复合萃取剂脱除废水中的活性红K-7B染料
文 晨,毛率先 ,刘文凤
(天津工业大学 环境与化学工程学院,天津300387)
随着人口增长和化工产业的快速发展,全球每天约有400 t染料以各种形式,如合成、加工和应用等排入自然水体中,由此导致了严重的水环境污染[1].众所周知,印染废水富含染料、表面活性剂和无机盐等.其中染料的分子结构均属于苯环、萘环和蒽醌类,在江河湖泊中会逐渐转化为有毒物质.因此,在排入受纳水体前必须对印染废水实施严格的有效处理[2].通常印染废水处理方法主要包含混凝沉淀法、吸附法、萃取法、膜分离法、高级氧化法和生化法等[3-12],而生化法是首选之一,这主要基于其环境友好和低成本.但是一些研究结果表明[13-18],生化法很难彻底降解染料分子,致使其出水残留颜色.为此,印染废水的脱色成为保护水环境和公众健康的重要课题之一.萃取法作为一种分离技术在富集与分离领域已得到广泛地应用,如废水脱色、脱酚和脱有机酸等[19-27].众所周知,萃取法的关键在于萃取剂的设计,也就是既要确保对溶质具有高度的亲和力,又要防止在溶剂中溶解或乳化.张奎等[28]报告了以三辛胺(TOA)作为萃取剂对CLT酸废水进行预处理,在最佳工艺条件下其萃取率高达99.27%.Homsirikamol等[19]设计了以三辛基甲基氯化铵、二(2-乙基己基)磷酸酯(P204)和磷酸三丁酯(TBP)为组分的复合萃取剂脱除废水中的阿莫西林,其研究显示,萃取率和分配系数分别达到了90.4%和9.44.因此,在印染废水处理领域,开发兼有良好溶质分配比和稳定性的复合萃取剂是极具挑战性的课题,对出水达标具有重要意义.本文构建了以三辛胺(TOA)和二(2-乙基己基)磷酸酯(P204)为主的二元复合萃取剂,探讨了在酸性条件下萃取活性红K-7B染料的行为及其实现最佳脱色率的工艺条件,为印染废水达标排放提供了有力的技术支持.
1 实验部分
1.1 实验材料与仪器
主要材料:三辛胺(TOA)、二(2-乙基己基)磷酸酯(P204)和磺化煤油(SK),河南洛阳奥达化工有限公司产品;氢氧化钠和碳酸钠,分析纯,天津市福晨化学试剂公司产品;活性红K-7B,山东如意数码印染公司产品,其特性显示在表1;实验用水为蒸馏水.
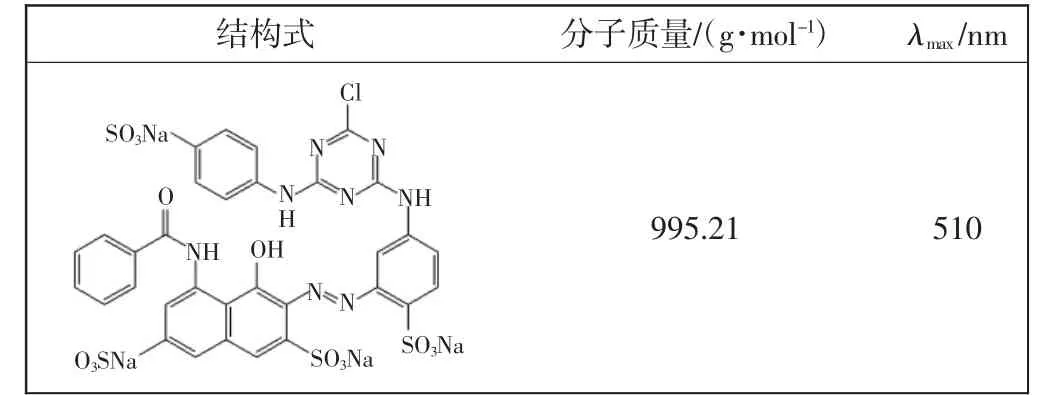
表1 活性红K-7B染料特征Tab.1 Characteristics of reactive red 4
主要仪器:TU-1900/1901型可见-紫外分光光度仪,北京普析通用仪器有限责任公司产品;PHS-25型数显pH计,上海雷磁公司产品;DF-101S智能集热恒温加热磁力搅拌器,河南巩义仪器公司产品.
