In vitro propagation of conifers using mature shoots
Mostafa K.Sarmast
Introduction
Plant tissue culture ideally allows the rapid proliferation of selected trees in a limited time frame,although it is desirable to select individual plants with greater growth rates or individuals that are more resistant to diseases.These could basically provide more options for breeding purposes(Thorpe 1985).In long-lived trees such as conifers,the genetic fidelity of plantlets derived by indirect organogenesis should be tested through several techniques before establishing clonally propagated offspring in the field.Despite considerable amounts of research,most gymnosperm species are still regarded as extremely dif ficult to regenerate.For clonal production of recalcitrant trees,not only the explant position of the mother trees is important,but also the seasonal effects should be taken into consideration because such factors play decisive roles in the regeneration response(Bonga et al.2010).Apart from a few successful examples with optimized procedures of micropropagation,mostclonalpropagation attempts encounter dif ficulties,either in the culture media or thereafter.Dif ficulties may include the inability to continue elongation,necrosis,low rooting ef ficiency,morphological changes,vitri fication,low regeneration,and excessive phenolic exudation,especially in older tissues.In somatic embryogenesis(SE),somatic cells undergo a developmental process similar to the development of zygotic embryos.However,somatic embryos must be tested for genetic stability before being established in the field.The SE technology for many conifers has yet to be evaluated in detail.In contrast to SE,the in vitro establishment of conifers through axillary and adventitious shoots—as they generally originate from mature differentiated stems—have a much lower chance of in vitro variation(Sarmast 2016).However,information on explant disinfestation and shoot multiplication through axillary and adventitious buds in conifers is sparse.Therefore,here we review the available literature on surface sterilization and in vitro propagation of conifers,exclusively from axillary and adventitious shoots.
Explant disinfestation
Success in plant tissue culture depends on obtaining aseptic mother plants;thus,controlling microbial contamination is the first and most important step for in vitro plant propagation and genetic transformation.Pathogen-free plants are also required for germplasm storage and moving living materials across international borders.Tissue culture regeneration of plants without viruses,bacteria,and fungi via shoot tip culture has been widely used since the 1960s(Thorpe 2007).However,meristem culture is now commonly used to remove viruses from infected plants.Epiphytic and endophytic microorganisms may cause severe problems for in vitro cultures(Cassells 1991;Debergh and vanderschaeghe 1988;Leifert et al.1991).In some cases,no signs or symptoms of infection are visible,yet infection can stop the growth of explants(Leifert et al.1989;1992).Bacterial contamination is especially hard to treat and dif ficult to detect in old woody plant species and conifers,because bacterial infections are mostly untraceable inside the plant tissues(Viss et al.1991;Buckley et al.1995).In contrast,however,fungal contamination is,in most cases,easily removable by ethanol and sodium hypochlorite.Internal contamination is often present in many in vitro propagation systems(Chanway 1998;Ewald and Suss 1993;Ewald et al.1997,2000;Laukkanen et al.2000;Traore et al.2005,Sarmast et al.2011).Generally,the surface sterilization of seeds is easier than other approaches such as sterilizing shoots or roots.Explants from fieldgrown perennial plants,especially explants obtained during the fall and winter or from tissues close to or inside the soil,are particularly dif ficult to disinfest due to the possible presence of epiphytic and endophytic microbes(Buckley et al.1995).It is common knowledge that the highest number of decontaminant explants,mostly reported in percentages,must be achieved with the least possible injury during establishment.Even a minor injury caused by a chemical is likely to act as a barrier for future growth and developmentofthe explants.Ethanoland sodium hypochlorite are two common chemicals used to decontaminate woody species,but explants from mature conifers are dif ficult to sterilize without injury(Keathley 1984;Timmis and Ritchie 1984).Gupta and Durzan(1985)obtained 90%aseptic explants from shoots of mature trees ofPinus lambertianaDougl.andPseudotsuga menziesii(Mirb.)Franco by successively applying H2O2(30%for 15 min),bleach(10%for 10 min),then HgCl2(0.05%for 10 min).Vegetative buds ofP.menziesiiwere surfacesterilized by bleach(20%and 100%)and flaming(for 3,5 s,or until the flame self-extinguished).According to this study,spring buds have higher contamination rates than winter buds.However,3–5 s of flaming,in combination with bud dissection,was preferred to bleach treatments for sterilizing buds in a highly contaminated environment before the in vitro culture is initiated.Increasing the duration of flaming apparently causes a decrease in the percentage of healthy,actively growing buds obtained(Traore et al.2005).An improved method of sterilization was reported,but it involved the use of toxic agents such as mercuric chloride(HgCl2),which should be avoided.Previous reports suggest that ethanol and sodium hypochlorite cannot completely controlErwinia herbicolacontamination when treating in vitro cultures ofAraucaria excelsaR.Br.,and the use of common antibiotics(i.e.,submersing in 200 mg L-1antibiotic solutions of cefotaxime,gentamicin,amoxicillin,ampicillin or tetracycline for 60 and 120 min after surface sterilization)can have dramatic side effects on the explants ofA.excelsa(Sarmast et al.2011).However,adding silver nanoparticles(AgNPs)is quite effective in controllingP. fluorescens,as the treatment drastically decreasedE.herbicolacontamination.The authors suggested that AgNPs,which induce physiochemical changes,have several modes of action and a higher surface area per mass,enabling them to prevent bacterial infections and actively block ethylene through the release of silver ions(Sarmast et al.2015).The presence of elevated levels of ethylene may also reduce explant growth and development,depending on the species(Sarmast et al.2010,2011,2015).
