Effects of soil compaction on growth variables in Cappadocian maple(Acer cappadocicum)seedlings
Meghdad Jourgholami
Introduction
Forest soil plays a vital role in the cycling of nutrients,water and energy flows in forests to ensure their productivity and sustain biodiversity(Cambi et al.2015;Etehadi Abari et al.2017),but the soil is sensitive to inappropriate forest management,to machines used in forest management,in particular heavy logging machinery(Ampoorter et al.2007;Jourgholami et al.2014;Cambi et al.2015)and to human and animal trampling.With soil compaction,soil pores are compressed or destroyed(Kozlowski 1999),and subsequently the particles become redistributed into an arrangement that has a higher bulk density(BD;mass per unit volume)(Kozlowski 1999;Gomez et al.2002;Ampoorter et al.2011;Jourgholami et al.2014).
Soil compaction can have many different effects on different plants such as decrease length of primary roots,decreased water absorption,increased leaf water de ficits,decreased nutrient absorption and photosynthetic rate,and general growth reduction(Misra and Gibbons 1996;Kozlowski 1999;Alameda and Villar 2009,2012;Bejarano et al.2010).
Increasing soil strength generally increases resistance to root development(Aca´cio et al.2007;Ampoorter et al.2007).Seedling root elongation and penetration decreases as soil penetration resistance increases,which then leads to de ficiency in water and nutrient uptake(Greacen and Sands 1980;Gomez et al.2002).In soils with a higher BD and penetration resistance,plants may require a greater budget for root elongation,thus boosting energy costs for seedlings and potentially reducing their growth rate and productivity(Bejarano et al.2010).
Roots must overcome the strength of the soil to penetrate pores of smaller diameters than those of the roots.Because soil compaction increases soil strength and decreases the number of macropores,the rate of root elongation and therefore root length is reduced(Greacen and Sands 1980).Typically elongation rate is reduced exponentially as soil strength(as measured by resistance to a penetrometer)increases(Greacen and Sands 1980;Bejarano et al.2010).Severe compaction of soil not only shortens and thickens roots,but also may alter their branching patterns(Misra and Gibbons 1996;Kozlowski 1999;Gomez et al.2002;Ampoorter et al.2007)and typically decreases absorption of major mineral nutrients(Alameda and Villar 2009,2012;Bejarano et al.2010;Pe´rez-Ra´mos et al.2010).For most plants,there is a nonlinear inverse relation between the rate of root elongation and soil penetration resistance(Misra and Gibbons 1996;Kozlowski 1999;Siegel-Issem et al.2005;Fleming et al.2006;Alameda and Villar 2009).
Numerous studies have documented changes in woody plant growth following experimentally set compaction levels(Gomez et al.2002;Tubeileh et al.2003;Bassett et al.2005;Bulmer and Simpson;Siegel-Issem et al.2005;Fleming et al.2006;Alameda and Villar 20092005,2012;Bejarano et al.2010;Pe´rez-Ra´mos et al.2010).Also,seedling growth reductions in controlled laboratory conditions have been reported for many species,includingPinus contorta(Bol.)(Corns 1988;Conlin and van den Driessche 1996),Pinus sylvestris(L.)(Wasterlund 1985),Picea glauca(Corns 1988),Picea abies(L.)Karsr(Wasterlund 1985),Quercus pyrenaicaWilld.(Bejarano et al.2010),Pistacia lentiscus(Verdu and Garcıa-Fayos 1996),Fraxinus angustifoliaVahl.(Alameda and Villar 2012),andAraucaria angustifolia(Mosena and Dillenburg 2004).
The structure of leaves,stems and roots can be altered by some environmental factors(Misra and Gibbons 1996;Zou et al.2001;Bulmer and Simpson 2005;Kabzems and Haeussler 2005;Siegel-Issem et al.2005;Blouin et al.2008;Skinner et al.2009;Bejarano et al.2010;Pe´rez-Ra´mos et al.2010;Alameda and Villar 2012;Tracy et al.2012;Kormanek et al.2015).Total height and periodic annual height growth of ponderosa pine were negatively correlated with increasing adjusted bulk densities and unadjusted bulk densities(Cochran and Brock 1985).Soil compaction had a strong,negative effect on the length of the main root;thus,at maximum soil compaction,the length was roughly one-half that observed at lower compaction levels(Bejarano et al.2010).In addition,the speci fic root length(root length to root mass ratio)of the main root was reduced by roughly one-half in highly compacted soils.Bassett et al.(2005)found that soil compaction negatively affected stem and root growth ofCordyline australis.Alameda and Villar(2009)found thatQuercus suberL.,Ailanthus altissimaMill.andF.angustifoliahad a higher total biomass with a moderate increase in soil compaction,possibly due to a greater root–soil contact.
Because plants absorb water and different nutrients from the soil,physical characteristics of the soil such as texture,porosity and structure are biologically noteworthy(Alameda and Villar 2009).One of the vital variables closely related to the physical properties of soil is soil compaction.Soil compaction can strongly stress plant performance almost the same as other stresses(Bejarano et al.2010),negatively affecting growth,with a signi ficant role in impeding forest succession after harvesting(Tracy et al.2012).Soil compaction can impact roots directly or indirectly by imposing mechanical resistance to root penetration(Kormanek et al.2015),reducing the soil matric potential(Kozlowski 1999;Ampoorter et al.2011;Cambi et al.2015),decreasing water permeability and water availability to plants(Gomez et al.2002),increasing soil bulk density(Kozlowski 1999;Ampoorter et al.2007),lowering saturated hydraulic conductivity(Bejarano et al.2010),disrupting gaseous exchange(Misra and Gibbons 1996)and worsening the effects of drought(Alameda and Villar 2012).With a decline in soil porosity,soil strength(Bejarano et al.2010)and penetration resistance(Kormanek et al.2015)rise,so root growth may be hampered(Greacen and Sands 1980),potentially leading to a change in species composition in the forest flora(Blouin et al.2008)and seedling or regeneration problems(Siegel-Issem et al.2005).