拟定不同浓度的活性红K-7B染料溶液,依次测定其吸光度与浓度的工作曲线,在中性水中得到Y=38.11X+0.567,R2=0.999 5;在 pH 值为 3时 Y=38.14X+0.556,R2=0.999 1.可见,加入一定酸剂对活性红K-7B溶液的吸光度无影响.
1.2 实验方法
1.2.1 萃取实验
在煤油中依次加入不同体积比的TOA和P204充分混合后得到系列复合萃取剂.配置1 g/L活性红K-7B水溶液,调节pH值=3与复合萃取剂按水油比为5∶1置于烧杯中,在恒温磁力搅拌器中搅拌15 min后静置分层,取水相用UV-vis分析残存活性红K-7B染料的吸光度.按式(1)、(2)和(3)计算脱色率 Dr、萃取率Er、分配比D和协同系数R:
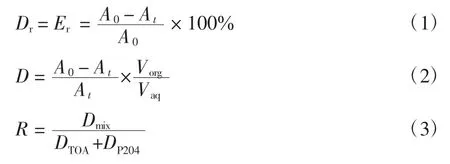
式中:A0、At分别为萃取前后水相的吸光度;Vorg、Vaq分别为复合萃取剂与染料溶液的体积(mL).
1.2.2 复合萃取剂再生实验
取上述条件下得到的负载染料的复合萃取剂与碱剂按油碱比为4∶1置于烧杯中,在恒温磁力搅拌器中搅拌15 min后静置分层,取水相用UV-vis分析仪分析残存活性红K-7B染料的吸光度.按式(4)计算复合萃取剂脱附率(Sr):

式中:Aaq为在碱剂中K-7B的吸光度;Aorg为拟再生的复合萃取剂中染料吸光度,Aorg=(A0-At)-Aaq;Vaq和Vorg分别为碱剂和拟再生的复合萃取剂体积(mL).
2 实验结果与讨论
2.1 萃取剂组分的体积比对萃取率影响
在恒定TOA与P204总体积分数为30%前提下,考察了复合萃取剂中TOA与P204的体积比(以XTOA表示,XTOA=[TOA]/[TOA]+[P204])对活性红K-7B的萃取率(脱色率)和协同系数的影响,其结果如图1所示.
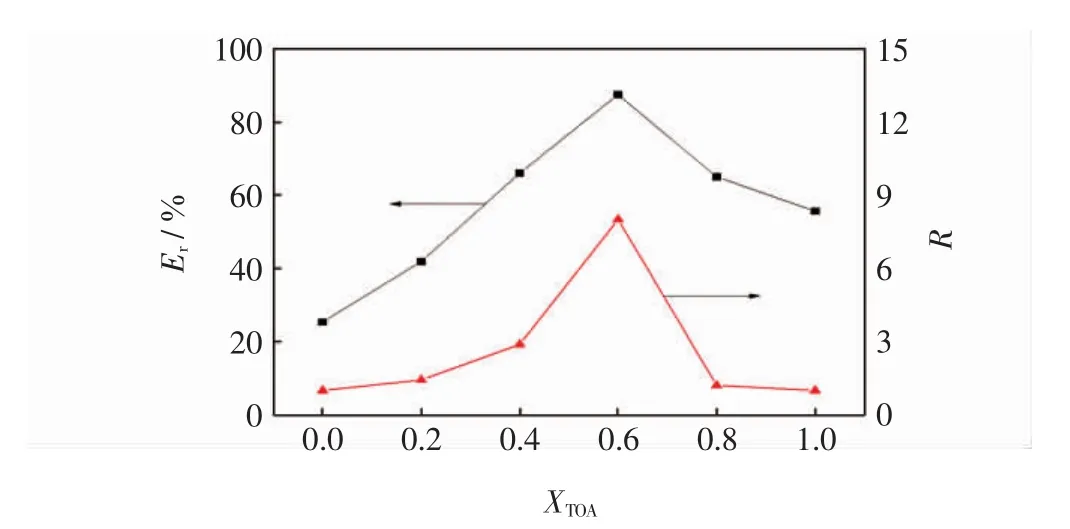
图1 复合萃取剂对活性红K-7B的萃取行为Fig.1 Behavior of composite extraction on removal reactive red 4