Endogenous bacterial infections are apparently inevitable during the culture conifers in vitro and may persist even after several subcultures(Ewald et al.2000).Ideal antibiotics to eliminate these bacteria are soluble in water,heat and light stable,unaffected by pH and the media used and are deleterious only on bacteria and fungi,not to the plant cells.Preferably they are active against a broad range of bacteria,and bactericidal rather than bacteriostatic.They ought to be optimally effective,even when used in combination with other antibiotics.Ideal antibiotics are expected to be non-resistance inducing,inexpensive,nontoxic to humans and other nontarget organisms and should have at least two modes of action(Falkiner 1990).However,common antibiotics are phytotoxic or may disrupt explant growth by inhibiting metabolic processes(Dodds and Roberts 1981;Falkiner 1990;Leifert et al.1992).For the genetic transformation ofPinus pineaL.,the application of cefotaxime,vancomycin,and ticarcillin is frequently advised to eliminate excessive growth ofAgrobacterium tumefaciensafter co-cultivation.These antibiotics are essentially nontoxic to stone pine cotyledons and enable significantly more buds to form on cotyledons(Humara and Ordas 1999).However,carbenicillin has inhibitory effects during somatic embryogenesis of Sitka spruce[Picea sitchensis(Bong.)Carr.;Sarma et al.(1995)]and Norway spruce[Picea abies(L.)Karst.;Mala´et al.(2009)].For conifers,the results are inconsistent.Cefotaxime appears to be detrimental to general health following the regeneration ofPinus radiatacotyledons(Holland et al.1997),whereas it had little negative effect on explant growth and development ofPicea glauca(Moench)Voss(Ellis et al.1989),Picea abies(Hood et al.1990),Pinus nigraJ.F.Arnold(Lo´pez 2000),Larix kaempferi×L.decidua(Levee et al.1997)andPinus pineaL.(Humara and Ordas 1999).These reports are in contrast with many other reports on nonconiferous plant species(Holford and Newbury 1992;Yepes and Aldwinckle 1994;Nauerby et al.1997;Ogawa and Mii 2005;Mendes et al.2009)in which the negative effect of cefotaxime on shoot regeneration was con firmed.Mala´et al.(2009)recommended amoxicillin and meropenem to eliminate infections byAgrobacteriumin explants ofPicea abiesduring their somatic embryogenesis because it did not adversely affect growth.When Le-Feuvre et al.(2013)was usingAgrobacteriumto transformP.radiata,even 400 mg L-1timentin did not inhibitAgrobacteriumgrowth after cocultivation ofP.radiataembryonal masses withA.tumefacienswhen cultured on modi fied Murashige and Skoog(MSG)medium(Becwar et al.1990).
The procedures for in vitro sterilization of many conifers are summarized in Table 1.The decontamination procedure is now mostly aimed at mature tissues of conifers.Recent reports have focused on the micropropagation of conifers via somatic embryogenesis through seed-based materials,which are not dif ficult to sterilize.Yet,new approaches have not been developed,and we still have little information on the decontamination of mature tissues in conifers other than several procedures that can reduce bacterial contamination in plant tissue culture systems.Such measures include sanitation of growth rooms,use of laminar flow hoods,and proper autoclaving and training of operators to reduce contamination of samples from mother plants from the field.Proper prewashing of explants with different antifungal compounds,mechanical shaking,and applying multiple decontaminants via vacuum in filtration can improve the effectiveness of the decontamination.Heat treatment of whole plants or plant parts(for up to 4 h at 43.5–57 °C),thermotherapy (exposure forweeksat 37–38 °C)or heat combined with meristem excision(0.10–0.15 mm in length)can most likely be effective for excluding bacteria and viruses.Visual inspection and specialized media(indexing cultures)should also be used to detect slow-growing bacteria,viruses,endophytes,or bacteria that do not grow on plant tissue culture media.Media supplemented with ribavirin or tribavirin can partially be successful in producing virus-free plants.Finally,a widespread range of antibiotics at different concentrations should be tested to determine the most effective concentrations with minimal phytotoxicity(Reed and Tanprasert 1995).