In general,the impact of soil compaction on seedling and establishing regeneration is negative;thus,it is one of the main factors leading to biomass reduction for broad species categories(Bulmer and Simpson 2005).Roots can generally adapt to soil compaction by decreasing length to biomass ratio(Kabzems and Haeussler 2005).Although root length is a necessary trait in connection with growth and ecological features(Skinner et al.2009),until recently,few studies have focused on the effect of soil strength and bulk density on root growth(Tracy et al.2012).Most of these studies have shown that woody species are affected by soil compaction,but the effects on different trees traits vary;for example,the length of the main and lateral roots decrease,but root diameter increases,and nutrition and water uptake,photosynthetic rate,and thus usually total biomass decline(Misra and Gibbons 1996;Kozlowski 1999;Pe´rez-Ra´mos et al.2010).Further,these effects differ for various species and differentlevels of compaction.In fact,total biomass of several deciduous and evergreen tree species responded positively to increased soil strength(Alameda and Villar 2009).
In spite of extensive studies on the performance of herbaceous species in relation to soil compaction(Tracy et al.2012),detailed research about the in fluence of soil compaction on seedling morphology and architecture,and seedling growth or biomass is rare.Several studies,however,have investigated the effects of soil compaction on seedling growth of a variety of tree species in PVC pots in greenhouses(Conlin 1996;Mosena and Dillenburg 2004;Siegel-Issem et al.2005;Blouin et al.2008;Kormanek et al.2015).
In addition,soil compaction studies have primarily focused on agricultural and herbaceous species,whereas research on woody forest species is not common,with even lessresearch in Iran,which hasmountain forests.Machinery operation is less common in these forests,and most logs are transported from the forest interior by ground skidding;thus,soil compaction is high.All in all,machines(tractors and skidders passages,tire pressure)and human and animal trampling are the main factors of forest soil compaction(e.g.,Misra and Gibbons 1996;Kozlowski 1999;Siegel-Issem et al.2005;Fleming et al.2006;Alameda and Villar 2009).
Alameda and Villar(2009)found that the effects of moderate soil compaction vary among woody species,from a null effect to positive or negative linear effects,or a bellshaped response for growth and growth components.Brais(2001)found that on coarse-textured soils,competition severity in skid trails decreased with the number of skidding cycles,resulting in signi ficant increases in black spruce(Picea marianaMill.)and jack pine(Pinus banksianaLamb.)growth.Brais(2001)found that growth of white spruce,black spruce and jack pine were either negatively affected by high macroporosity values or positively by high microporosity values,indicating that water retention may be the limiting factor in these soils.
The focus of the present study,Cappadocian maple(Acer cappadocicumGled.),is a light-demanding and fastgrowing tree.It grows as individuals and in small groups and better tolerates shallow,alkaline and dry soils than velvet maple(Sagheb-Talebi et al.2014).Also,Cappadocian maple is one of the most popular trees for plantations and the restoration of different forest stands in the Caspian region and is widely produced in nurseries.The species holds promise for restoring the Hyrcanian forest in northern Iran,where soil disturbance and compaction after groundbased skidding operation has led to serious forest regeneration problems.Because the effects of soil penetration resistance on different growth variables of seedlings have not yet been examined in the Hyrcanian forest,here we studied the response ofA.cappadocicumseedlings to soil compaction in the greenhouse to test the hypotheses that(1)seedling growth will decrease with increasing soil compaction and(2)the magnitude of the growth decrease will increase as soil compaction increases.
Materials and methods
Seeds of Cappadocian maple(A.cappadocicum)trees with the best morphology characteristics were chosen from the seed center of Amol,then transported to the seed laboratory at the Natural Resources Faculty in Karaj to sort seeds and were preserved in a refrigerator at 4°C.On 25 December 2014,four types of seeds were sown at a depth of 1–3 mm in loam or clay loam soils in plastic pots(20×15 cm)and grown with constant moisture,controllable temperature,and humidity without fertilizer to avoid any possible confounding effects of different soil water regimes(Souch et al.2004).Pots were irrigated every day for 70 days in a greenhouse at the College in Agriculture and Natural Resources at the University of Tehran,Karaj.
After 15 days,seedlings began to sprout.On 29 February 2015,seedlings were transplanted into larger pots(20×30 cm)to avoid any space limitations for root growth during the experiment(Alameda and Villar 2012).Six soil compaction treatments were applied manually with a compaction hammer from a height of 45.7 cm 4.736 kg weight with 0,1,2,4,6 or 8 blows at each layer for the lowest intensity(no compaction),very low,low,medium,high,and highest compaction,respectively.Three replicates per treatment were made.By raising the soil mass,we can increase soil compaction because soil bulk density is the reference for creating compaction treatments(Alameda and Villar 2012).Soil penetration resistance(SPR)was measured every 10 cm along the soil pro file with a handheld penetrometer;the six values were averaged for each pot.
