients with depressed LVEF: (49 ± 5)%,range 36–54%.In this group, LVEF increased by(7 ± 7)% after PVR (P < 0.0001).By multivariate analysis to assess CMR predictors for increased LVEF after PVR, the only independent variable that was significantly associated with a postoperative increase in ejection fraction was a low LVEF before PVR, as seen in Figures 3 and 4 (regression coefficient −0.7,R2= 0.59, P < 0.0001).In the entire cohort (n= 40), the LV end-diastolic volume index (LVEDVI) after PVR increased from 66 ± 20 mL/m2to 71 ± 19 mL/m2(P = 0.03), with
no significant change in LV end-systolic volume index (LVESVI): 31 ± 12 mL/m2before PVR and 34 ± 14 mL/m2after PVR (P = 0.15) (Table 4).LVEF increased to (56.3 ± 5.8)% after PVR in patients with a baseline low pre-PVR LVEF and increased to (58.7 ± 5.7)% in patients who had a preserved LVEF of 55% or greater (P = 0.22) (Table 4).
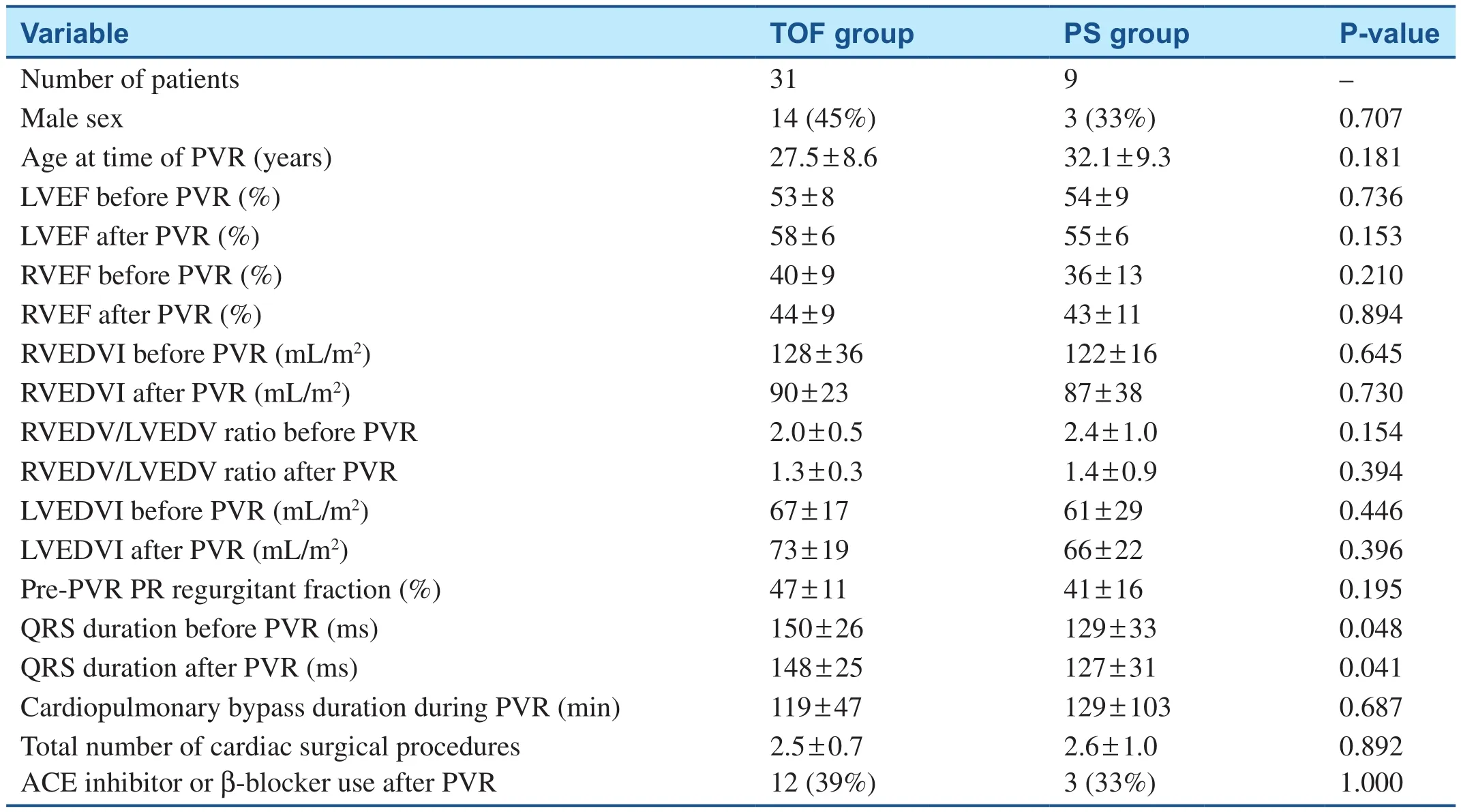
Table 3 Comparison of Cardiac Magnetic Resonance Parameters between the Tetralogy of Fallot (TOF) Group and the Pulmonary Stenosis (PS) Group.