由图1可知,当仅有P204(TOA体积为0)时,对活性红K-7B的萃取率和协同系数均较低,仅为25.4%和1.0,表明P204对活性红K-7B萃取能力很低,仅仅是分子间力的作用;但随着TOA体积比增加,对活性红K-7B的萃取率和协同系数也随之提高,当XTOA=0.6时达到最大值,即Er(Dr)=87.6%和R=8.0,此时相当于18%TOA与12%P204混合呈现出最优的萃取能力;而当仅有TOA(P204体积为0)时,对活性红K-7B的萃取率明显下降至55.7%,这意味着TOA在复合萃取剂中起主导作用,其原因可归结为:在酸性条件下活性红K-7B染料显负电,而TOA与P204组成的复合萃取剂因TOA的氨基质子化表面带正电,分子间强烈的静电力利于萃取染料;而随TOA量体积增加,质子化数密集化,更易于萃取活性红K-7B,从而提高了萃取率.然而当TOA体积分数超过0.6时(XTOA>0.6),复合萃取剂萃取效率逐渐降低,这是基于2种考虑:①增加TOA体积,提高了体系粘度,阻碍了分子间的相互碰撞;②过量TOA能中和溶液中氢离子,使自身的质子化数减少,因而降低了萃取效率.此外,从该二元复合萃取体系看,其协同系数(R)代表了组分间的协同关系,通常该值大于1,且R越大表明组分间的协同效应越强[29].基于上述实验结果,该复合萃取剂TOA和P204的体积比确定为18∶12.
图2给出了萃取前后不同TOA与P204体积比对活性红K-7B染料紫外-可见光谱的影响,样品稀释10倍,得到吸光度值乘10.
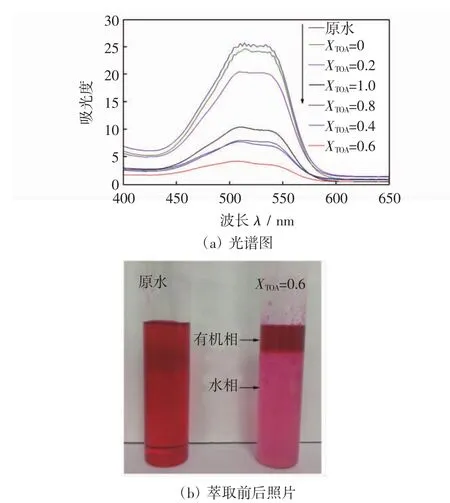
图2 萃取前后不同TOA体积比对活性红K-7B染料紫外-可见光谱的影响Fig.2 UV-vis spectra of dye solution before and after extraction by different TOA volume fractions
由图2(a)可知,活性红K-7B的最大吸收波长λmax=510 nm的吸光度随TOA与P204体积比变化逐渐降低,这再次证明在复合萃取剂中适宜的TOA与P204体积比对染料脱色起重要作用.图2(b)显示了位于XTOA=0.6的复合萃取剂萃取活性红K-7B前后的出水照片.可见,在达到萃取平衡时,其油水分层清晰,出水色度比原水明显地下降.
2.2 复合萃取剂的协同效应
本研究考察了TOA和P204不同浓度萃取活性红K-7B染料对其分配比的影响,以评价其协同效应,结果如图3所示.
