Impacts of low-intensity prescribed fire on microbial and chemical soil properties in a Quercus frainetto forest
Serdar Akburak •Yowhan Son •Ender Makineci •Meric¸C¸akir
Introduction
Prescribed fire is a common method to decrease combustible biomass and wild fire risk as an economic and effective forestry practice.Prescribed fires are usually of low intensity and burn the understory plant cover and partially the organic soil layers;the risk to trees is low(Ferran et al.2005).Furthermore,low-intensity prescribed fires sustain required soil nutrients(e.g.calcium,potassium,manganese,magnesium,sodium)for the healthy growth of forest ecosystems(Bento-Gonc¸alves et al.2012).
Prescribed burning retains the vigorous state of oak forest ecosystems by impeding epidemic pathogens,controlling competition by exotic species and supporting oak regeneration(Meentemeyer et al.2008).The impacts of regular prescribed burnings are of great importance especially for oak forest management(Gonzalez-Perez et al.2004;Williams et al.2012).
It is important to understand the effects of fire on soil properties for better soil management through the recovery dynamics of burned forests ecosystems(Berber et al.2015),and in ecological restoration studies.Fires can cause impacts on microbial soil properties with changes in microbial composition,microbial activities and the availability of carbon(C)and nutrients(Ilstedt et al.2003).However,Wang et al.(2012)reported that previous research demonstrated inconsistent results regarding the effects of fire on the microbial biomass.Speci fic indicators with respect to soil microbiota such as microbial C biomass(Cmic),soil microbial respiration(RSM)and the metabolic quotient(qCO2)also appear to be potentially essential to characterize the extent of soil perturbation in relation to fire(Vega et al.2013).
The effects of fire on forest soils have been studied extensively(Certini 2005;Nave et al.2011).Furthermore,numerous studies of various forest ecosystems have also evaluated the effects of prescribed fire on soils(Pereira et al.2011).Fire affects all chemical characteristics.Different studies have revealed that chemical soil properties decrease,increase or remain the same(Gonzalez-Perez et al.2004).However,there are a relatively few studies that have focused on the status of soil properties after lowintensity surface fires(Certini 2005;Berber et al.2015).This issue is of major importance because soil properties will determine available nutrients,important for plant growth and therefore ecosystem restoration(Pereira et al.2011).
Oak forests constitute a large part of forests in Turkey;therefore,they are of great importance in terms of biodiversity,area covered,and forestry management.Prescribed burning is not a common ecological restoration or rehabilitation practice in Turkish forestry,although its effective use is well-known around the world.Some controlled fires have been carried out in several Turkish forests such as Calabrian pine(Pinus brutia,Ten)(Yildiz et al.2010),Lebanon cedar(Cedrus libani,A.Rich.)(Kantarcı 1989),Austrian pine(Pinus nigra,Arnold)(Tu¨fekc¸iog˘lu et al.2006)and Turkish chestnut+Oriental beech+Austrian pine(Castanea sativa,Miller+Fagus orientalis,Lipsky+P.nigra,Arnold spp.)to study the effects of fire on soils(Berber et al.2015).
This study is the first of its kind to explore the effects of prescribed fire on soil in a Hungarian oak(Quercus frainettoTen.)forest.The objectives are to evaluate the impacts of low-intensity prescribed fire on chemical properties,such as acidity(pH),electrical conductivity(EC),carbon(C),nitrogen(N),C/N ratio,exchangeable cations(calcium(Ca),magnesium(Mg),sodium(Na),potassium(K),and manganese(Mn),and on microbial properties such as carbon biomass(Cmic),respiration(RSM)and metabolic quotient(qCO2)in the top soil layer(5 cm),through monthly samplings from unburned control and burned plots over the 1-year research period.The following hypothesis was tested:low-intensity prescribed fire would have no effect on chemical properties(exchangeable cations,soil acidity,electrical conductivity,carbon and nitrogen content,C/N ratio)and microbial properties(microbial respiration,microbial biomass carbon and qCO2)of topsoil in Hungarian oak forest.