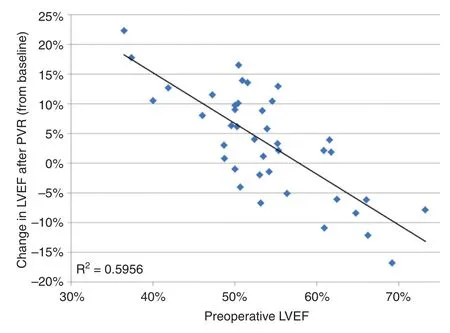
Figure 4 Change in Left Ventricular Ejection Fraction(LVEF) as a Function of LVEF before Pulmonary Valve Replacement (PVR).
The CMR volumetric and functional (LVEF)parameters were also compared in patients after PVR, with the patients divided into two main groups: patients with normal LVEF and those with low LVEF (<55%) after PVR.For patients with low LVEF after PVR, there was no signif icant change in LVEF in this subgroup: pre-PVR LVEF of (52.8 ± 8)% and post-PVR LVEF of(51.2 ± 2.6)% (P = 0.46) (Table 5).However, in patients with low LVEF after PVR, RVEF signif icantly increased from (36.0 ± 7.1) to (42.5 ± 9.2)%(P = 0.01) after PVR (Table 5).There were no statistically significant changes in the diastolic or systolic LV volumetric indices before or after PVR in patients who had either a low LVEF or preserved LVEF after PVR.The degree of PR before PVR did not correlate with either RV or LV diastolic/systolic indices or RVEF or LVEF in either group after PVR (Table 5).
Discussion
Although the right ventricle has been the primary focus of attention in patients with rTOF, LV dysfunction is not an uncommon finding in these patients, and may play a role in determining longterm morbidity and mortality.The presence of LV systolic dysfunction in adults with rTOF has been increasingly recognized, and was reported in multiple recent studies [6–9].It has been suggested that the reduced volume load on the right ventricle after PVR results in the rapid improvement of RV systolic function and in a more gradual recovery of RV diastolic function [17].Although the right ventricle and left ventricle are separate chambers, their interdependence has been described in the past.The ventricular chambers are anatomically linked in several ways, including by spiral muscle fi bers that ensure synchronous contraction, with evidence for hemodynamic impact of the right ventricle on the left ventricle, and vice versa.This has been demonstrated in several studies in both the normal heart and the diseased heart [18, 19].Since the right ventricle is more compliant than the left ventricle, a significant degree of RV volume overload is required to adversely affect LV compliance and geometrywith consequent LV dysfunction.Such ventricular changes are often seen in patients with rTOF secondary to long-standing severe PR.It therefore stands to reason that LV function may improve following restoration of pulmonary valve competence.This functional recovery can differ depending on the preoperative status of LV function [19].

Table 4 Comparison of Cardiac Magnetic Resonance Parameters in Patients with Baseline Low Left Ventricular Ejection Fraction (LVEF) (≤55%) and Normal LVEF (≥55%) before Pulmonary Valve Replacement (PVR).