Seedlings grown in the larger pots were carefully harvested from the pots after 176 days(transplanted in late February,harvested in late August),then the roots were washed with water and gently dried.Fresh mass of leaves,stems,and roots of each seedling were measured separately.Leaves,stems,and roots were then dried at 70°C to measure dry mass.Morphological variables of seedlings such as stem diameter and height were measured with a digital Collis(0.01 mm accuracy)and scaled ruler(0.1 cm accuracy),respectively.Height was measured at the beginning and end of the growth period to calculate height growth.Lengths of the longest main and lateral root were measured.Other variables were measured such as(1)morphological(size)responses(cm):stem length,stem diameter,main root length,lateral root length,root diameter;(2)growth[fresh and dry mass(g)]:shoot biomass,root biomass,total biomass;and(3)architecture:speci fic stem length(SSL);speci fic root length(SRL);stem mass ratio(SMR);root mass ratio(RMR);root to shoot ratio(R/S).
The experimental design was a completely randomized factorial design.Data were analyzed with a one-way ANOVA to relate seedling growth responses to soil penetration resistance. The Kolmogorov–Smirnov test(α=0.05)was used to check data for normal distribution.Homogeneity of variance among classes was veri fied by Levene’s test(α =0.01).Post-hoc comparisons of compaction intensity group means were performed using Duncan’s multiple range test at 95 and 99%con fidence levels.Treatment effects were considered statistically signi ficant whenp≤0.05 andp≤0.01.The SPSS(release 15.0;SPSS,Chicago,IL,USA)statistical package was used for all analyses.
Results
Soil compaction and porosity
Soil penetration resistance at the different soil compaction levels significantly increased from(0.39±0.05)MPa in the no-compaction class to(1.95±0.1)MPa in the highest compaction class(p≤0.01)and bulk density from(1.08±0.03)g cm-3in no-compaction to(1.38±0.03)g cm-3in the highest class(p≤0.01;Table 1).Total porosity significantly decreased from(51.13±1.36)%in the no-compaction to 37.41±1.38%in the highest class(p≤0.01).
Seedling morphology(size)and growth(biomass)
The total morphologic(size)and growth(biomass)variables forA.cappadocicumseedlings changed significantly with increasing soil penetration resistance(allp≤0.01;Table 2).Comparing amounts of the morphological variables in the uncompacted class(control)to the highest compaction showed a decrease of 43%in stem lengths,36%in stem diameters,49.8%in main root lengths,and an increase of 101%in lateral root lengths(Table 2).Except for lateral root lengths,morphological variables showed a negative quadratic relationship with increasing soil penetration resistance(SPR).Stem lengths of were much shorter as soil penetration resistance increased to the highest class(p≤0.001)(Fig.1a).Similarly,stem diameter were characterized by two trends as SPR increased;first,it increased gradually up to the medium soil penetration resistance class(up to about 1.1 MPa),then with increasing compaction to the highest class,it did not change significantly(p≤0.01)(Fig.1b).Lateral root length also had two trends as SPR increased,but it first decreased gradually as SPR increased to the medium compaction class(up to about 1.0 MPa),then as soil penetration resistance increased to the highest class,it significantly increased along a quadratic curve(p≤0.01)(Fig.1c).Main root lengths,however,decreased signi ficantly as soil compaction increased from uncompacted to the highest SPR(p≤0.001)(Fig.1d).
Duncan’s multiple range test did not show any signi ficant differences in stem length responses among uncompacted,very low,low,and medium SPR,but it did detect signi ficant differences between the medium and heavy SPR.However,there was no signi ficant difference in response between the high and highest SPR(Table 2).Duncan’s test did not detect signi ficant differences in stem diameter variables among nocompacted,very light and light or among medium,high and highest intensity SPR,but it a signi ficant difference between the light and medium SPR(Table 2).
The relationship of main root length to increasing SPR showed negative quadratic curves(Fig.1d),indicating that decrease in the variables were greatest between the light and the very light intensity SPR.Duncan’s test showed signi ficant differences in main root length among the very light,light,medium,and highest SPR,but did not detect signi ficant differences between uncompacted and very light SPR(Table 2).Lateral root length increased as SPR increased,indicating that increases in the responses were lower among the nocompacted,very light and the light intensity SPR and the highest increases were found from the medium to highest SPR.Further,lateral root length did not significantly differ among uncompacted,light,andmedium SPR,but it did differ significantly among the medium,high and highest SPR(Table 2).

Table 1 Means(±standard deviation)for soil physical properties in each of the six levels of compaction classes

Table 2 Means for variables for seedling morphology(size),growth(biomass),and architecture(allocation ratios)of Acer cappadocicum seedlings at different soil penetration resistance classes
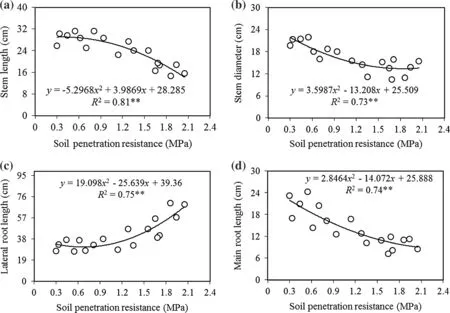
Fig.1 Relationship between SPR and morphological variables of Acer cappadocicum seedlings:stem length(a),stem diameter(b),lateral root length(c),and main root length(d).The level of signi ficance of the model is indicated by*p<0.05,**p<0.01,ns not signi ficant(p≥0.05)
The biomass variables ofAcer cappadocicumdecreased significantly with increasing SPR(allp≤0.05;Table 2)with the exception of lateral root biomass.Comparing amountsforthe differentbiomassvariablesin the uncompacted class with the high SPR showed decreases of 15%in shoot,12%in stem,18%in leaf,18%in main root,an increase of 54%in lateral root and 4%in total biomass(Table 2).The relationships of increasing SPR to some biomass variables(total,stem,total root and main root)followed a negative quadratic curve,while SPR in relation to lateral root biomass followed a positive quadratic shape,indicating that reductions in lateral root biomass were greatest among the very light,light and the medium SPR,and reductions were smaller as SPR increased beyond medium(Fig.2a,c–f).