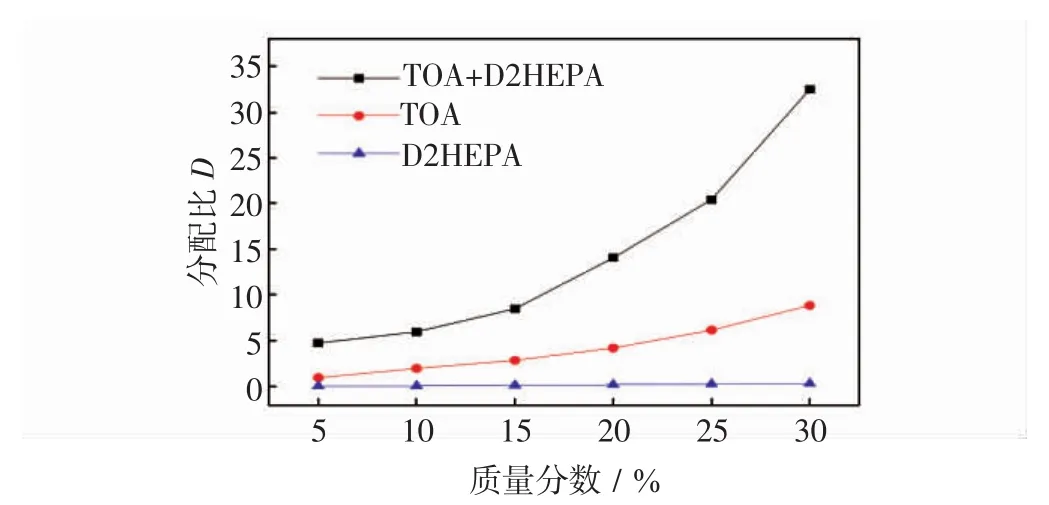
图3 不同组分浓度对萃取活性红K-7B分配系数的影响Fig.3 Effect of extractant concentration on distribution ratio of reactive red 4 using TOA,P204 and the mixture
由图3可知,随TOA、P204和其混合物浓度增加,各分配比也随之增大.然而,与TOA和P204单独萃取活性红K-7B染料的分配比相比较,混合物的分配比显著地增大,如当仅有TOA或P204,且质量分数为30%时,其分配比依次为8.8和0.3,尤其P204对活性红K-7B染料分配比接近0;但将它们以一定比例混合后,其萃取活性红K-7B的分配比明显地提高至32.5,远高于单独任何组分,这进一步表明TOA与P204的协同效应促进了对活性红K-7B染料萃取,类似的其他研究也证明了二者的协同关系[30].
2.3 复合萃取工艺参数优化
2.3.1 萃取时间对脱色率的影响
用复合萃取剂萃取活性红K-7B染料,其萃取时间对染料脱色的影响如图4所示.
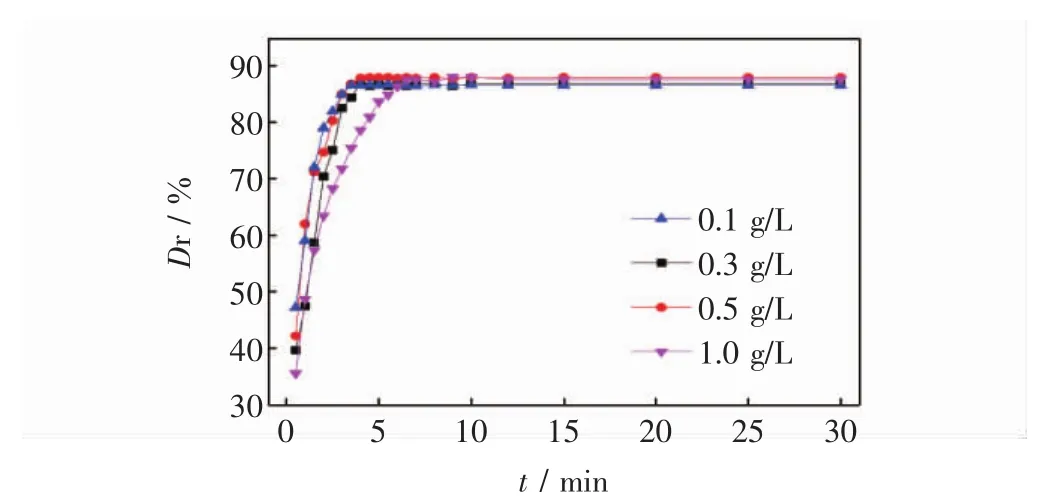
图4 萃取时间对活性红K-7B脱色率的影响Fig.4 Effect of extraction times on removal reactive red 4
从图4可看出,尽管活性红K-7B初始质量浓度不同(0.1、0.3、0.5 和 1.0 g/L),但随萃取时间其脱色率均快速增加,当时间超过7 min时,基本达到萃取平衡,且不同初始浓度的脱色率无明显地差异,这表明该复合萃取剂萃取活性红K-7B染料属于快速达到平衡的过程;同时其萃取能力与被萃取物的初始浓度在一定范围内无相关性.因此,在本实验条件下,确定最佳萃取时间为10 min.
2.3.2 水油比对脱色率的影响
萃取过程中水油比是影响萃取效率的关键因素,它不仅影响萃取效率,而且关系到运行成本.图5显示了不同水油比对脱色率的影响.