Materials and methods
Study site
The study was conducted in the Research Forest of Istanbul University,Faculty of Forestry located in the Belgrade Forest between 41°09′15′′–41°11′01′′N and 28°59′17′′–29°32′25′′E in Istanbul-Turkey.Mean annual precipitation is 1111.4 mm and mean annual temperature 12.7°C.The altitude of the research area is 90 m,the slope is 3–5%located in the west–northwest aspect.The soil group in the research area is Luvisol according to the World Reference Base for Soil Resources(WRB).Soils are well-drained,moderately deep and lime-free with no carbonate reaction.The general texture is loamy clay.The research was in a pure Hungarian oak(Q.frainettoTen)stand with homogeneous site characteristics(altitude,aspect,soil type and slope)and structure(basal area,density,height and diameter at breast height(DBH))before fire treatment.The main characteristics before treatment were as follows:the number of trees was 1112 ha-1,basal area 22.7 m-2ha-1,mean tree height 15.3 m,mean tree diameter(dbh)15.8 cm and mean forest floor mass(oven dry weight dried at 70°C to constant weight)was 857.9 g m-2.
Prescribed fire treatment
Four experimental plots(20×20 m)were established for prescribed burning and control,with each replicated twice.The number of replications were limited to two due to the inhomogeneity of the site conditions in the surrounding area.A 20-m buffer zone was created between the burning and control plots to reduce edge effects(Marcos et al.2009).Five quadrats(2×2 m)were uniformly distributed around each plot for sampling.In total,ten quadrats( five replications in each burning plot)were burnt by a low intensity prescribed fire in October 2012 when there was a large litterfall.An average of 857.95 g m-2of litter was burnt during the burning process.The burn took approximately 20 min in each plot.Ash color can be a good indicator to determine the intensity of fire(Bento-Gonc¸alves et al.2012).Although we did not measure the soil surface temperatures,the black color of soil after the prescribed fire demonstrated that the heat achieved was insufficient to absolutely deplete the forest floor and organic soil material on the surface which showed that the intensity of the fire was possibly low(Fig.1).A total of ten in five replications in each plot was used as unburned control plots.
Soil sampling and analysis of chemical properties
Only the top layers were sampled because the main effects of fire on soil organic matter are usually limited to the forest floor and a few centimeters of the upper mineral.Moreover,the effect of prescribed fire on water-soluble elements(Ca,Na,K,Mg)have a heterogeneous and complex mosaic of effects.Prescribed fire generally increases the main elements for plant growth(Pereira et al.2011).Samples were taken from 0 to 5 cm in the mineral soil with steel soil cores(100 cm3).Five replicated samples were taken systematically and composited into a single sample at each plot and sampling time.Samples were oven-dried at 105°C,ground and sieved with a two-mm screen,and all roots and coarse materials higher than 2 mm were separated.Acidity was determined by a microprocessor pH meter(Hanna-HI 221),and WTW-Inolab(cond level 1)electrical conductivity(EC)meter used for electrical conductivity.All samples were analyzed for their C and N concentrations by means of dry combustion using a LECO Truspec CN-2000 analyzer(LECO Corporation,St.Joseph,MI,USA).Exchangeable cations(K,Ca,Mg,Na,Mn)in 5-g samples were extracted with a standard neutral(i.e.pH=7)1 N ammonium acetate solution at an extracting ratio of 1:5(w/v;soil to solution),shaken for 20–30 min and then filtered.The concentrations of the individual cations were determined by ICP/OES(Perkin Elmer).
Analysis of microbial properties
Microbial respiration(RSM)was determined by placing 30 g samples adjusted to 50–55%water holding capacity into 500-ml beakers and incubating in dark at 25°C within sealed incubation vessels along with 10 ml of 1 M NaOH.The CO2–C released was measured every 7 days by adding BaCl2and subsequently titrating with 1 M HCl(Alef and Nannipieri 1995).For microbial biomass carbon(Cmic),the substrate-induced respiration(SIR)method was used.SIR was obtained with an amendment rate of 60 mg glucose corresponding to an oven-dry matter of 20 g.CO2was trapped in 0.05 M NaOH for 4-h incubation at 25°C and measured by titration(Alef and Nannipieri 1995).Microbial respiration(RSM)and microbial biomass carbon(Cmic)ratios were used to calculate the metabolic quotient(qCO2),which is the amount of CO2–carbon produced per unit of microbial biomass carbon(Anderson and Domsch 1986).
Statistical analysis
The results were subjected to independentt-tests at α:0.05 to determine the statistically signi ficantdifferences between control and fire plots within the research period.The results were evaluated monthly and annually.Correlation analysis and Pearson correlation coefficient were used to determine the interaction and to compare the relationship between the variables.MiniTab 16.0 was used for all statistical evaluations.