Clonal multiplication
The three common methods for clonal propagation include the production of plantlets via axillary shoots with the lowest somaclonal variation,direct and indirect adventitious bud induction,and somatic embryogenesis(Murashige 1974).An efficient in vitro regeneration procedure is a prerequisite forcommercialmicropropagation and genetic transformation.Immature or mature embryos of conifers are a popular explant for clonal production because regenerations from other types of conifer explants such as leaf and mature shoots are too laborious.Tang et al.(2006)reported cotyledon-derived embryos ofPinus elliottiion severalbasalmedia;higherfrequencies(34–46%)of callus induction were obtained on B5,SH,and TE media than on DCR,LP,MSG and MS media.On shoot formation media,adventitious shoots were regenerated from callus on B5,SH and TE within 6–12 weeks,with higher frequencies of callus induction(26–35%)on SH and TE,but with low frequencies(6–9%)on B5.They induced the formation of roots on adventitious shoots in media supplemented with 0.01 mg L-1indole-3-acetic acid(IAA)and 0.01 mg L-1indole-3-butyric acid(IBA),but the frequency of rooting was not mentioned.Tang and Newton(2005)also indued calli ofPinus strobuson PS medium(Tang and Newton 2005)supplemented with high concentrations of 2,4-Dichlorophenoxyacetic acid(2,4-D),naphthaleneacetic acid(NAA)or IAA(15 μM)within 6 weeks.They reported that the 6 μM thidiazuron(TDZ)was more effective than 6-benzyladenine(BA)and 6(Dimethylallylamino)purine(2iP)regarding the formation of adventitious shoots.They also concluded that treating the callus cultures at 4°C increases shoot formation,the average number of shoots per gram of callus and the rooting frequency in the PS medium.In addition,putrescine(0.01–1 μM)had positive effects on callus induction and shoot formation,most probably by decreasing lipid peroxidation in callus cultures.However,in protocols where the callus is the basal source of regeneration,the genetic fidelity of regenerated plantlets is more questionable than those that are directly derived from axillary shoots,which are used for many woody species.Burrows et al.(1988)reported that explants had a negative response to high concentrations of plant growth regulators(PGRs),
especially BA in the culture media.These results agree with observations by Traore et al.(2005),Selby et al.(2005)and Sarmast et al.(2012a).Furthermore,Stojicic et al.(1999)reported that the average number of buds per explant,average shoot length,and the explant survival percentage ofPinus heldrichiiChrist.grown on GD medium(Gresshoff and Doy 1972)was better than growth on LP or MS.In their experiment,BA was the best PGR,and 1 mM IBA pulse treatments of shoots propagated in vitro was ideal for inducing roots.Stojicic and Budimir(2004)later reported that a 1 h pulse treatment with 222 μM BA of explants from 30-day-oldP.heldreichiiseedlings on GD medium resulted in an average of 4.6 shoots per explant,but only 10%of the shoots formed roots.Adventitious shoot formation(caulogenesis)by cotyledons excised from mature embryos ofP.pineacan take place directly in the presence of BA.Nonetheless,adventitious shoot formation not only depends on the applied concentration of BA in addition to the genotype,but also on the extent of differentiation within the cotyledon after BA treatment(Valde´s et al.2001).According to Moncalea´n et al.(2005)and Alonso et al.(2007),BA-mediated caulogenic gene expression depends on BA concentration.Loureiro et al.(2007)evaluatedJuniperus phoeniceaL.on five media with different PGRs and found that the best elongation was achieved on Driver and Kuniyuki(DKW)medium supplemented with kinetin,either alone or with NAA.When well-developed internodes ofJuniperus phoeniceaL.were exposed to 2.4 μM IBA for 5 min,40%rooting was obtained after they were transferred to OM-free PGR medium(Rugini 1984).Combining BA and NAA can increase the percentage of responsive explants,and the mean number of buds per explant(Zhu et al.2010).Furthermore,the explants were superior to those on media without NAA;this situation can be of signi ficance compared to when media is supplemented with BA alone(Zhu et al.2010).Cortizo et al.(2009a)reported the first successful procedure for micropropagation ofPinus pineaadult trees via axillary buds using TDZ in LP medium(Quoirin and Lepoivre 1977).De Diego et al.(2010)