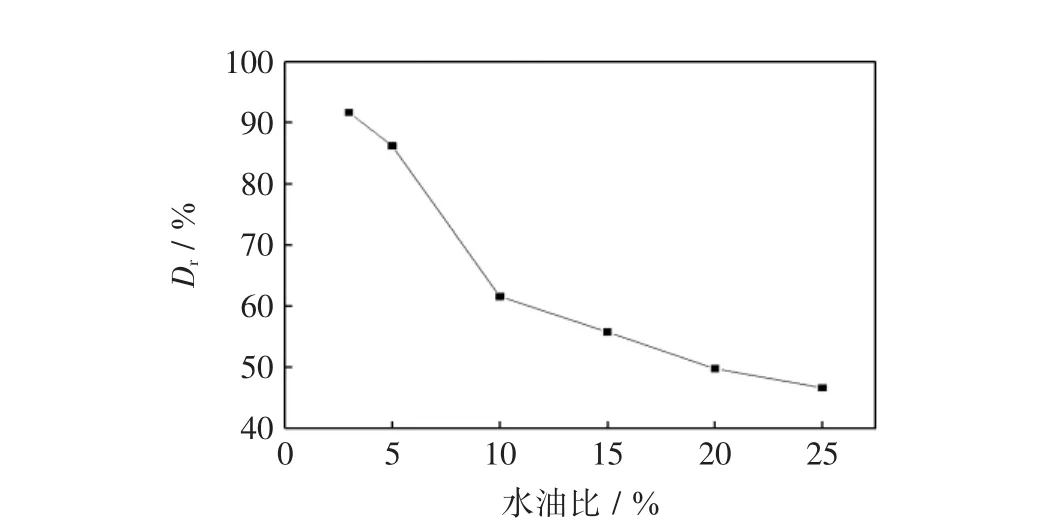
图5 水油比对活性红K-7B脱色率的影响Fig.5 Effect of phase ratios on removal reactive red 4
由图5可见,随溶液体积增加,染料脱色率逐渐地降低,这是基于增加水油比使复合萃取剂的有效浓度减少,从而降低了单位时间内萃取剂对活性红K-7B的传质通量,从而导致其脱色率低下.因此,在本实验条件下,确定最佳水油比为5∶1.
2.3.3 pH对脱色率的影响
在萃取过程中废水pH也是影响萃取效率的关键因素,因为溶液中氢离子浓度直接关联到TOA的质子化,从而影响分子间作用力.在调节溶液pH值为2.0~5.0范围内,复合萃取剂萃取活性红K-7B染料脱色率的结果如图6所示.
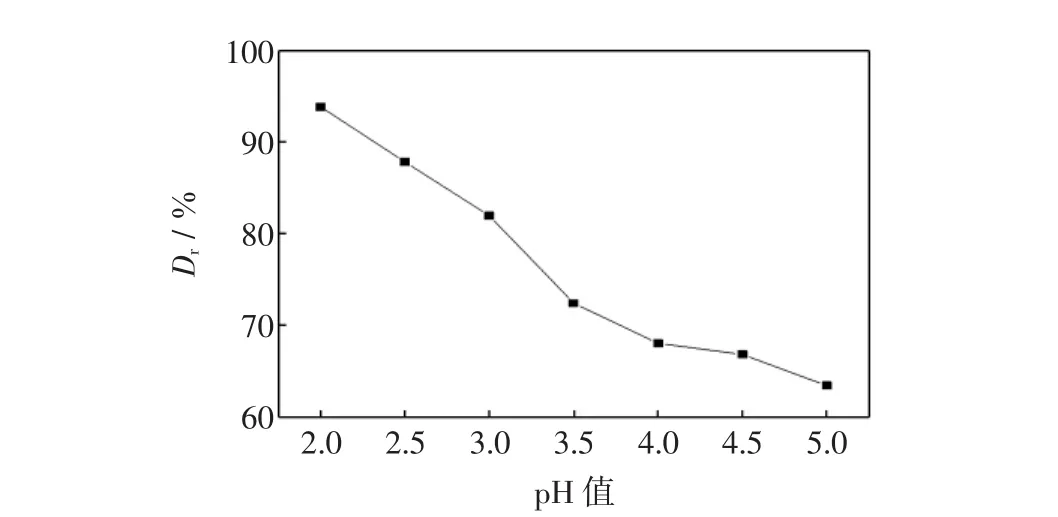
图6 pH对活性红K-7B脱色率的影响Fig.6 Effect of pH on removal reactive red 4
由图6可以看出,当溶液pH值从2.0升至5.0时,脱色率由93.8%降至63.4%,表明酸性条件有利于萃取过程的进行.因此,在本实验条件下,确定初始废水pH值为3.