Fig.1 Low-intensity prescribed burning treatment in plots
Results
Effects of prescribed fire on chemical soil properties
The mean monthly soil carbon concentration ranged from 5.26 to 7.12%in the burned plots,and between 5.20 and 6.19%in control plots.The highest soil carbon concentration was determined 1 month after the fire(November 2012)in the burnt plots,and the lowest in September 2013(11 months following the fire) (Fig.2). Monthly differences and mean annual comparisons of carbon were not statistically signi ficant between burned and unburned plots(Table 1).
Soil nitrogen concentration showed a similar trend in both the burned and control plots(Fig.2).Monthly differences(except September 2013,Fig.2)and mean annual comparisonsofnitrogen did notsignificantly differ between burned and unburned controls(Table 1).
The ratio of soil C/N showed signi ficant(P<0.022)annualdifferencesdespite no signi ficantdifferences between carbon and nitrogen concentrations in annual and monthly changes.Soil C/N ratio changed from 24.3:1(October 2012)and 32.9:1(May 2013)in the burned plots,and from 22.1:1(January 2013)to 32.2:1(July 2013)in control plots.The mean annual value of the C/N ratio was significantly different between burned(28.5:1)and control plots(27.0:1)(Table 1)despite no signi ficant differences in monthly comparisons(except January 2013)(Fig.2).
Soil pH tends to increase after a prescribed fire and burned plots had higher pH values every month;however,comparing only in 2 months(second and fifth months after the fire)and the annual mean comparison showed statistically signi ficant differences between burned and control plots(Table 1,Fig.3).Neither monthly nor mean annual comparison was significantly different(Table 1,Fig.3)with respect to soil electrical conductivity(EC).
The changes in exchangeable cations(K,Ca,Mg,Na,Mn)are presented in Table 1.The temporal variation of all cations usually had a similar trend in both unburned and burned plots.There were no clear fire-induced changes in the temporal variation of these cations.Monthly comparisons were significantly different for potassium(K)calcium(Ca)and sodium(Na)in 3 months,for magnesium(Mg)in 2 months and for manganese(Mn)in only 1 month within the research period(Fig.3).The mean annual values of all cations did not differ significantly between the controls and burned plots(Table 1).
Effects of prescribed fire on microbial properties
The mean monthly soil microbial respiration(RSM)varied from 0.59 to 2.26 μg CO2–C g-1h-1in the burned plots,and from 0.83 to 2.86 μg CO2–C g-1h-1in the control plots(Fig.2).Prescribed fire may slightly alter RSMbecause signi ficantdifferences were found only in 3 months—the first(October 2012),sixth(April 2013)and twelfth months(September 2013)after the fire(Fig.2),and annual comparison did not show a signi ficant difference between controls and burned plots(Table 1).Microbial biomass carbon(Cmic)showed a similar trend in both thecontrol (0.41–1.23 mg g-1) and burned plots(0.52–1.61 mg g-1)(Fig.2).Prescribed fires may cause a slight decrease because burned plots almost always have lower values compared to the control plots;however,signi ficant differences were found in only 5 months,usually in the spring and summer(October,April,May,July and August)(Fig.2).The mean annual Cmic was significantly(P<0.000)lower in the burned plots(Table 1).Prescribed fire-induced changes in qCO2had slightly higher values(1.0–4.13 μg CO2–C mg Cmic h-1)in the burned plots than in the controls(0.89–3.82 μg CO2–C mg Cmic h-1).The mean annual value(2.56 μg CO2–C mg Cmic h-1)of the burned plots was significantly higher than that of the control plots(Table 1);however,monthly differences were not signi ficant t(except January 2013)(Fig.2).