reported an efficient biotechnological approach for micropropagating adultPinus sylvestris;the highest organogenesis was obtained by culturing dormant shoot buds on DCR(Gupta and Durzan 1985)and WPM(McCown and Lloyd 1980).Media supplemented with 25 μM meta-topolin[6-(3-hydroxybenzylamino)purine]was also effective for organogenesis.De Diego et al.(2008)revealed thatPinus pinastershoot buds generated the most axillary shoots when cultured on DCR medium supplemented with 25 μM zeatin and meta-topolin,and in combination with 25 or 50 μM 6-benzyladenine.When Cortizo et al.(2009b)investigated the cause of reductions in the cytokinin-induced formation of adventitious shoots from embryos ofP.pinea,they postulated that differing concentrations of BA/[9R]BA inside the explants is the most probable cause of this germination-related loss in the cotyledon capacity to produce buds.The germination process not only decreases the endogenous concentration of BA/[9R]BA,but also hinders the occurrence of the peak where free bases and ribosids become evident(the active forms of BA metabolites).The procedures for in vitro shoot induction pertaining to the majority of conifers are summarized in Table 2.

Table 1 Treatments for surface sterilization of some conifer species
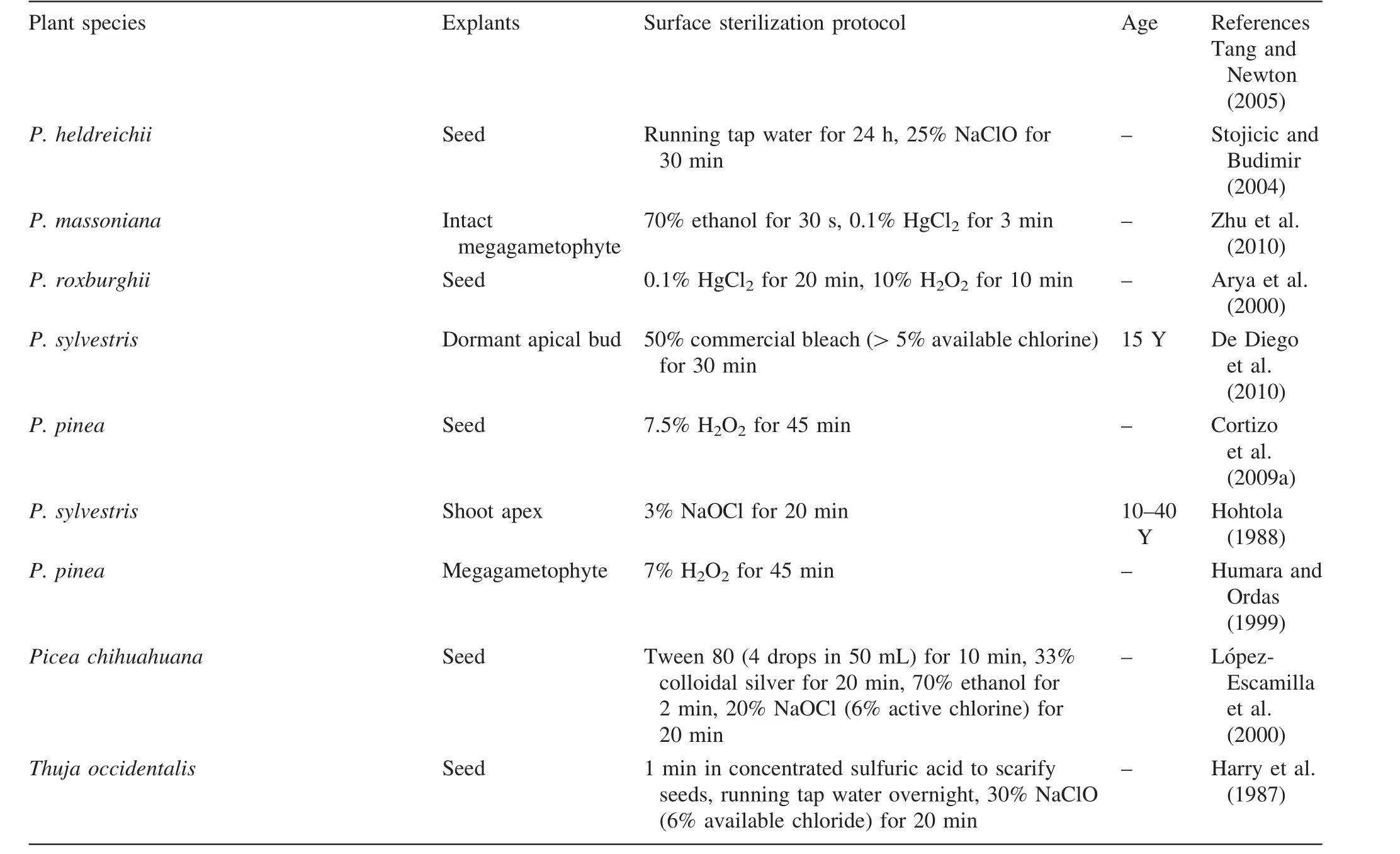
Table 1 continued
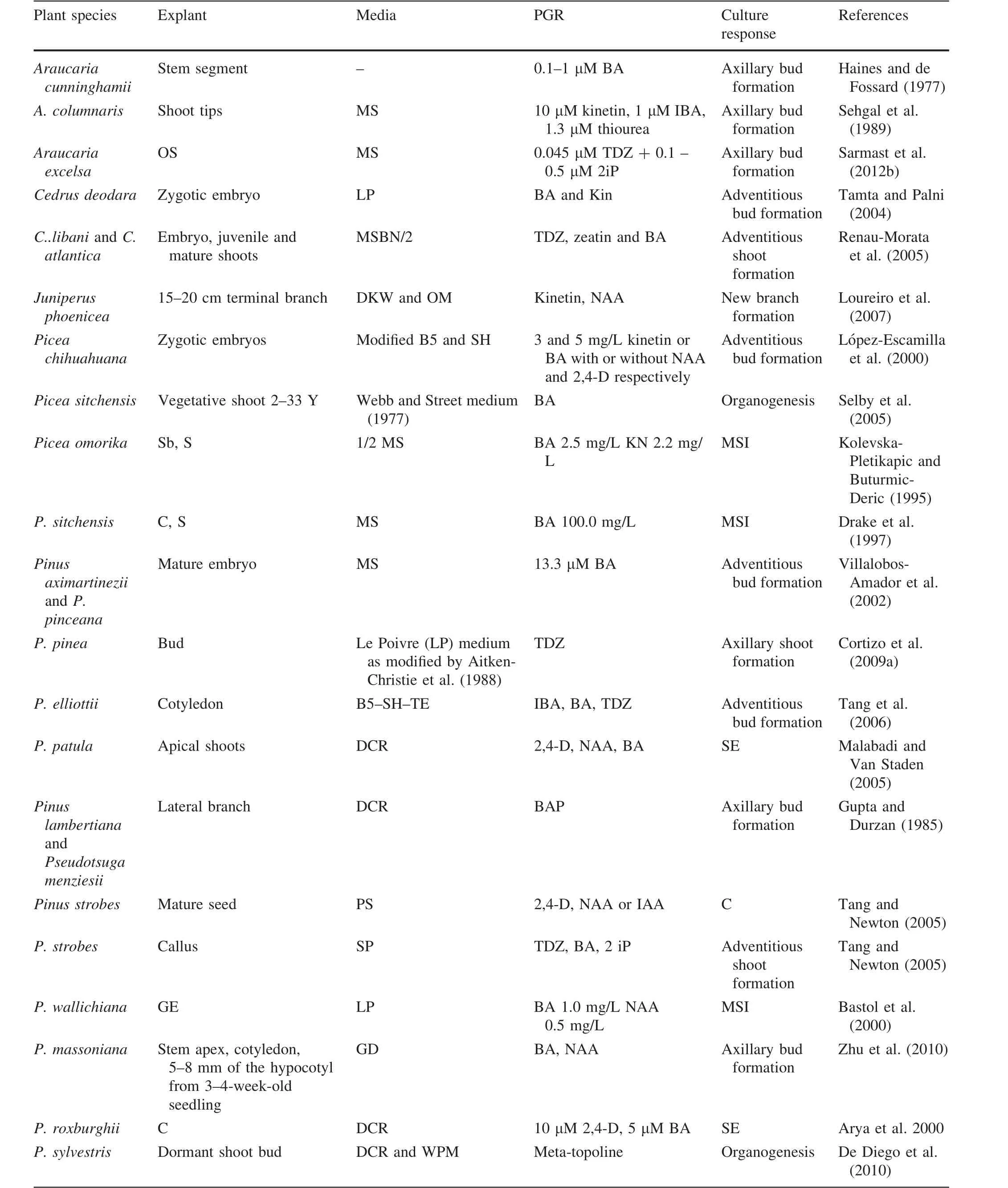
Table 2 Summary of treatments used to induce tissue production by various explants from conifers species
Renau-Morata et al.(2005)and Piola et al.(1999)achieved the highest proliferation rate(nearly 4.5 buds per explants)inCedrus libaniafter pulse treatment of microcuttings with 2.2 μM BA and 0.1 mM BA,respectively.In one report,adventitious shoots were regenerated fromC.atlanticaembryos,achieving 4.2 buds per embryo after 60 days.They were cultured on MSBN/2 media(halfstrength MS salt and half-strength Bourgin and Nitsch[1967]vitamins and Skoog[1944]amino acids)when preliminarily exposed to 9.0 μM zeatin for the induction stage,and 4.6 μM for further development.The adventitious shoots then continued to elongate on cytokinin-free media.However,Tamta and Palni(2004)reported a mean of 33.75 shoots per explant forC.deodaraon LP medium after exposure to 0.1 μM BA and kinetin for 28 days before transfer to PGR-free LP.But these shoots turned yellow during proliferation and elongation and,in most cases,they did not survive.For overcoming dehydration of shoots produced in subsequent subcultures,explants should be inverted on LP supplemented with 20.0 μM BA for 10 days,then shifted back to their typical position on PGR-free medium.Montalba´n et al.2011 established an efficient procedure whereby 100 mg embryonic tissue can produce 1700 rooted shoots via a combined pathway of SE and organogenesis inPinus radiata.The researchers reported significantly more shoots per explant in freshly collected somatic embryos that were exposed to 4.4 μM BA for 3 or 4 weeks,when compared to somatic embryos that were a week overdue for germination.The evaluation of two variables together,i.e.,the percentage of embryo formingshoots(EFS)and the number of shoots per embryo,called the shoot elongation capacity(SEC),provides a realistic assessment of the ef ficiency of the culture conditions(Pulido et al.1992).Freshly collected embryos ofPinus radiatatreated with suitable levels of BA for 3–4 weeks,resulted in a high ef ficiency of induction(Montalba´n et al.2011).Stump sprouts were produced from the basal zone of the juvenile crown of old trees and were successfully used as explant sources to micropropagateSequoia sempervirens(Boulay 1979)andCunninghamia lanceolataLamb.(Bigot and Engelmann 1987).Epicormic shoots have also been as an explant source for micropropagation(Burrows et al.1988;Vidal et al.2003).