2.4 复合萃取剂再生与回用
活性红K-7B废水经萃取工艺处理后,部分染料分子由水相迁移到复合萃取剂(油相)中,只有将该复合萃取剂进行脱附处理后方能再利用.为此,实验选择2种碱剂(NaOH和Na2CO3)作为洗脱剂,以不同初始浓度的碱液分别洗脱染料,其结果如图7所示.
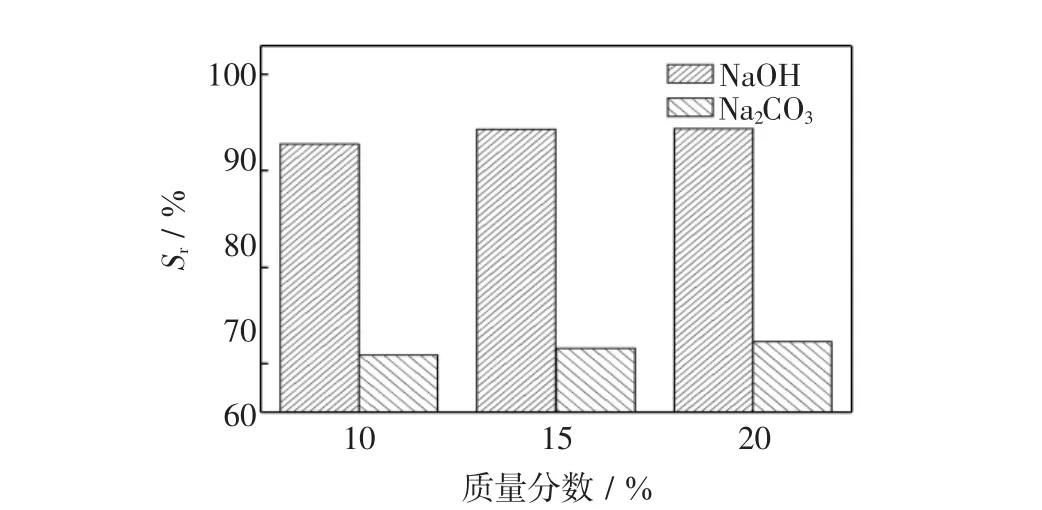
图7 碱剂浓度对脱附率的影响Fig.7 Effect of alkaline solution concentration on stripping rate
由图7可见,当用15%NaOH溶液洗脱后,约94.3%染料被脱除,该值远高于Na2CO3溶液的洗脱率.因此,在本实验条件下,最佳洗脱液是15%NaOH溶液.
图8给出了再生复合萃取剂重新萃取含活性红K-7B染料废水的脱色效果.
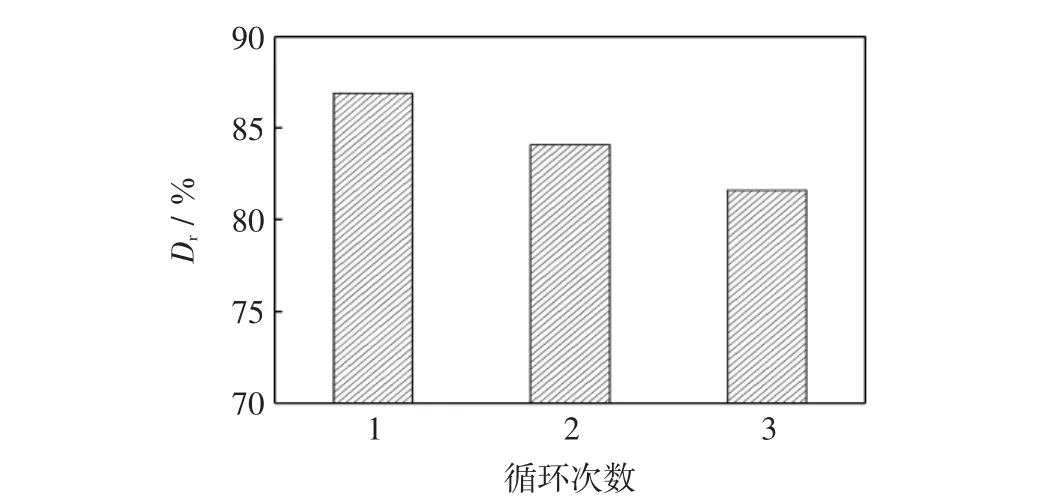
图8 再生复合萃取剂循化萃取对脱色率影响Fig.8 Effect of cycles by spent extractant on removal reactive red 4
由图8可见,当循环使用3次后再生复合萃取剂对活性红K-7B染料的脱色率仍保持在80%以上.因此,进一步表明借助15%NaOH溶液脱附染料后可使复合萃取剂获得再生与回用.