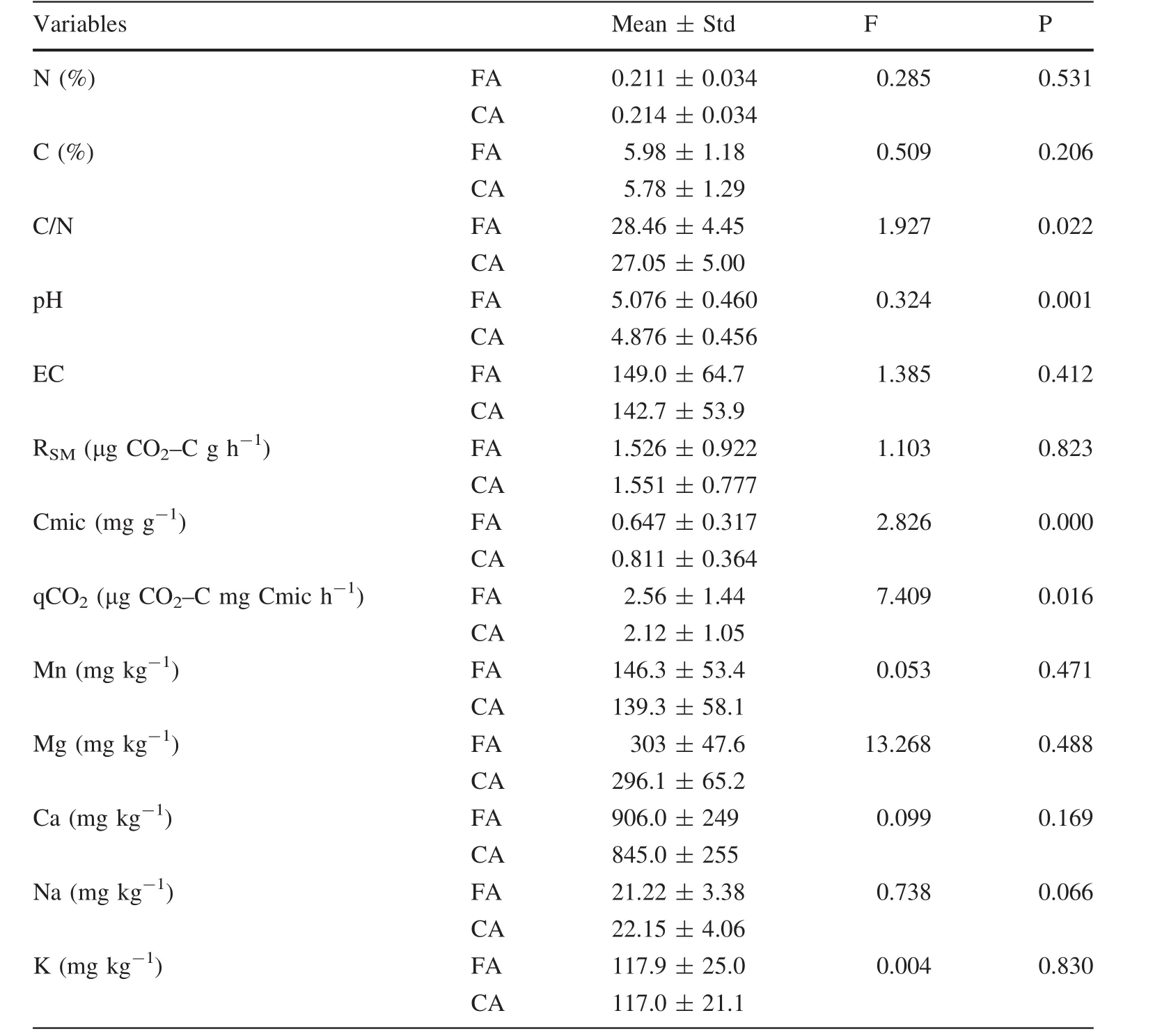
Table 1 Means of investigated parameters and comparison of prescribed burned and control plots with independent samples t test at 0.05 signi ficance level

Fig.3 Temporal variation of exchangeable cations between prescribed fire and control areas.Lines on bars indicate standard errors.Values followed by same letter(a,b)in each month are not statistically signi ficant at 0.05 level according to independent samples t test.(Mn manganese,Mg magnesium,K potassium,Ca calcium,Na sodium,FA prescribed fire area,CA control area)
Relationships between variables
In general,similar relationships between the investigated variables may be observed in both control and burned plots(Table 2).However,soil nitrogen concentrations were negatively correlated with C/N ratios;soil nitrogen had a significantly positive relationship with magnesium(Mg),calcium(Ca)and potassium(K)in the burned plots although these relationships were not signi ficant in control plots(Table 2).Soil C/N ratios had a significantly negative correlation with pH,qCO2,manganese(Mn),magnesium(Mg),potassium(K)and calcium(Ca)in the burned plots;however,a significantly negative correlation was found only in exchangeable calcium(Ca)in the control plots(Table 2).Exchangeable sodium(Na)was not significantly correlated with pH in the burned plots;however,it had a significantly positive correlation in control plots.On the other hand,it had a significantly negative correlation with Cmic and a significantly positive correlation with qCO2in burned plots.Such relationships were not observed in the controls(Table 2).Another striking result regarding the relationships amongst variables was that Cmic showed a significantly positive correlation with RSMand exchangeable calcium(Ca)in the control plots(Table 2).