All the biomass variables,except lateral root,followed negative quadratic curves in relation to increasing SPR.The biomass variables(total,stem,total root and main root)of maple seedlings showed two trends to increasing soil penetration resistance; first,they increased slowly to the medium compaction class(up to about 1.2 MPa),then as compaction increased to the very heavy class,they declined quickly(p≤0.01).In contrary,the lateral root biomassofmapleseedlingsshowed two trendsto increasing SPR; first,increased to the heavy compaction class(up to about 1.7 MPa),then with increasing soil penetration resistance to very heavy class,significantly decreased as quadratic curves(p≤0.05)(Fig.2b).
Duncan’s multiple range test showed signi ficant differences in total biomass variables among all SPR classes(Table 2),but did not detect any signi ficant differences in shoot biomass variables between uncompacted and very light SPR.Signi ficant differences were found the light,medium,high and highest SPR(Table 2).Further,it did not detect signi ficant differences in stem biomass variables among uncompacted,very light and high SPR,but did detect signi ficant differences among the light,medium,and highest SPR(Table 2).
In addition,Duncan’s test did not detect signi ficant differences in leaf biomass variables among uncompacted,very light and medium SPR,but signi ficant differences were found among the light,medium,high and highest SPR(Table 2).For main root biomass variables,no signi ficant differences were found between very light and medium SPR or between the high and highest,but significant differences were detected among the uncompacted,very light and light(Table 2).Lateral root biomass did not significantly vary among the SPR classes except between the very light and light.
Seedling architecture(allocation ratios)
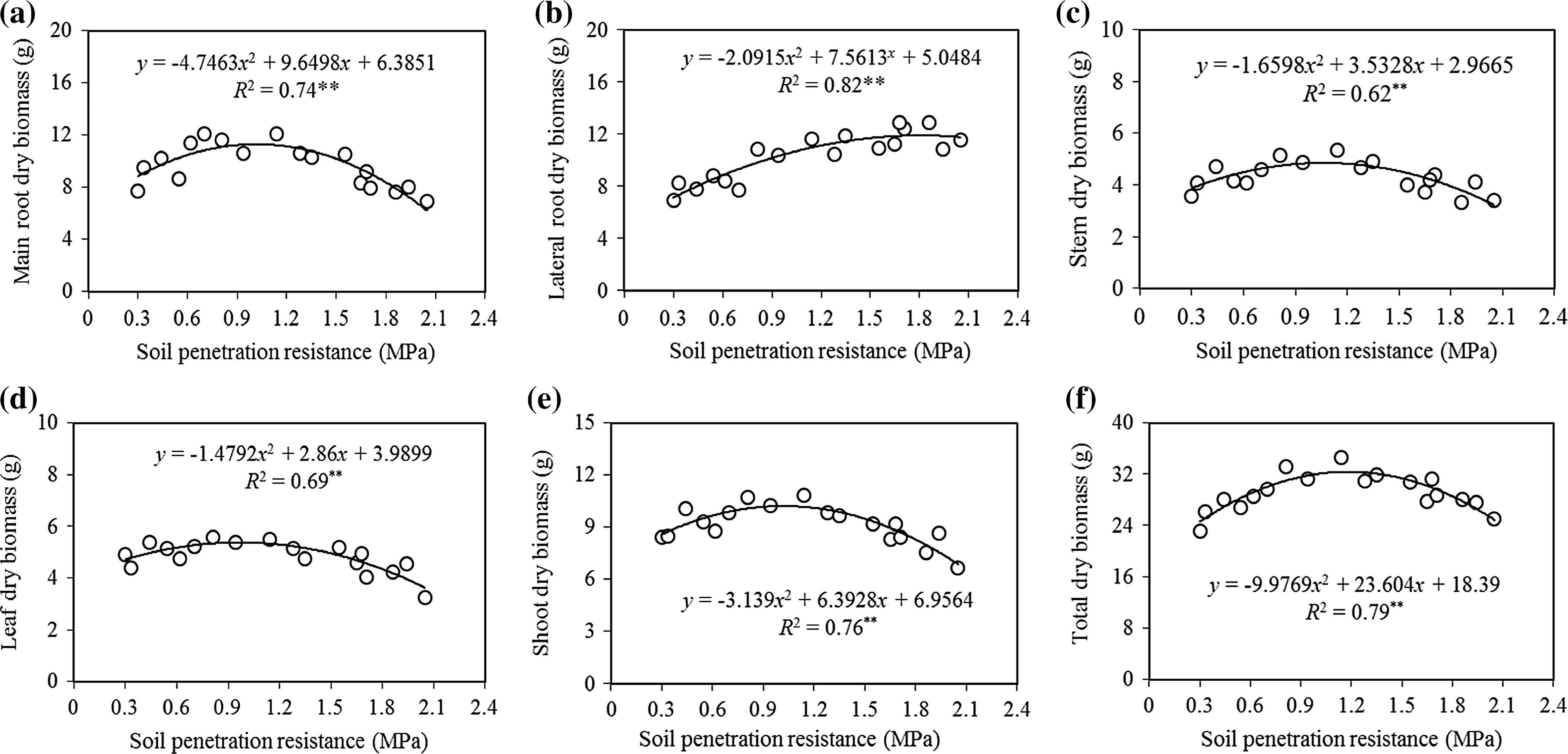
Fig.2 Relationship between SPR and biomass variables of Acer cappadocicum seedlings:main root dry(a),lateral root dry(b),stem dry(c),leaf dry(d),shoot dry(e),and total dry mass(f).The level of signi ficance of the model is indicated by*p<0.05,**p<0.01,ns not signi ficant(p≥0.05)
There was a signi ficant relationship between the speci fic stem length(SSL)and soil increasing penetration resistance(Fig.3a).In addition,SSL differed significantly among soil penetration resistance classes(Table 2).The speci fic root length(SRL)of the maple seedlings showed two trends in response to increasing SPR; first,it decreased as the soil penetration resistance class increased to the medium class(up to about 1.2 MPa),then as soil penetration resistance increased to the highest class,SRL increased significantly(p≤0.01)(Fig.1b).Also,SRL differed significantly amongthesoilpenetrationresistanceclassesexceptbetween the light and medium and between the high and highest soil penetration resistance classes(Table 2).