3 结论
(1)构建了TOA-P204-SK的二元复合萃取剂,在TOA、P204与SK体积分数为18%、12%与70%,水油比为5∶1,初始废水pH值为3和萃取时间为10 min等最佳工艺条件下,对活性红K-7B溶液脱色率可达87.6%,且获得了32.5分配比.
(2)在复合萃取剂中,TOA和P204的协同作用是实现高效脱除活性红K-7B染料的关键因素,只有在TOA中加入适宜量的P204,才能明显地提高活性红K-7B的萃取率.
(3)以15%NaOH作洗脱剂可完成对复合萃取剂的回收与再利用,其回收率达94.3%.
[1]LI K,ZHANG H,HE Y,et al.Novel wedge structured rotating disk photocatalytic reactor for post-treatment of actual textile wastewater[J].Chemical Engineering Journal,2015,268:10-20.
[2]PUNZI M,NILSSON F,ANBALAGAN A,et al.Combined anaerobic-ozonation process for treatment of textile wastewater:Removal of acute toxicity and mutagenicity[J].Journal of Hazardous Materials,2015,292:52-60.
[3]MEERBERGEN K,CRAUWELS S,WILLEMS K A,et al.Decolorization of reactive azo dyes using a sequential chemical and activated sludge treatment[J].Journal of Bioscience and Bioengineering,2017,124:668-673.
[4]REDDY C N,KUMAR A N,MOHAN S V.Metabolic phasing of anoxic-PDBR for high rate treatment of azo dye wastewater[J].Journal of Hazardous Materials,2018,343:49-58.
[5]VENKATA Mohan S,SURESH Babu P,SRIKANTH S.Azo dye remediation in periodic discontinuous batch mode operation:Evaluation of metabolic shifts of the biocatalyst under aerobic,anaerobic and anoxic conditions[J].Separation and Purification Technology,2013,118:196-208.
[6]NARESH Kunmar A,NAGENDRANATHA Reddy C,HARI Prasad R,et al.Azo dye load-shock on relative behavior of biofilm and suspended growth configured periodic discontinuous batch mode operations:Critical evaluation with enzymatic and bio-electrocatalytic analysis[J].Water Research,2014,60:182-196.
[7]LI X,ZHOU M,PAN Y,et al.Highly efficient advanced oxidation processes (AOPs)based on pre-magnetization Fe0for wastewater treatment[J].Separation and Purification Technology,2017,178:49-55.
[8]TAN K B,VAKILI M,HORRI B A,et al.Adsorption of dyes by nanomaterials:Recent developments and adsorption mechanisms[J].Separation and Purification Technology,2015,150:229-242.
[9]NATARAJAN S,BAJAJ H C,TAYADE R J.Recent advances based on the synergetic effect of adsorption for removal of dyes from waste water using photocatalyticprocess[J].Journal of Environmental Sciences,2017,17:181-187.
[10]MITTERSTEINER M,SCHMITZ F,BARCELLOS I O.Reuse of dye-colored water post-treated with industrial waste:Its adsorption kinetics and evaluation of method efficiency in cotton fabric dyeing[J].Journal of Water Process Engineering,2017,17:181-187.
[11]MOHAMMED S,FASNABI P A.Removal of dicofol from waste-water using advanced oxidation process[J].Procedia Technology,2016,24:645-653.
[12]AN A K,GUO J,JEONG S,et al.High flux and antifouling properties of negatively charged membrane for dyeing wastewater treatment by membrane distillation[J].Water Research,2016,103:362-371.
[13]ISIK M,SPONZA D T.Fate and toxicity of azo dye metabolites under batch long-term anaerobic incubations[J].Enzyme and Microbial Technology,2007,40(4):934-939.
[14]MURALI V,ONG S A,HO L N,et al.Evaluation of integrated anaerobic-aerobic biofilm reactor for degradation of azo dye methyl orange[J].Bioresource Technology,2013,143:104-111.