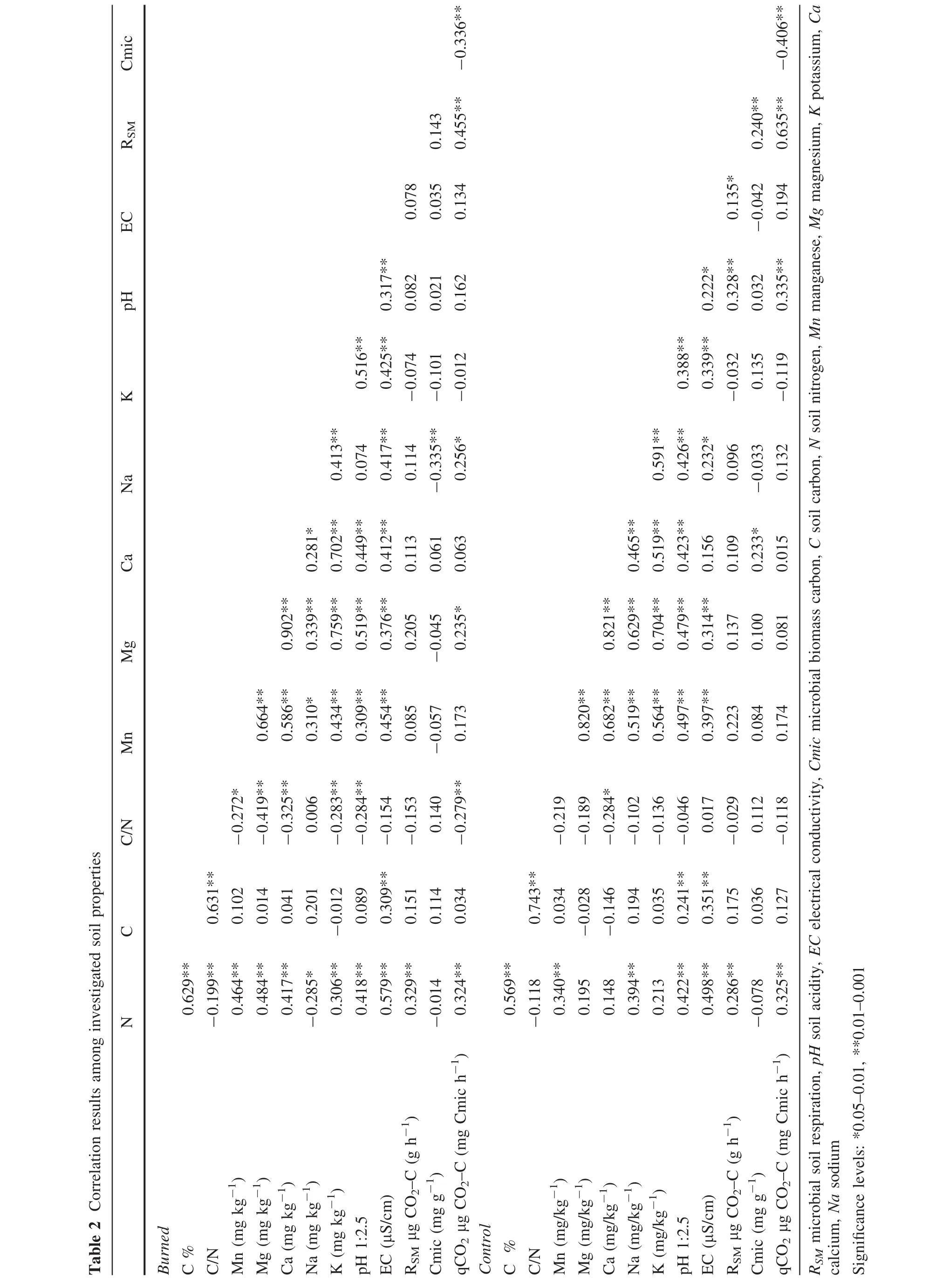
?