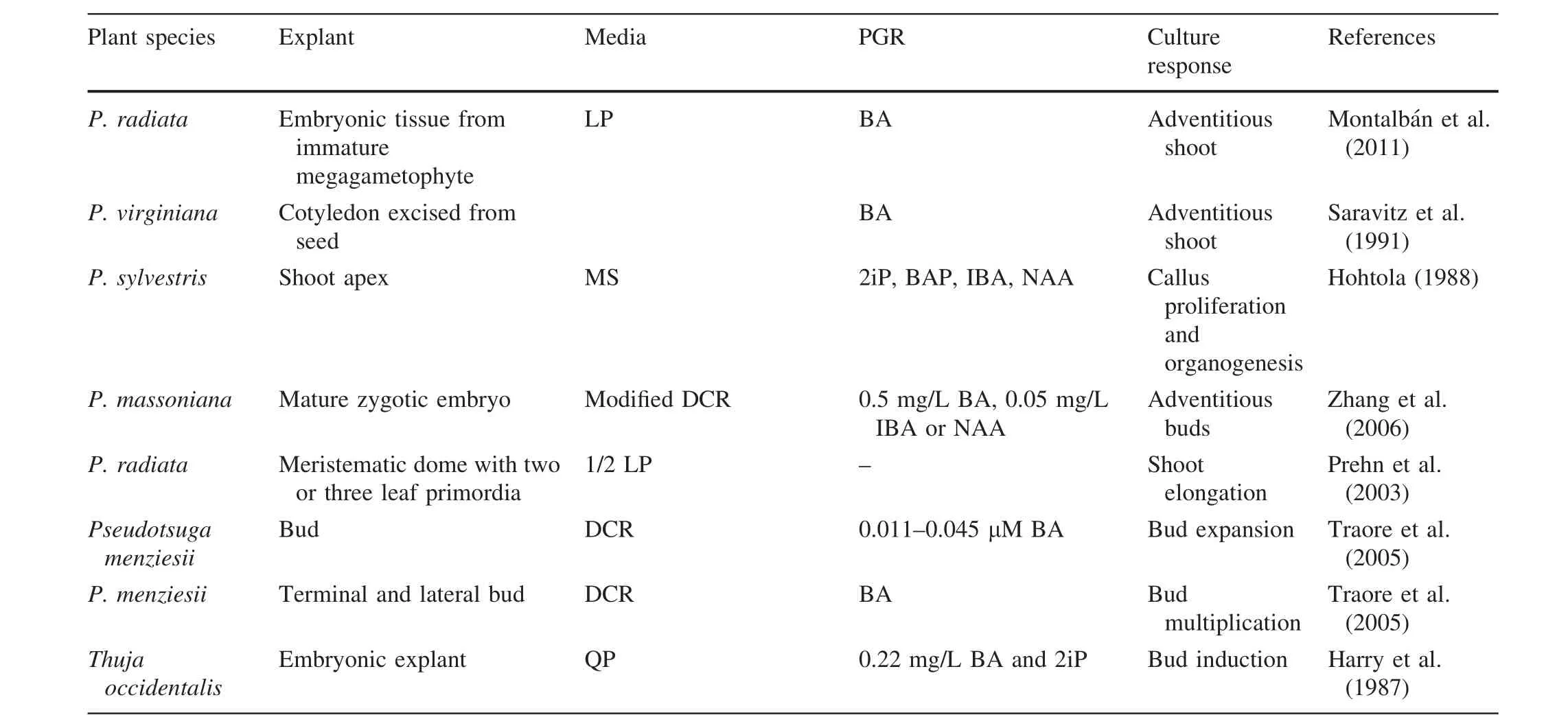
Table 2 continued
Conclusion
Proper elimination of unwanted microbial contamination concerning in vitro conditions could contribute to a better establishment of mature shoots in conifers.Before surface sterilization,elements such as selection of explant source,physiological activity of explants are pivotal,and proper exposure and combination of decontaminants during surface sterilization are critical for removing surface contaminants.SE protocols for many conifer species are not adequate;therefore,mature shoots of conifers offer a good alternative for micropropagation.Although there are dif ficulties in the clonal multiplication of conifers,such as higher levels of contamination on mature shoots,low rates of regeneration,and excessive phenolic exudation,plantlets derived from axillary shoots and adventitious shoots of conifers generally have a much lower incidence of somaclonal variation,making this procedure more reliable than callus-derived techniques.
AcknowledgementsI thank Dr.Jenna E.Gallegos(Department of Molecular and Cellular Biology,University of California,Davis)for critically reading the manuscript.Mohsen Hamedpour-Darabi is thanked for editing the English.