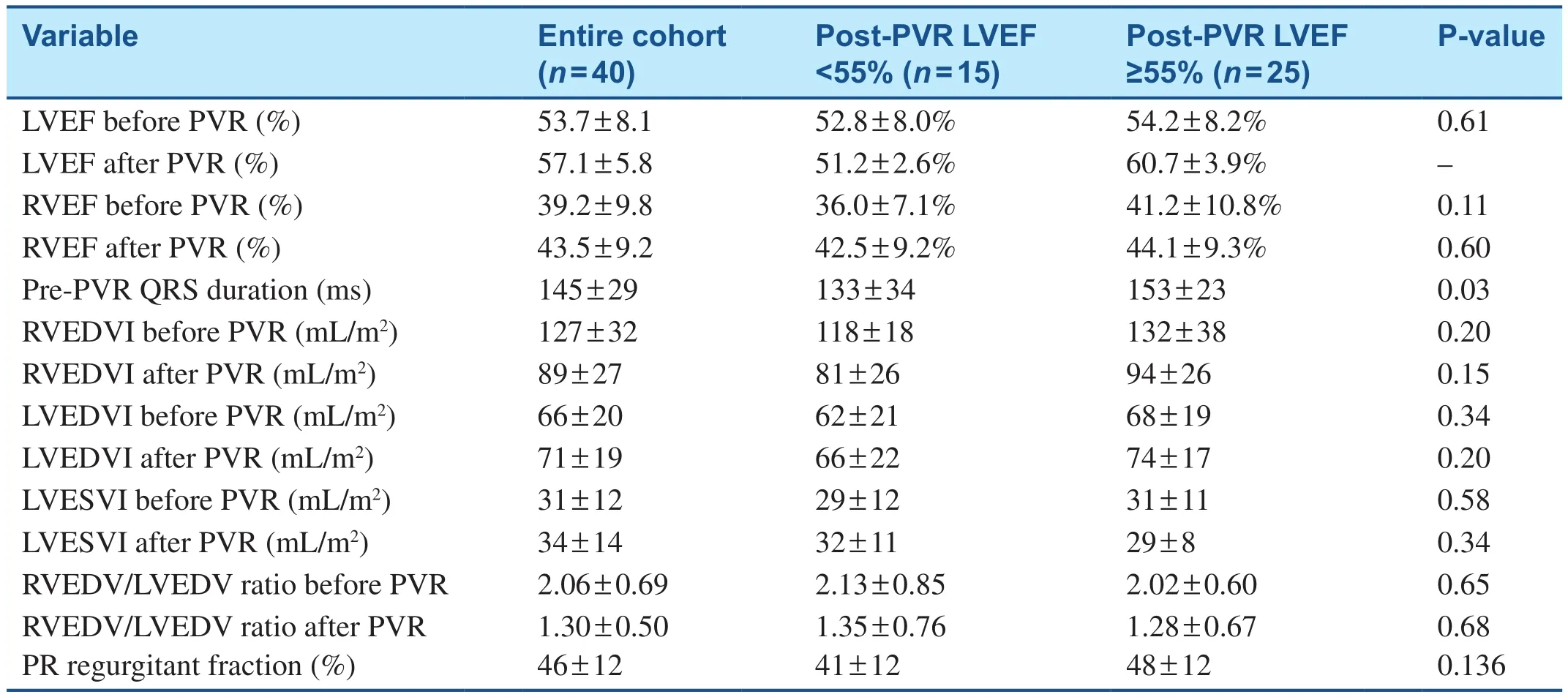
Table 5 Comparison of Cardiac Magnetic Resonance Parameters in Patients with Low Left Ventricular Fraction (LVEF) and Normal LVEF After Pulmonary Valve Replacement (PVR).