Discussion
In this study,mean annual evaluation revealed that only four parameters(C/N ratio,soil pH,Cmic and qCO2)showed signi ficant differences between control and burned plots over the 1-year research period.In general,other parameters in the mean annual comparison and all parameters in monthly comparisons were not significantly different.
Effects of prescribed fire on chemical soil properties
Low-intensity fires may cause ash incorporation,increasing the availability of nutrients in the soil and increasing the pH slightly.Moreover,significantly different C/N ratios in annual comparisons con firm that pH has a positive correlation with exchangeable magnesium(Mg),calcium(Ca),manganese(Mn)and potassium(K)in burned plots.However,all exchangeable nutrients did not show any signi ficant differences following fire in this study,possibly due to other unanalyzed nutrients may be in fluential in soil pH changes.In this context,previous research has described soil pH increases after fire resulting from the mixing of ash material into the soil.This is because of the chemical composition of ash consists of carbonates,oxides and base cations(Pereira et al.2011).Furthermore,Certini(2005)reported that the pH of the top soil changed drastically immediately after fire in aQuercus engelmanniiforest(Certini 2005),while such changes demonstrate that pH increase was mainly a result of the production of hydroxides,carbonates and oxides of K and Na.
Consistent with Nave et al.(2011),we did not find any signi ficant impacts of prescribed fire on mineral soil carbon(C)and nitrogen(N).On the other hand,the mean annual C/N ratio showed a signi ficant difference,with higher carbon(C)in the burned plots.This may be due to increased deposition of dry leaves and charred plant materials(Gonzalez-Perez et al.2004).Williams et al.(2012)found,similar to our study,that C/N ratios were higher in burned areas.On the other hand,the increase in soil C/N ratios in our study was inconsistent with results reported by Vega et al.(2013)and Berber et al.(2015).Higher proportions of carbon(C)lost relative to nitrogen(N)due to fire may decrease the C/N ratio(Pourreza et al.2014).However, fire-induced effects on soil carbon and nitrogen were highly variable and controversial.This may be attributed to different characteristics of vegetation,soil properties,sampling and fire severity(Wang et al.2012).
There was not a signi ficant change in soil electrical conductivity(EC).This is consistent with Granged et al.(2011)who reported that soil EC remained stable and returned to pre- fire values after 1 year.However,other researchers found that EC increased after fire,likely resulting from the release of combustible organic ions(Scharenbroch et al.2012).
All exchangeable cations were not significantly different between burned and control plots,and the fire-induced changes were often slight and transitory.The literature contains highly variable fire-induced results of concerning exchangeable cations.Certini(2005)reported that available calcium,magnesium,and potassium in aQuercus rubra–Populus grandidentataforest were significantly higher 1 month after a fire;however,these almost disappeared 3 months later.Increases in available forms of calcium(Ca),magnesium(Mg),sodium(Na)and potassium(K)after fire have been observed in several studies(Scharenbroch et al.2012).No changes in the concentration of these elements have been reported(Vega et al.2013).Contrary to our results,Pereira et al.(2011)found that prescribed fire led to a signi ficant calcium rise in soils in an oak forest and they reported higher calcium(Ca)solubility between pH 7–8.This may explain low changes in this cation in this study because soil pH in the research area was approximately 5.0,which was not within the range of calcium(Ca)solubility.Speci fically for manganese(Mn),Certini(2005)noted that its behavior with reference to fire was not well-known due to the absence of speci fic studies.However,similar to our results,Parra et al.(1996)found that the exchangeable manganese did not show any variations inPinus pinaster.On the other hand,Pereira et al.(2011)described an important decrease in the water-soluble manganese in ash after fire,and they emphasized that manganese concentrations were considerable in pine species.
Effects of prescribed fire on microbial soil properties
By the end of the study period,mean annual Cmic was significantly lower in burned plots.Contrary to our study,Scharenbroch et al.(2012)reported that moderate-and low-intensity fires did not affect soil microbial biomass significantly.Similarly,Vega et al.(2013)did not observe a signi ficant reduction in soil microbial biomass immediately after a low-intensity fire.The actual impact of fire is the reduction in the biomass of soil microorganisms(Williams et al.2012).Eivazi and Bayan(1996)suggested that reduced Cmic in burned plots could be related to the reduction in nutrient availability.Similarly,in our study,higher Cmic in the control plots had signi ficant linear correlation with calcium(Ca)concentration;lower Cmic in the burned plots had a significantly negative correlation with sodium(Na).Fire induced alterations in the microbial biomass of soil have been indicated to be more complicated(Wang et al.2012).