References
Aitken-Christie J,Singh A,Davies H(1988)Multiplication of meristematic tissue:a new tissue culture system for radiata pine.In:Hanover JW,Keathley DE(eds)Genetic manipulation of woody plants,vol 44.Plenum Publishing Corporation,New York,pp 413–432
Alonso P,Cortizo M,Canto´n FR,Ferna´ndez B,Rodrı´guez A,Centeno ML,Ca´novas FM,Orda´s RJ(2007)Identi fication of genes differentially expressed during adventitious shoot induction inPinus pineacotyledons by subtractive hybridization and quantitative PCR.Tree Physiol 27:1721–1730
Arya S,Kalia RK,Arya ID(2000)Induction of somatic embryogenesis inPinus roxburghiiSarg.Plant Cell Rep 19:775–780
Bastol B,Jasik J,Mantell S(2000)In vitro propagation of a Himalayan pineP.wallichianaA.B.Jacks.Curr Sci 78:338–341
Becwar M,Nagmani R,Wann S(1990)Initiation of embryogenic cultures and somatic embryo development in loblolly pine(Pinus taeda).Can J For Res 20:810–817
Bigot C,Engelmann F(1987)Vegetative propagation in vitro ofCunninghamia lanceolata(Lamb.)Hook.In:Bonga JM,Durzan DJ(eds)Cell and tissue culture in forestry,case histories,gymnosperms,angiosperms and palms,vol 3.Martinus Nijhoff,The Hague,pp 114–127
Bonga JM,Klimaszewska KK,von Aderkas P(2010)Recalcitrance in clonal propagation,in particular of conifers.Plant Cell Tissue Organ Cult 100:241–254
Boulay M(1979)Multiplication et clonage rapide du Sequoia sempervirens par la culture in vitro.Annales de Recherche Sylvicole,AFOCEL,pp 49–55
Bourgin JP,Nitsch JP(1967)Obtention de Nicotiana haploides a`partir d’e´tamines cultive´es in vitro.Ann Physiol Ve´g 9:337–338
Buckley PM,DeWild TN,Reed BM(1995)Characterization and identi fication of bacteria isolated from micropropagated mint plant.In Vitro Cell Dev Biol Plant 31:58–64
Burrows GE,Doley DD,Haines RJ,Nikles DG(1988)In vitro propagation ofAraucaria cunninghamiiand other species of the Araucariaceae via axillary meristems.Aust J Bot 36:665–676
Cassells AC(1991)Problems in tissue culture:culture contamination.In:Debergh PC,Zimmerm RH(eds)Micropropagation technology and application.Kluwer Academic Publishers,Dordrecht,pp 31–44
Chanway C(1998)Bacterial endophytes:ecological and practical implication.Sydowia 50:149–170
Cortizo M,Cuestam C,Centeno ML,Rodriguez A,Fernandez B,Ordas R(2009a)Benzyladenine metabolism and temporal compedence ofPinus pineacotyledons to form buds in vitro.J Plant Physiol 166:1069–1076
Cortizo M,De Diego N,Orda´s R,Moncalea´n P(2009b)Micropropagation of adult Stone Pine(Pinus pineaL.).Trees 23:835–842
De Diego N,Montalba´n IA,Ferna´ndez E,Moncalea´n P(2008)In vitro regeneration ofPinus pinasteradult trees.Can J For Res 38:2607–2615
De Diego N,Montalba´n IA,Moncalea´n P(2010)In vitro regeneration of adultPinus sylvestrisL.trees.S Afr J Bot 76:158–162
Debergh PC,Vanderschaeghe AM(1988)Some symptoms indicating the presence of bacterial contaminants in plant tissue culture.Acta Hortic 255:77–81
Dodds JH,Roberts WL (1981)Some inhibitory effectors on gentamicin on plant tissue culture.In Vitro 17:467–470
Dos Santos ALW,Silveria V,Steinr N,Vidor M,Guerra MP(2002)Somatic embryogenesis in Parana Pine(Araucaria angustifolia(Bert.)O.Kuntze).Braz Arch Biol Technol 45:97–106
Drake PMW,John A,Power JB,Davey MR(1997)Expression of the gus A gene in embryogenic cell lines of Sitka spruce followingAgrobacterium-mediated transformation.J Exp Bot 48:151–155
Driver JA,Kuniyuki AH(1984)In vitro propagation of paradox walnut root stocks.Hortic Sci 19:507–509
Ellis DD,Lazaroff WR,Roberts DR,Flinn BS,Webb DT(1989)The effect of antibiotics on elongation and callus and bud formation from embryogenic tissue ofPicea glauca.Can J For Res 19:1343–1346
Ewald D,Suss R(1993)A system for repetable formation of elongating adventitious buds in Norway spruce tissue cultures.Silvae Genetica 42:169–175
Ewald D,Kretzschmar U,Chen Y(1997)Continuous micropropagation of juvenile larch from different species via adventitious bud formation.Biol Plant 39:321–329
Ewald D,Zaspel I,Naujoks G,Behrendt U(2000)Endogenous bacteria in tissue cultures of conifers—appearance and action.Acta Hortic 530:137–144
Falkiner,FR(1990)The criteria for choosing an antibiotic for control of bacteria in plant tissue culture in TCL.Assoc Plant Tiss Cult Newsl 60:13–23
Gamborg OL,Miller RA,Ojima K(1968)Nutrient requirements of suspension cultures of soybean root cells.Exp Cell Res 50:151–158
Gresshoff PM,Doy CH(1972)Development and differentiation of haploidLycopersicon esculentum(tomato).Planta 107:161–170 Gupta PK,Durzan DJ(1985)Shoot multiplication from mature trees of Douglas- fir(Pseudotsuga menziesii)and sugar pine(Pinus lambertiana).Plant Cell Rep 4:177–179
Haines RJ,de Fossard RA (1977)Propagation of Hoop pine(Araucariacunninghamii)by organ culture.Acta Hortic 78:297–302
Harry IS,Thompson MR,Lu CY,Thorpe TA(1987)In vitro plantlet formation from embryonic explants of eastern white cedar(Thuja occidentalis L.).Tree Physiol 3:273–283
Hohtola A (1988)Seasonal changes in explant viability and contamination of Scots pine tissue cultures from mature Scots pine.Plant Cell Tissue Organ Cult 15:211–222
Holford P,Newbury HJ(1992)The effects of antibiotics and their breakdown products on the in vitro growth ofAntirrhinum majus.Plant Cell Rep 11:93–96
Holland L,Gemmell JE,Chanty JA,Walter C(1997)Foreign gene transfer intoPinus radiatacotyledons byAgrobacterium tumefaciens.NZ J Forest Sci 27:289–304