Despite signi ficant quadratic regression equations that described a curvilinear response curve of root mass ratio(RMR)with increasing SPR and with lowest RMR at uncompacted to light soil penetration resistance classes and the highest RMR at the medium to highest SPR.Root mass ratio ranged between 65 and 72%and differed significantly among the SPR classes except among the light,medium and high classes(Table 2,Fig.3c).
Despite signi ficant quadratic regression equations that indicate that a curvilinear response curve of the root to shoot ration(R/S)with increasing penetration resistance and with lowest R/S at uncompacted to light SPR and highest R/S at medium to highest SPR.The R/S ranged from 1.87 to 2.56 and differed significantly among the SPR classes except between the very light and light and between the medium and high SPR classes(Table 2,Fig.3d).
Signi ficant quadratic regression equations described the curve response of stem mass ratio(SMR)to increasing penetration resistance,with highest SMR at uncompacted to light SPR and lowest SMR at the medium to highest SPR.SMR ranged between 16 and 14%and did not differ significantly among the SPR classes except between the uncompacted and very light and between the medium and high SPR classes(Table 2,Fig.3e).There was a signi ficant relationship between the ratio of lateral root to total root biomass and the SPR as quadratic regression equation,which was indicated by lowest values at uncompacted to medium SPR and greatest values at the high and highest SPR(Fig.3f).The ratio of lateral root to total root biomass did not differ significantly among the SPR except between uncompacted and light and between the high and highest SPR(Table 2,Fig.3f).

Fig.3 Relationship between SPR and speci fic stem length(a),speci fic root length(b),root mass ratio(c),root/shoot ratio(R/S)(d),stem mass ratio(e),lateral root/main root dry mass(f),leaf mass ratio (g),and lateral root length/main root length(h)in Acer cappadocicum seedlings.The level of signi ficance of the model is indicated by*p<0.05,**p<0.01,ns not signi ficant(p≥0.05)
Despite a signi ficant quadratic regression equation of leaf mass ratio(LMR)with increasing soil penetration resistance,the greatest LMR was found for the uncompacted to medium SPR(Fig.3g).LMR,which ranged between 10 and 15%,differed significantly among the SPR classesexcept between the light and medium SPR(Table 2).The ratio of lateral to main root length ranged from 1.64 in the uncompacted class to 6.56 in the highest SPR and significantly increased as de fined by a quadratic regression equation(p≤0.001).The highest values for LMR were found at the medium to highest SPR(Fig.3h).The ratio of lateral to main root length did not differ significantly among the SPR classes except among the medium,high and highest classes(Table 2,Fig.3h).
Discussion
Inmostplants,growthanddevelopmentwilldecreaseorstop when roots encounter compacted soil and high mechanical resistance(Kozlowski 1999;Alameda and Villar 2009).When penetration resistance reaches 700–1500 kPa,root growth decreases by 50%and will stop at 4000 kPa(Siegel-Issem et al.2005).Of the few studies on ecological aspects on soil penetration resistance,most have been done on herbaceous or woody plants of agricultural importance,rather than on wild,woody species(Mosena and Dillenburg 2004;Bassett et al.2005;Pe´rez-Ra´mos et al.2010).
In the present greenhouse study onA.cappadocicumseedlings,we found that the relationships of the growth in stem length,stem diameter,and main root length to increasing soil penetration resistance followed negative quadratic curves,but growth in lateral root length did not.Similarly,numerous studies on woody species have shown that elongation of the main root was restricted by soil compaction(Gomez et al.2002;Mosena and Dillenburg 2004;Bassett et al.2005;Bulmer and Simpson 2005;Siegel-Issem et al.2005;Bejarano et al.2010;Pe´rez-Ra´mos et al.2010;Ampoorter et al.2011;Alameda and Villar 2012).Alameda and Villar(2012)found that mean seedling root length and root diameters were negatively correlated with SPR inF.angustifolia(i.e.,means for growth variables decreased as SPR increased).Soil compaction also had a negative effect on root growth and distribution(Bejarano et al.2010;Alameda and Villar 2012;Tracy et al.2012).