Although LV systolic dysfunction has been reported in rTOF, there are only sparse data regarding its prevalence, associated factors, and etiological cause [9].Factors playing a role in decreased LV function can include abnormalities of the ventricular septum due to surgical patch material and septal or myocardial fi brosis from prior surgical repair, all of which would be difficult to reverse with PVR [14].Our findings of an LVEF increase for all patients and a significantly larger LVEF increase for patients with more depressed LVEF at the baseline (before PVR) are similar to those of recent studies by Tobler et al.[19] using CMR imaging and Kane et al.[14] using echocardiography.The findings of Tobler et al.[19] imply that there was an increase in LV contractility, rather than an increase in LV end-diastolic volumes, driving the increase in LVEF after PVR in patients with an abnormal LVEF at the baseline.An alternative hypothesis to explain LVEF increase after PVR is that restored pulmonary valve competence results in increased forward fl ow through the pulmonary vasculature and thus increased pulmonary venous return to the left atrium and left ventricle, thereby increasing LVEDVI without changing contractility.
Studies in young adults have demonstrated a significant increase in LVEDVI with a significant improvement in CMR or echocardiographic indices of LV systolic function following PVR [14,20].We found a statistically significant, but only mild, increase in LVEDVI of 5 mL/m2in the entire cohort (P = 0.03).There was a statistically signif icant increase in LVEDVI in the low-LVEF group,but this trend was not demonstrated in patients with preserved LVEF before PVR.There was no significant change in LVESVI in either the low-LVEF group or the preserved-LVEF group.Our study did not show any relationship between the severity of PR and LV dysfunction (P = 0.136).
Since all rTOF patients had a ventricular septal defect patch as part of their complete intracardiac repair, the mechanics of the interventricular septum are likely very different in rTOF patients compared with PS patients given that a ventricular septal defect patch has no contractility.However, both disease processes (rTOF and PS) are associated with hemodynamically significant PR and resultant RV dilation and dysfunction, which are thought to affect LV function adversely.
Limitations
We are limited by the retrospective nature of this study.Because this was a retrospective study, data were limited to those available from medical record review.Referral bias is expected because patients were selected from a single established tertiary care adult congenital heart disease center.This was a single-center study with a small number of patients and, thus, limited statistical power.
Conclusions
Although LV volume and systolic function can be abnormal in adults late after TOF repair, PVR may have a beneficial effect on LV systolic function.We found that the only independent CMR predictor of postoperative improvement in LV systolic function is preoperative LV systolic dysfunction.This may be secondary to normalization of interventricular interactions after PVR, but the exact mechanisms responsible are as yet unknown.Larger studies are needed to further analyze our findings and determine accurate predictors associated with increased LVEF following PVR.
Author Contributions
Both authors designed the study, contributed to data acquisition, analysis, and interpretation, and read and approved the final manuscript.
Conflict of lnterest
The authors declare no conflict of interest.
REFERENCES
1.Hoffman JI.Incidence of congenital heart disease: I.Postnatal incidence.Pediatr Cardiol 1995;16:103–13.
2.Therrien J, Siu SC, McLaughlin PR,Liu PP, Williams WG, Webb GD.Pulmonary valve replacement in adults late after repair of tetralogy of Fallot: are we operating too late?J Am Coll Cardiol 2000;36:1670–5.
3.Murphy JG, Gersh BJ, Mair DD,Fuster V, McGoon MD, Ilstrup DM, et al.Long-term outcome in patients undergoing surgical repair of tetralogy of Fallot.N Engl J Med 1993;329:593–9.
4.Wald RM, Lyseggen E, Oechslin EN, Webb GD, Silversides CK.Variability in surgical referral patterns for pulmonary valve replacement in adults with repaired tetralogy of Fallot.Congenit Heart Dis 2009;4:231–8.
5.Eyskens B, Brown SC, Claus P,Dymarkowski S, Gewillig M,Bogaert J, et al.The influence of pulmonary regurgitation on regional right ventricular function in children after surgical repair of tetralogy of Fallot.Eur J Echocardiogr 2010;11:341–5.
6.Broberg CS, Aboulhosn J, Mongeon FP, Kay J, Valente AM, Khairy P,et al.Prevalence of left ventricular systolic dysfunction in adults with repaired tetralogy of Fallot.Am J Cardiol 2011;107:1215–20.
7.Geva T, Sandweiss BM, Gauvreau K, Lock JE, Powell AJ.Factors associated with impaired clinical status in long-term survivors of tetralogy of Fallot repair evaluated by magnetic resonance imaging.J Am Coll Cardiol 2004;43:1068–74.