In this present study,qCO2in the burned plots was significantly higher than in the control plots.Increase of qCO2was interpreted as a microbial response to adverse environmental stresses or disturbances after fire(Wardle and Ghani 1995).In agreement with our study,De Marco et al.(2005)observed the increase of the metabolic quotient for a short time after fire in burned soils,attributed to changes in soil nutrients.In this study,this case can be explained by fire-induced changes in magnesium(Mg)and sodium(Na)concentrations,and in the C/N ratios.
Again,in agreement with our results,Scharenbroch et al.(2012)declared no signi ficant changes in microbial respiration in an oak forest soil.On the contrary,Wang et al.(2012)found that fire reduced soil Cmic and respiration because fire decreased the microbial activity.Fire can affect the life of microorganisms directly or alter the availability of soil nutrients and environmental conditions in forest ecosystems;thus,it causes short or long-term effects on soil microbial activity(Pourreza et al.2014).
Conclusions
Our results showed that low-intensity prescribed fire caused very short-lived changes in soil chemical and biological parameters over the 1-year period following treatment.It is also obvious that the negative effects of highseverity fires,such as loss of soil C,soil N,nutrient amounts,microbial parameters,are not observed in these low-severity fires.The soils do not reach extremely high temperatures,as the effect of fire quickly passes and temperatures reached during combustion are low and balanced.On the other hand,our results reveal that annual low-intensity prescribed fires have signi ficant effects on C/N ratios,pH,Cmic and qCO2in the top soil.Especially,it surmised from this study that the increase in soil pH can enhance soil fertility primarily in the study area in which soils are slightly acidic.These factors have importance in terms of the monitoring of ecosystems process since qCO2and Cmic used as indices of environmental stability instantly react to soil disturbances.
Prescribed fire has a number of bene ficial effects,however,understanding of these effects on soil and ecosystem processes require long-term monitoring by combining management practices such as thinning,clearcutting,and fertilization.In future years,this study will provide signi ficant master data to evaluate short-term and long-term impacts after combining prescribed burning and forest management treatments.
AcknowledgementsThis work was supported by the Scienti fic Research Projects Coordination Unit of Istanbul University,Project number:International Research Projects:IRP-27803,as a part of international collaboration between Istanbul University,Istanbul-Turkey and Korea University,Seoul-Korea.
References
Alef K,Nannipieri P(eds)(1995)Estimation of microbial activites.In:Methods in applied soil microbiology and biochemistry.Cambridge,Academic press,p 214–216
Anderson TH,Domsch KH(1986)Carbon assimilation and microbial activity in soil.J Plant Nutr Soil Sci 149:457–468
Bento-Gonc¸alves A,Vieira A,U´beda X,Martin D(2012)Fire and soils:key concepts and recent advances.Geoderma 191:3–13
Berber AS,Tavs¸anog˘lu C¸,Turgay OC(2015)Effects of surface fire on soil properties in a mixed chestnut-beech-pine forest in Turkey.Flamma 6(2):78–80
Certini G(2005)Effects of fire on properties of forest soils:a review.Oecologia 143(1):1–10
De Marco A,Gentile AE,Arena C,de Virzo Santo AV(2005)Organic matter,nutrient content and biological activity in burned and unburned soils of a Mediterranean marquis area of southern Italy.Int J Wildland Fire 14:365–377
Eivazi F,Bayan MR(1996)Effects of long-term prescribed burning on the activity of select soil enzymes in an oak–hickory forest.Can J For Res 26:1799–1804