Many studies have shown that different trees vary in their growth responses to compacted or high-density soils depending on whether they are woody or herbaceous(Alameda and Villar 2009).Compaction is not always severe enough to obstruct root advancement and proliferation(Bejarano et al.2010;Pe´rez-Ra´mos et al.2010).For example,Zisa et al.(1980)found that root in filtration byPinus nigrain sandy loam soil was not significantly restricted by bulk density up to 1.60 g cm-3;in silt loam soil,however,root in filtration decreased distinctively at soil bulk density of 1.40 g cm-3.Small changes in bulk density as a consequence of soil compaction may also have a greater impact on the root biomass of evergreen tree species than on deciduous trees(Twum and Nii-Annang 2015).Likely,compaction may cause a particular physical condition in root expansion and development as seen in Fig.3.Deciduous trees were also shown in different studies to be more resistant to stress conditions such as soil compaction(Kormanek et al.2015).A decline in different properties of seedlings of other species as a result of soil compaction has been related to a reduction in NH4-N content(Fonseca et al.2004;Kormanek et al.2015),and the decline for evergreen trees was greater than for deciduous trees.Seedlings ofPinus taedacultivated in a greenhouse also grew more poorly in compacted soils(Hatchell et al.1970).
Evergreen trees are less plastic than deciduous trees to changesindifferentsituations(Valladaresetal.2000).Other studies of soil compaction have shown that the effects are in fluenced by the experimental conditions such as soil textureand water content(Smith etal.2001;Souch etal.2004).InPinusponderosa(Gomezetal.2002),thein fluenceofsoil compaction on saplings can be either negative or positive depending on soil texture and water content.
In the present study,the length of the lateral root was in fluenced by soil penetration resistance.Similarly,Bejarano et al.(2010)found that soil compaction in fluenced the development of root systems.Although we found that the main root length was reduced as SPR increased,the increase in lateral root length is not consistent with previous research(Zou et al.2001;Bulmer and Simpson 2005;Kabzems and Haeussler 2005;Siegel-Issem et al.2005;Blouin et al.2008;Skinner et al.2009;Pe´rez-Ra´mos et al.2010;Alameda and Villar 2012;Kormanek et al.2015).
We found that the relationships of all the biomass variables to increasing soil penetration resistance followed a negative quadratic curve except for lateral root biomass.Biomass variables such as total,stem,total root and main root of the maple seedlings showed two trends as SPR increased; first,they increased slowly up to the medium SPR class(about 1.2 MPa),then declined quickly as compaction increased to the highest treatment,agreeing with the results of Alameda and Villar(2009),who found that total dry biomass increased from lighter to moderate SPR levels and then decreased with greater compaction.Others have suggested that moderate soil compaction can create greater contact between the root and the substrate,enabling greater absorption ofwaterand nutrients(Arvidsson 1999;Gomez et al.2002;Mosena and Dillenburg 2004;Bassett et al.2005;Bejarano et al.2010;Pe´rez-Ra´mos et al.2010;Alameda and Villar 2012).Also,Mosena and Dillenburg(2004)demonstrated that shoot extension and overall plant mass increased with soil compaction,perhaps as a result of closer contact between roots and soil particles.In contrast,Alameda and Villar(2012)found that speci fic root length(SRL)was negatively associated with soil compaction inF.angustifoliaseedlings(64%decrease with a 25%increment in bulk density).
In the present study,SPR affected seedling growth variables ofA.cappadocicumseedlings above 1.2 MPa of SPR.However,as SPR increased to the medium level(about 1.2 MPa),total dry biomass increased(about 1.2 MPa).Alameda and Villar(2009)also studied the effects of soil compaction on woody plants growth from 0.1 to 1.0 MPa,whereas Bassett et al.(2005)studied maximum values of 1.2–1.4 MPa.In comparison,Blouin et al.(2008)proposed the critical value for mechanical impedance of conifer roots waslikelybelow2.5 MPa.Inthepresentstudy,however,the lateralrootbiomassofmapleseedlingsshowedtwotrendsto increasing SPR; first,lateral root biomass increased the high compaction treatment(about 1.7 MPa),then as from high compaction to highest,significantly decreased following a quadratic curves.
There was also a signi ficant relationship between speci fic stem length(SSL)and increasing SPR:SSL decreased as SPR increased.Speci fic root length of the maple seedlings also showed two trends to increasing SPR; first,it decreased until the moderate compaction treatment(about 1.2 MPa),then increased significantly as compaction increased from high to highest treatment.Previous studies have shown that the structural traits of stems and roots also change with soil penetration resistance(Arvidsson 1999;Kozlowski 1999;Gomez et al.2002;Mosena and Dillenburg 2004;Alameda and Villar 2009;Bejarano et al.2010).
Stem mass ratio(SMR)and leaf mass ratio(LMR)were also reduced with higher SPR;however,root mass ratio(RMR)increased with higher SPR.Arvidsson(1999)and Alameda and Villar(2009)found that soil compaction has a bell effect on biomass.
Seedling architecture variables ofAcer cappadocicumseedlings(i.e.,root/shoot ratio(R/S),lateral root/main root dry mass,and lateral root length/main root length)were also significantly in fluenced by SPR,increasing with increasing soil compaction.Root allocation patterns were found to change inQuercus coccifera,Q.suberandQ.fagineain responsetothesoilcompactionlevelsthatweused(Alameda and Villar 2009).Further,root/shoot ratio(R/S)typically increases with decreasing water availability(Gower et al.1992;Schenk and Jackson 2002),but after soil compaction,R/S responses change dramatically.In contrast,the root biomassratioforfour(Q.coccifera,Q.suber,Q.fagineaandRhamnus alaternus)of 17 species(23%)decreased as SPR increased(Alameda and Villar 2009).