8.Babu-Narayan SV, Kilner PJ, Li W,Moon JC, Goktekin O, Davlouros PA, et al.Ventricular fi brosis suggested by cardiovascular magnetic resonance in adults with repaired tetralogy of Fallot and its relationship to adverse markers of clinical outcome.Circulation 2006;113:405–13.
9.Ghai A, Silversides C, Harris L,Webb GD, Siu SC, Therrien J.Left ventricular dysfunction is a risk factor for sudden cardiac death in adults late after repair of tetralogy of Fallot.J Am Coll Cardiol 2002;40:1675–80.
10.Rocchini AP, Keane JF, Freed MD, Castaneda AR, Nadas AS.Left ventricular function following attempted surgical repair of tetralogy of Fallot.Circulation 1978;57:798–802.
11.Borow KM, Green LH, Castaneda AR, Keane JF.Left ventricular function after repair of tetralogy of Fallot and its relationship to age at surgery.Circulation 1980;61:1150–8.
12.Knauth AL, Gauvreau K, Powell AJ,Landzberg MJ, Walsh EP, Lock JE,et al.Ventricular size and function assessed by cardiac MRI predict major adverse clinical outcomes late after tetralogy of Fallot repair.Heart 2008;94:211–6.
13.Khairy P, Harris L, Landzberg MJ, Viswanathan S, Barlow A,Gatzoulis MA, et al.Implantable cardioverter-defibrillators in tetralogy of Fallot.Circulation 2008;117:363–70.
14.Kane C, Kogon B, Pernetz M,McConnell M, Kirshbom P, Rodby K, et al.Left ventricular function improves after pulmonary valve replacement in patients with previous right ventricular outflow tract reconstruction and biventricular dysfunction.Tex Heart Inst J 2011;38:234–7.
15.Cheung EW, Liang XC, Lam WW, Cheung YF.Impact of right ventricular dilation on left ventricular myocardial deformation in patients after surgical repair of tetralogy of Fallot.Am J Cardiol 2009;104:1264–70.
16.van der Hulst AE, Delgado V,Holman ER, Kroft LJ, de Roos A, Hazekamp MG, et al.Relation of left ventricular twist and global strain with right ventricular dysfunction in patients after operative“correction” of tetralogy of Fallot.Am J Cardiol 2010;106:723–9.
17.Mueller M, Rentzsch A, Hoetzer K,Raedle-Hurst T, Boettler P, Stiller B,et al.Assessment of interventricular and right-intraventricular dyssynchrony in patients with surgically repaired tetralogy of Fallot by twodimensional speckle tracking.Eur J Echocardiogr 2010;11:786–92.
18.Spadaro J, Bing OH, Gaasch WH, Weintraub RM.Pericardial modulation of right and left ventricular diastolic interaction.Circ Res 1981;48:233–8.
19.Tobler D, Crean AM, Redington AN, Van Arsdell GS, Caldarone CA, Nanthakumar K, et al.The left heart after pulmonary valve replacement in adults late after tetralogy of Fallot repair.Int J Cardiol 2012;160:165–70.
20.Frigiola A, Redington AN,Cullen S, Vogel M.Pulmonary regurgitation is an important determinant of right ventricular contractile dysfunction in patients with surgically repaired tetralogy of Fallot.Circulation 2004;110(11 Suppl 1):II-153–7.
 Cardiovascular Innovations and Applications2018年2期
Cardiovascular Innovations and Applications2018年2期
- Cardiovascular Innovations and Applications的其它文章
- Pulmonary Arterial Hypertension Medical Management of the Adult Patient with Congenital Heart Disease
- The Pulmonary Hypertension Story
- The Surgical Management of Ebstein Anomaly
- Pregnancy in Congenital Heart Disease:A Review for the General Cardiologist
- Atrial Arrhythmias lncluding Atrial Fibrillation in Congenital Heart Disease: Mechanisms,Substrate ldentification and lnterventional Approaches
- Heart Transplantation for Adult Congenital Heart Disease: Overview and Special Considerations