[15]GARCíA-MARTíNEZ Y,BENGOA C,STüBER F,et al.Biodegradation of acid orange 7 in an anaerobic-aerobic sequential treatment system[J].Chemical Engineering and Processing:Process Intensification,2015,94:99-104.
[16]MANAVI N,KAZEMI A S,BONAKDARPOUR B.The development of aerobic granules from conventional activated sludge under anaerobic-aerobic cycles and their adaptation for treatment of dyeing wastewater[J].Chemical Engineering Journal,2017,312:375-384.
[17]VENKATA MOHAN S,SURESH Babu P,SRIKANTH S.Azo dye remediation in periodic discontinuous batch mode operation:Evaluation of metabolic shifts of the biocatalyst under aerobic,anaerobic and anoxic conditions[J].Separation and Purification Technology,2013,118:196-208.
[18]SHAW C B,CARLIELL C M,WHEATLEY A D.Anaerobic/aerobic treatment of coloured textile effluents using sequencing batch reactors[J].Water Research,2002,36(8):1993-2001.
[19]HOMSIRIKAMOL C,SUNSANDEE N,PANCHAROEN U,et al.Synergistic extraction of amoxicillin from aqueous solution by using binary mixtures of Aliquat 336,D2EHPA and TBP[J].Separation and Purification Technology,2016,162:30-36.
[20]SULAIMAN R N R,OTHMAN N.Synergistic green extraction of nickel ions from electroplating waste via mixtures of chelating and organophosphorus carrier[J].Journal of Hazardous Materials,2017,340:77-84.
[21]CHEN Z,WANG W T,SANG F N,et al.Fast extraction and enrichment of rare earth elements from waste water via mi-crofluidic-based hollow droplet[J].Separation and Purification Technology,2017,174:352-361.
[22]ONG L K,TRAN NGUYEN P L,SOETAREDJO F E,et al.Kinetic evaluation of simultaneous waste cooking oil hydrolysis and reactive liquid-liquid Cu extraction from synthetic Cucontaining wastewater:Effect of various co-contaminants[J].Separation and Purification Technology,2017,187:184-192.
[23]KESIEME U K,ARAL H.Application of membrane distillation and solvent extraction for water and acid recovery from acidic mining waste and process solutions[J].Journal of Environmental Chemical Engineering,2015,3(3):2050-2056.
[24]DEVA A N,ARUN C,ARTHANAREESWARAN G,et al.Extraction of peroxidase from waste Brassica oleracea used for the treatment of aqueous phenol in synthetic waste water[J].Journal of Environmental Chemical Engineering,2014,2(2):1148-1154.
[25]FISCHER L,FALTA T,KOELLENSPERGER G,et al.Ionic liquids for extraction of metals and metal containing compounds from communal and industrial waste water[J].Water Research,2011,45(15):4601-4614.
[26]MA L,ZHAO Z,DONG Y,et al.A synergistic extraction strategybyCyanex572andCyanex923forTh(IV)separation[J].Separation and Purification Technology,2018,191:307-313.
[27]WEI Q F,REN X L,GUO J J,et al.Recovery and separation of sulfuric acid and iron from dilute acidic sulfate effluent and waste sulfuric acid by solvent extraction and stripping[J].Journal of Hazardous Materials,2016,304:1-9.
[28]张奎,袁慎峰,尹红,等.络合萃取法预处理CLT酸生产废水研究[J].水处理技术,2014,40(1):33-36.ZHANG K,YUAN S F,YIN H,et al.Study on the pretreatment of clt acid production wastewater by complexing extraction[J].Technology of Water Treatment,2014,40(1):33-36(in Chinese).
[29]SHI Q,ZHANG Y,HUANG J,et al.Synergistic solvent extraction of vanadium from leaching solution of stone coal using D2EHPA and PC88A[J].Separation and Purification Technology,2017,181:1-7.
[30]孙盈,李艳玲,权新军,等.二-(2-乙基己基)磷酸P204与三烷基叔胺N235协同萃取钼 [J].应用化学,2009,26(11):1353-1356.SUN Y,LI Y L,QUAN X J,et al.Synergistic extraction of molybdenum using acid-base coupling extractants of Di-2-ethylhexyl phosphoric acid P204 and trialkylamine N235[J].Chinese Journal of Applled Chemistry,2009,26(11):1353-1356(in Chinese).