Growth rate will decline when the environment is unsuitable,and soil compaction limits growth,shoot diameter and shoot dry mass(Mosena and Dillenburg 2004;Bejarano et al.2010;Alameda and Villar 2009;Kormanek et al.2015).For instance,unfavorable conditions for underground parts of plants often disrupt the balance between abscisic acid(ABA),cytokinins and gibberellins and before any direct harmful effect from an environmental change alters growth rate(Bassett et al.2005;Bejarano et al.2010;Pe´rez-Ra´mos et al.2010;Alameda and Villar 2012).Shorter roots with less biomass are found in very heavily compacted soils,similar to the results of other studies that have shown that denser soils cause a reduction in root growth(Conlin and Van den Driessche 1996;Bassett et al.2005;Bejarano et al.2010).A decrease in root penetration also limits nutrition access and water absorption(Blouin et al.2008).
The results of our study are consistent with previous findings that suggest increasing soil density limits root growth(Bejarano et al.2010)and reduces root penetration,which in turn may limit access to and absorption of nutrients and water(Blouin et al.2008).As a result,leaves undergo a water de ficit and decline in the rate of photosynthesis,reducing the size and growth of stems in seedlings and trees,and limiting seedling survival during droughts(Gomez et al.2002;Pe´rez-Ra´mos et al.2010).
Conclusion
In this study of growth variables ofAcer cappadocicumseedlings in a greenhouse in a loamy soil with a soil penetrationresistancefrom0.4to1.9 MPa,biomassvariableshad two responses:with increasing soil compaction to moderate level(to about 1.2 MPa),total dry biomass increased;with increasing soil compactionfrom moderate to very high level of compaction,total dry biomass decreased.Increasing soil strengthchangedbiomassallocationpatternstotherootsand shoots,thus changing seedling architecture.
AcknowledgementsThis paper is a one of the results of the research project No.93014726.The authors would like to acknowledge the financial support of the Iran National Science Foundation(INSF).We thank two anonymous reviewers for helpful comments to improve the manuscript.
References
Aca´cio V,Holmgren M,Jansen PA,Schrotter O(2007)Multiple recruitment limitation causes arrested succession in Mediterranean cork oak systems.Ecosystems 10:1220–1230
Alameda D,Villar R(2009)Moderate soil compaction:implications on growth and architecture in seedlings of 17 woody plant species.Soil Tillage Res 103:325–331
Alameda D,Villar R(2012)Linking root traits to plant physiology and growth inFraxinus angustifoliaVahl.seedlings under soil compaction conditions.Environ Exp Bot 79:49–57
Ampoorter E,De Frenne P,Hermy M,Verheyen K(2011)Effects of soil compaction on growth and survival of tree saplings:A metaanalysis.Basic Appl Ecol 12:394–402
Ampoorter E,Goris R,Cornelis WM,Verheyen K(2007)Impact of mechanized logging on compaction status of sandy forest soils.For Ecol Manag 241:162–174
Arvidsson J(1999)Nutrient uptake and growth of barley as affected by soil compaction.Plant Soil 208:9–19
Bassett IE,Simcock RC,Mitchell ND(2005)Consequences of soil compaction for seedling establishment:implications for natural regeneration and restoration.Aust Ecol 30:827–833
Bejarano MD,Villar R,Murillo AM,Quero JL(2010)Effects of soil compaction and light on growth ofQuercus pyrenaicaWilld.(Fagaceae)seedlings.Soil Tillage Res 110:108–114
Blouin VM,Schmidt MG,Bulmer CE,Krzic M(2008)Effects of compaction and water content on lodgepole pine seedling growth.For Ecol Manag 255:2444–2452
Brais S(2001)Persistence of soil compaction and effects on seedling growth in northwestern Quebec.SoilSciSoc Am J 65:1263–1271
Bulmer CE,Simpson DG(2005)Soil compaction and water content as factors affecting the growth of lodgepole pine seedlings on sandy clay loam soil.Can J Soil Sci 85:667–679
Cambi M,Certini G,Neri F,Marchi E(2015)The impact of heavy traffic on forest soils:a review.For Ecol Manag 338:124–138
Cochran PH,Brock T(1985)Soil compaction and initial height growth of planted ponderosa pine.USDA Forest Service.Research Note PSW 434,Portland,Oregon,1–2
Conlin TSS(1996)Soil compaction studies.FRDA Rep.No.264.Canadian Forest Service,Victoria,British Colombia,11–12
Conlin TSS,van den Driessche R(1996)Short-term effects of soil compaction on growth ofPinus contortaseedlings.Can J For Res 26:727–739
Corns GW(1988)Compaction by forestry equipment and effects on coniferous seedling growth on four soils in the Alberta foothills.Can J For Res 18:75–84
Etehadi Abari M,Majnounian M,Malekian A,Jourgholami M(2017)Effects of forest harvesting on runoff and sediment characteristics in the Hyrcanian forests,northern Iran.Eur J For Res 136:375–386
Fleming RL,Powers RF,Foster NW,Kranabetter JM,Scott DA,Ponder FJ,Berch S,Chapman WK,Kabzems RD,Ludovici KH,Morris DM,Page-Dumroese DS,Sanborn PT,Sanchez FG,Stone DM,Tiarks AE(2006)Effects of organic matter removal,soil compaction,and vegetation control on 5-year seedling performance:a regional comparison of long-term soil productivity sites.Can J For Res 36:529–550