Hood EE,Clapham DH,Ekberg I,Johanson T(1990)T-DNA presence and opine production in tumors ofPicea abies(L.)Karst induced byAgrobacterium tumefaciensA281.Plant Mol Biol 14:111–117
Humara JM,Ordas RJ(1999)The toxicity of antibiotics and herbicides on in vitro adventitious shoot formation onPius pineaL.cotyledone.In Vitro Cell Dev Biol Plant 35:339–343
Keathley DE(1984)In:Proceedings of the international symposium of recent advances in forest biotechnology,Michigan Biotechnology Institute,Traverse City,Michigan,pp 58–63,10–13 June 1984
Kolevska-Pletikapic B,Buturovic-Deric Z(1995)Regeneration ofPicea omarikaplants via organogenesis.Plant Cell Tissue Organ Cult 41:189–192
Laukkanen H,Soini H,Kontunen-Soppela S,Hohtola A,Viljanen M(2000)A mycobacterium isolated from tissue cultures of maturePinus sylvestrisinterferes with growth of Scots pine seedlings.Tree Physiol 20:915–920
Le-Feuvre R,Trivin˜o C,Sabja AM,Bernier-Cardou M,Moynihan MR,Klimaszewska K(2013)Organic nitrogen composition of the tissue culture medium in fluencesAgrobacterium tumefaciensgrowth and the recovery of transformedPinus radiataembryonal masses after cocultivation.In Vitro Cell Dev Biol Plant 49:30–40
Leifert C,Waites WM,Nicholas LR(1989)Bacterial contamination of micropropagated plant tissue cultures.J Appl Bacteriol 67:353–361
Leifert C,Camotla H,Wright SM,Waites B,Cheyne VA,Waites WM(1991)Elimination ofLactobacillus plantarum,Corynebacteriumspp.Staphylococcus saprophyticusandPseudomonas paucimobilisfrom micropropagatedHemerocallis,ChoisyaandDelphiniumcultures using antibiotics.J ApplBacteriol 71:307–330
Leifert C,Camotla H,Wailes WM(1992)Effect of combinations of antibiotics on micropropagatedClematis,Delphinium,Hosta,IrisandPhotinia.Plant Cell Tissue Organ Cult 29:153–160
Levee V,Lelu MA,Jouanin L,Cornu D,Pilati G(1997)Agrobacterium tumefaciens-mediated transformation of hybrid larch(Larix kaempferi×L.decidua)and transgenic plant regeneration.Plant Cell Rep 16:680–685
Lo´pez M,Humara JM,Rodrı´guez R,Orda´s RJ(2000)Factors involved in Agrobacterium tumefaciens-mediated gene transfer into Pinus nigra Arn.ssp.Salzmannii(Dunal)Franco.Euphytica 114:195–203
Lo´pez-Escamilla AL,Olguı´n-Santos LP,Ma´rquez J,Cha´vez VM,Bye R(2000)Adventitious bud formation from mature embryos ofPicea chihuahuanaMartı´nez,an endangered Mexican spruce tree.Ann Bot 86:921–927
Loureiro J,Capelo A,Brito G,Rodriguez E,Silva S,Pinto G,Santos C(2007)Micropropagation ofJuniperus phoeniceafrom adult plant explants and analysis of ploidy stability using flow cytometry.Biol Plant 51:7–14
Mala´J,Pavingerova´D,Cvrcˇkova´H,Brˇı´za J,Dosta´l J,Sˇı´ma P(2009)Tolerance of Norway spruce(Picea abies[L.]Karst.)embryogenic tissue to penicillin,carbapenem and aminoglycoside antibiotics.J For Sci 55:156–161
Malabadi RB,Van Staden J(2005)Somatic embryogenesis from vegetative shoot apices of mature trees ofPinus patula.Tree Physiol 25:11–16
McCown BH,Lloyd G(1981)Woody plant medium(WPM)—a mineral nutrient formulation for microculture for woody plant species.Hortic Sci 16:453
Mendes AFS,Cidade LC,de Oliveira MLP,Otoni WC,Soares-Filho WS,Costa MGC(2009)Evaluation of novel beta-lactam antibiotics in comparison to cefotaxime on plant regeneration ofCitrus sinensisL.Osb.Plant Cell Tissue Organ Cult 97:331–336
Moncalea´n P,Alonso P,Centeno ML,Cortizo M,Rodrı´guez A,Ferna´ndez B,Orda´s RJ(2005)Organogenic responses ofPinus pineacotyledons to hormonal contents:BA metabolism and cytokinin content.Tree Physiol 25:1–19
Montalba´n IA,De Diego N,Igartua EA,Setie´n A,Moncalea´n P(2011)A combined pathway of somatic embryogenesis and organogenesis to regenerate radiata pine plants.Plant Biotechnol Rep 5:177–186
Murashige T(1974)Plant propagation through tissue culture.Ann Rev Plant Physiol 25:135–166
Murashige,T,Skoog F(1962)A revised medium for rapid growth and bioassays with tobacco tissue cultures.Physiologia Plantarum 15:473–497
Nauerby B,Billing K,Wyndaele R(1997)In fluence of the antibiotic timentin on plant regeneration compared to carbenicillin and cefotaxime in concentrations suitable forelimination ofAgrobacterium tumefaciens.Plant Sci 123:169–177
Ogawa Y,Mii M(2005)Evaluation of 12 b-lactam antibiotics forAgrobacterium-mediated transformation through in planta antibacterial activities and phytotoxicities.Plant Cell Rep 23:736–743
Piola F,Rohr R,Heizmann P(1999)Rapid detection of genetic variation within and among in vitro propagated cedar(Cedrus libaniLoudon)clones.Plant Sci 141:159–163
Prehn D,Serrano C,Mercado A,Stange C,Barrales L,Arce-Johnson P(2003)Regeneration of whole plants from apical meristems ofPinus radiata.Plant Cell Tissue Organ Cult 73:91–94
Pulido CM,Harry IS,Thorpe TA(1992)Optimization of bud induction in cotyledonary explants ofPinus canariensis.Plant Cell Tissue Organ Cult 29:247–255
Quoirin M,Lepoivre P(1977)Elude de milieu adaptes aux cultures in vitro dePrunus.Acta Hortic 78:437–442
Reed BM,Tanprasert P(1995)Detection and control of bacterial contaminations of plant tissue culture:a review of recent literature.Plant Tissue Cult Biotechnol 1:137–142
Renau-Morata B,Ollero J,Arrillagam I,Segura J(2005)Factor in fluencing axillary proliferation and adventitious budding in cedar.Tree Physiol 25:477–486
Rugini E(1984)In vitropropagation of some olive(Olea europaeaL.var.sativa)cultivars with different root-ability,and medium development using analytical data from developing shoots and embryos.Sci Hortic 24:123–134