Ferran A,Delitti W,Vallejo VR(2005)Effects of fire recurrence inQuercus cocciferaL.shrublands of the Valencia region(Spain):II.Plant and soil nutrients.Plant Ecol 177:71–83
Gonzalez-Perez JA,Gonzalez-Vila FJ,Almendros G,Knicker H(2004)The effect of fire on soil organic matter—a review.Environ Int 30:855–870
Granged AJP,Zavala LM,Jorda´n A,Ba´rcenas-Moreno G(2011)Post- fire evolution of soil properties and vegetation cover in a Mediterranean heathland after experimental burning:a 3-year study.Geoderma 164:85–94
Ilstedt U,Giesler R,Nordgren A,Malmer A(2003)Changes in soil chemical and microbial properties after a wild fire in a tropical rainforest in Sabah,Malaysia.Soil Biol Biochem 35:1071–1078
Kantarcı MD(1989)Sedir ormanlarının genc¸les¸tirilmesinde uygulanan yangın ku¨ltu¨ru¨ile ku¨ltu¨r bakımı yo¨ntemlerinin ekolojik deg˘erlendirilmesi,I˙.U¨.Orman Faku¨ltesiDergisiSeriA 39(2):95–118(in Turkish)
Marcos E,Villalo´n C,Calvo L,Luis-Calabuig E(2009)Short-term effects of experimental burning on soil nutrients in the Cantabrian heathlands.Ecol Eng 35:820–828
Meentemeyer RK,Rank NE,Anacker BL,Rizzo DM,Cushman JH(2008)In fluence of land-cover change on the spread of an invasive forest pathogen.Ecol Appl 18:159–171
Nave LE,Vance ED,Swanston CW,Curtis PS(2011)Fire effects on temperate forest soil C and N storage.Ecol Appl 21:1189–1201
Parra JG,Rivero VC,Lopez TI(1996)Forms of Mn in soils affected by a forest fire.Sci Total Environ 181:231–236
Pereira P,U´beda X,Martin D,Mataix-Solera J,Guerrero C(2011)Effects of a low severity prescribed fire on water-soluble elements in ash from a cork oak(Quercus suber)forest located in the northeastofthe Iberian Peninsula.Environ Res 111(2):237–247
Pourreza M,Hosseini SM,Sinegani AAS,Matinizadeh M,Dick WA(2014)Soil microbial activity in response to fire severity in Zagros oak(Quercus brantiiLindl.)forests,Iran,after one year.Geoderma 213:95–102
Scharenbroch BC,Nix B,Jacobs KA,Bowles ML(2012)Two decades of low-severity prescribed fire increases soil nutrient availability in a Midwestern,USA oak (Quercus)forest.Geoderma 183:80–91
Tu¨fekc¸iog˘lu A,Ku¨c¸u¨k M,Sag˘lam B,Bilgili E,Altun L,Ku¨c¸u¨k O¨(2006)In fluence of fire on root biomass dynamics and soil respiration rates in young corsican pine(Pinus nigra)stands in Turkey.For Ecol Manag 234:195
Vega JA,Fontu´rbel T,Merino A,Ferna´ndez C,Ferreiro A,Jime´nez E(2013)Testing the ability of visual indicators of soil burn severity to re flect changes in soil chemical and microbial properties in pine forests and shrubland. Plant Soil 369(1–2):73–91
Wang Q,Zhong M,Wang S(2012)A meta-analysis on the response of microbial biomass,dissolved organic matter,respiration,and N mineralization in mineral soil to fire in forest ecosystems.For Ecol Manag 271:91–97
Wardle DA,Ghani A(1995)Critique of the microbial metabolic quotient(qCO2)as a bioindicator of disturbance and ecosystem development.Soil Biol Biochem 27:1601–1610
Williams RJ,Hallgren SW,Wilson GW(2012)Frequency of prescribed burning in an upland oak forest determines soil and litter properties and alters the soil microbial community.For Ecol Manag 265:241–247
Yildiz O,Esen D,Sarginci M,Toprak B(2010)Effects of forest fire on soil nutrients in Turkish pine(Pinus brutia,Ten)ecosystems.J Environ Biol 31:11–13
 Journal of Forestry Research2018年3期
Journal of Forestry Research2018年3期
- Journal of Forestry Research的其它文章
- In vitro propagation of conifers using mature shoots
- ‘Relationships between relationships’in forest stands:intercepts and exponents analyses
- Effects of application date and rate of foliar-applied glyphosate on pine seedlings in Turkey
- Assaying the allelopathic effects of Eucalyptus camaldulensis in a nursery bed incorporated with leaf litter
- Effects of soil compaction on growth variables in Cappadocian maple(Acer cappadocicum)seedlings
- Variation and selection analysis of Pinus koraiensis clones in northeast China