Fonseca TF,Abreu CG,Parresol BR(2004)Soil compaction and chestnut ink disease.For Pathol 34:273–283
Gomez A,Powers RF,Singer MJ,Horwath WR(2002)Soil compaction effects on growth of young ponderosa pine following litter removal in California’s Sierra Nevada.Soil Sci Soc Am J 66:1334–1343
Gower ST,Vogt KA,Grier CC(1992)Carbon dynamics of rocky mountain Douglas- fir:in fluence of water and nutrient availability.Ecol Monogr 62:43–65
Greacen EL,Sands R(1980)Compaction of forest soils.Aust J Soil Res 18:163–189
Hatchell GE,Ralston CW,Foil RR(1970)Soil disturbances in logging.J For 68:772–775
Jourgholami M,Majnounian B,Etehadi Abari M(2014)Effects of tree-length timber skidding on soil compaction in the skid trail in Hyrcanian forests.For Syst 23:288–293
Kabzems R,Haeussler S(2005)Soil properties,aspen and white spruce responses five years after organic matter removal and compaction treatment.Can J For Res 35:2045–2055
Kormanek M,Głab T,Banach J,Szewczyk G(2015)Effects of soil bulk density on sessile oak Quercus petraea Liebl.Seedlings.Eur J For Res 134:969–979
Kozlowski TT(1999)Soil compaction and growth of woody plants.Scand J For Res 4:596–619
Misra RK,Gibbons AK(1996)Growth and morphology of eucalypt seedling roots in relation to soil strength arising from compaction.Plant Soil 182:1–11
Mosena M,Dillenburg LR(2004)Early growth of Brazilian pine(Araucaria angustifolia[Bertol.]Kuntze)in response to soil compaction and drought.Plant Soil 258:293–306
Pe´rez-Ra´mos IM,Go´mez-Aparicio LG,Villar R,Garcı´a LV,Maran˜o´n T(2010)Seedling growth and morphology of three oak species along field resource gradients and seed mass variation:a seedling age-dependent response.J Veg Sci 21:419–437
Sagheb-Talebi K,Sajedi T,Pourhashemi M(2014)Forests of Iran:a treasure from the past,a hope for the future.Springer,Berlin,pp 42–152
Schenk HJ,Jackson RB(2002)Rooting depths,lateral root spreads and below-ground/above-ground allometries of plants in waterlimited ecosystems.J Ecol 90:480–494
Siegel-Issem CM,Burger JA,Powers RF,Ponder F,Patterson SC(2005)Seedling root growth as a function of soil density and water content.Soil Sci Soc Am J 69:215–226
Skinner AK,Lunt ID,Spooner P,McIntyre S(2009)The effect of soil compaction on germination and early growth ofEucalyptus albensand an exotic annual grass.Aust Ecol 34:698–704
Smith KD,May PB,Moore GM(2001)The in fluence of compaction and soil strength on the establishment of four Australian landscape trees.J Arboric 27:1–7
Souch CA,Martin PJ,Stephens W,Spoor G(2004)Effects of soil compaction and mechanical damage at harvest on growth and biomass production of short rotation coppice willow.Plant Soil 263:173–182
Tracy SR,Black CR,Roberts JA,Sturrock C,Mairhofer S,Craigon J,Mooney SJ(2012)Quantifying the impact of soil compaction on root system architecture in tomato(Solanum lycopersicum)by X-ray micro-computed tomography.Ann Bot 110:511–519
Tubeileh A,Groleau-Renaud V,Plantureux S,Guckert A(2003)Effect of soil compaction on photosynthesis and carbon partitioning within a maize-soil system.Soil Tillage Res 71:151–161
Twum EKA,Nii-Annang S(2015)Impact of soil compaction on bulk density and root biomass ofQuercus petraeaL.at reclaimed post-lignite mining lite in Lusatia,Germany.Appl Environ Soil Sci 2015:1–5
Valladares FE,Martinez-Ferri L,Balaguer E,Perez-Corona Manrique E(2000)Low leaf-level response to light and nutrients in Mediterranean evergreen oaks:a conservative resource-use strategy?New Phytol 148:79–91
Verdu M,Garcıa-Fayos P(1996)Nucleation processes in a Mediterranean bird-dispersed plants.Funct Ecol 10:275–280
Wasterlund I(1985)Compaction of till soils and growth tests with Norway spruce and Scots pine.For Ecol Manag 11:171–189
Zisa RP,Halverson HG,Stout BB(1980)Establishment and early growth of conifers on compact soils in urban areas.Res.Pap.NE-451.Broomall,PA:U.S.Department of Agriculture,Forest Service,Northeastern Forest Experiment Station,2–7
Zou C,Penfold C,Sands R,Misra RK,Hudson I(2001)Effects of soil air- filled porosity,soil matric potential and soil strength on primary root growth of radiata pine seedlings.Plant Soil 236:105–115
 Journal of Forestry Research2018年3期
Journal of Forestry Research2018年3期
- Journal of Forestry Research的其它文章
- In vitro propagation of conifers using mature shoots
- ‘Relationships between relationships’in forest stands:intercepts and exponents analyses
- Effects of application date and rate of foliar-applied glyphosate on pine seedlings in Turkey
- Assaying the allelopathic effects of Eucalyptus camaldulensis in a nursery bed incorporated with leaf litter
- Variation and selection analysis of Pinus koraiensis clones in northeast China
- Non-aerated liquid culture promotes shoot organogenesis in Eucalyptus globulus Labill