Saravitz CH,Blazich FA,Amerson HV(1991)In vitro propagation of virginia pine from cotyledons.J Am Soc Hortic Sci 116:362–365
Sarma KS,Evans NE,Selby CH(1995)Effect of carbenicillin and cefotaxime on somatic embryogenesis of Sitka spruce(Picea sitchensis(Bong.)Carr.).J Exp Bot 46:1779–1781
Sarmast MK(2016)Genetic transformation and somaclonal variation in conifers—a review.Plant Biotechnol Rep 10:309–325
Sarmast MK,Salehi H,Khosh-Khui M,Niazi A,Bastani R(2010)Nano silver functionalization ofAgrobacteriummediated transformation with companionship of nanobiotechnology.Symposium on nanotechnologies applied to biosystems engineering and the environment.CIGR.Que´bec City,13–17 June,Canada
Sarmast MK,Salehi H,Khosh-Khui M(2011)Nano silver treatment is effective in reducing bacterial contamination ofAraucariaexcelsaR.Br.var.glauca explants.Acta Biol Hung 62:477–484
Sarmast MK,Salehi H,Ramzani Abolimoghadam AA,Niazi A,Khosh-Khui M(2012a)RAPD fingerprint to appraise the genetic if delity of in vitro propagatedAraucaria excelsaR.Br.var.glauca plantlets.Mol Biotechnol 50:181–188
Sarmast MK,Salehi H,Khosh-Khui M(2012b)Micropropagation ofAraucaria excelsaR.Br.var.glauca Carrie`re from orthotropic stem explants.Physiol Mol Biol Plants 18:265–271
Sarmast MK,Niazi A,Salehi H,Abolimoghadam A(2015)Silver nanoparticles affect ACS expression inTecomella undulatain vitro culture.Plant Cell Tiss Organ Cult 121:227–236
Sehgal L,Sehgal OP,Khosla PK(1989)Micropropagation ofAraucaria columnarisHook.Ann Sci For 46:158–160
Selby C,Watson S,Harvey BMR(2005)Morphogenesis inSitka spruce(Picea sitchensis(Bong.)Carr.)bud cultures—tree maturation and explants from epicormic shoots.Plant Cell Tissue Organ Cult 83:279–285
Skoog F(1944)Growth and organ formation in tobacco tissue cultures.Am J Bot 31:19–24
Stojicic D,Budimir S(2004)Cytokinin-mediated axillary shoots formation inPinus heldreichii.Biol Plant 48:477–479
Stojicic D,Budimir S,Cula fic L(1999)Micropropagation ofPinus heldrechii.Plant Cell Tissue Organ Cult 59:147–150
Tamta S,Palni LMS(2004)Studies of in vitro propagation of Himalayan cedar(Cedrus deodara)using zygotic embryos and stem segments.Indian J Biotechnol 3:209–215
Tang W,Newton RJ(2005)Plant regeneration from callus cultures derived from mature zygotic embryos in white pine(Pinus strobusL.).Plant Cell Rep 24:1–9
Tang W,Sederoff R,Whetten R(2001)Regeneration of transgenic loblolly pine(Pinus taedaL.)from zygotic embryos transformed withAgrobacterium tumefaciencs.Planta 213:981–989
Tang W,Newton RJ,Charles TM(2006)Plant regeneration through multiple adventitious shoot differentiation from callus cultures of slash pine(Pinus elliottii).J Plant Physiol 163:98–101
Tang W,Harris LC,Outhavong V,Newton RJ(2004)The effect of different plant growth regulators on adventitious shoot formation from Virginia pine(Pinus virginiana)zygotic embryo explants.Plant Cell Tiss Org Cult 78:237–240
Thorpe TA(1985)Application of tissue culture to forest tree improvement.For Chron 61:436–438
Thorpe TA(2007)History of plant tissue culture.Mol Biotech 37:169–180
Timmis R,Ritchie GA(1984)In:Proceedings of the international symposium of recent advances in forest biotechnology,Michigan Biotechnology Institute,Traverse City,Michigan,pp 37–46,10–13 June 1984
Traore A,Xing Z,Bonser A,Carlson J(2005)Optimizing a protocol for sterilization and in vitro establishment of vegetative bud from matureDouglas firtrees.HortScience 40:1464–1468
Valde´s AE,Orda´s RJ,Ferna´ndez B,Centeno ML(2001)Relationships between hormonal content sand the organogenic response inPinus pineacotyledons.Plant Physiol Biochem 39:377–384
Vidal N,Arellano G,San-Jose MC,Vieitez AM,Ballester A(2003)Developmental stages during the rooting of in vitro culturedQuercus roburshoots from material of juvenile and mature origin.Tree Physiol 23:1247–1254
Villalobos-Amador E,Rodrı´guez-Herna´ndez G,Pe´rez-Molphe-Balch E(2002)Organogenesis andAgrobacterium rhizogenes-induced rooting inPinus maximartineziiRzedowsky andP.pinceanaGordon.Plant Cell Rep 20:779–785
Viss PR,Brooks EM,Driver JA(1991)A simpli fied method for the control of bacterial contamination in woody plant tissue culture.In vitro Cell Dev Biol Plant 27:42
Webb KJ,Street HE(1977)Morphogenesis in vitro of Pinus and Picea.Acta Horticult 78:259–267
Yepes LM,Aldwinckle HS(1994)Factors that affect leaf regeneration ef ficiency in apple and effect of antibiotics in morphogenesis.Plant Cell Tissue Organ Cult 37:257–269
Zhang Y,Wei Z,Xi M,Shi J(2006)Direct organogenesis and plantlet regeneration from mature zygotic embryos of masson pine(Pinus massonianaL.).Plant Cell Tissue Organ Cult 84:119–123
Zhu LH,Wu XQ,Qu HY,Ji J,Ye J(2010)Micropropagation ofPinus massonianaand mycorrhiza formation in vitro.Plant Cell Tissue Organ Cult 102:121–128
 Journal of Forestry Research2018年3期
Journal of Forestry Research2018年3期
- Journal of Forestry Research的其它文章
- ‘Relationships between relationships’in forest stands:intercepts and exponents analyses
- Effects of application date and rate of foliar-applied glyphosate on pine seedlings in Turkey
- Assaying the allelopathic effects of Eucalyptus camaldulensis in a nursery bed incorporated with leaf litter
- Effects of soil compaction on growth variables in Cappadocian maple(Acer cappadocicum)seedlings
- Variation and selection analysis of Pinus koraiensis clones in northeast China
- Non-aerated liquid culture promotes shoot organogenesis in Eucalyptus globulus Labill
