Effects of a nitric oxide donor and nitric oxide scavengers on Sorbus pohuashanensis embryo germination
Ling Yang•Dongyan Zhang•Hongnan Liu•Cheng Wei•Jianan Wang•Hailong Shen
Introduction
Mountain ash(Sorbus pohuashanensis),a small deciduous tree in the Rosaceae family,has very high ornamental and economic value(Yang and Shen 2011,2012).However,its poor ability to naturally regenerate and its sensitivity to interspeci fic competition have threatened itsgenetic diversity(Zheng 2007;Xu et al.2010).Therefore,effective measures should be taken to conserveS.pohuashanensisgermplasm.Traditionally,the main breeding strategy used forS.pohuashanensisis sexual reproduction(Shen 2006);there have been few studies on tissue culture(Yang et al.2010,2012),stem cuttings(Zhang 2015)and grafting(Tang et al.2014).Furthermore,the deep dormancy ofS.pohuashanensisseeds increases the dif ficulty of sexual reproduction(Yang et al.2008;Shen 2006).
Nitric oxide(NO)promotes the emergence of seeds from dormancy and regulates subsequent germination(Yang et al.2008;Wang et al.2015;Ma et al.2016).For example,NO has been shown to assist in breaking dormancy and improve the germination ofMalus pumilaandArabidopsis thalianaseeds(Krasuska et al.2014;Arc et al.2013).Moreover,pharmacological studies have provided additional information about NO donors and scavengers(Yang 2013).Sodium nitroprusside(SNP)and 2-phenyl-4,4,5,5-tetram-ethylimidazoline-1-oxyl-3 oxide(PTIO)or carboxy-PTIO potassium salt(cPTIO)have been widely used as a NO donor and NO scavenger.The germination percentage of seeds from many different species,such asArabidopsis thaliana,Hordeum vulgare,has been improved by application of SNP to seeds(Gniazdowska et al.2010).Although PTIO can inhibit germination of the embryo,the inhibition can be reversed by SNP(Bethke et al.2004;Yang 2013).Yang(2013)carried the study on the effects of SNP and PTIO on embryo germination ofS.pohuashanensisand found that the treatment of 5 mmol L-1SNP decreased the embryo germination and combined treatment with both SNP and PTIO increased the embryo germination percentage compared with the control.The effect of exogenous NO on embryo germination varies depending on the concentral;a low NO concentration promotes germination,whereas a high level of NO suppresses germination(Yang 2013).The ideal concentration of exogenous NO to promoteS.pohuashanensisembryo germination is unknown.The germination of plant seeds is a complex physiological process,and this process is con fined by many factors(Yin and Liu 2004).The mechanism by which NO removes seed dormancy and facilitates germination is also unclear and requires more intensive study.
In this study,zygotic embryos ofS.pohuashanensiswere treated with an NO donor(SNP)and scavenger(PTIO or cPTIO)to determine concentrations suitable to break embryo dormancy.The percentage germination after each treatment was calculated.Our results provided insight into the physiological effect and regulatory mechanism of exogenous NO onS.pohuashanensisembryo germination.
Materials and methods
Experimental materials
Plant material was collected on September 18,2011 from 10 wild,mature mother trees ofS.pohuashanensisgrown in the Fenghuang mountain forest farm,Shanhetun Forestry Bureau,Wuchang City,Heilongjiang Province,P.R.China.Fruits ofS.pohuashanensiswere treated with an arti ficial modulation water test(Deng et al.2010)to obtain seeds,which were then stored at 4°C.Seeds from mother trees 1 and 2 were used.Embryos were excised using the method described in Yang(2013).
Seed treatments
Seeds were fumigated with NO according to the method of Yang(2013).Brie fly,peeled embryos were put in a 500 mL beaker,and a smaller beaker containing 5 mL of freshly prepared SNP at different concentrations was placed beside the 500 mL beaker.The mouth of the large beaker was then sealed with plastic wrap.For embryos originating from mother tree 1,SNP was prepared at concentrations ranging from 1 to 5 mmol L-1SNP.For embryos originating from mother tree 2,SNP was prepared at concentrations ranging from 0.5 to 2 mmol L-1SNP.Small beakers containing 5 mL of water were used for the control.Peeled embryos were fumigated for 3 h at room temperature under natural light.
Embryo germination
Treated and untreated control embryos were placed in a 9-cm-diameter Petri dish(30 embryos per dish)at room temperature under a light intensity of 60 μmol m-2s-1.A germination bed production method was used,where the inside of each dish was covered with two layers of filter paper and 3 mL of distilled water.
Combination treatment of SNP and PTIO or cPTIO
Embryos were fumigated for 3 h with a 2 mmol L-1SNP solution using the method described above.Afterward,they were soaked with a filter paper wetted with 3 mL of either 300 μmol L-1PTIO or cPTIO.Germination conditions were as described above.Three treatment and control replicate experiments were carried out.
Data analysis and statistics
Percentage embryo germination was calculated according to the following formula:(number of germinated embryos/total number of embryos tested)×100.Mean germination speed,germination index,and germination potential were calculated according to the method of Yang and Zhang(2011).Microsoft(Redmond,WA,USA)Excel 2003 was used for data processing and generating plots.A DPS data processing system was used for analysis of variance,Duncan’s multiple comparison tests,and correlation analysis.Percentages were arcsinetransformed before statisticalanalysis.Alldata are reported as the means of three replicates±standard deviation(SD).
Results
Effects of SNP treatment on S.pohuashanensis embryo germination
Morphological effects of different reagents onS.pohuashanensisembryo germination are shown in Fig.1.Percentage germination of embryos 8 days after treatment with SNP at different concentrations significantly increased over that of the controls(p<0.05,Fig.2).The highest germination was 91.1%(Fig.2a)and 64.4%(Fig.2b)after treatment with 2 mmol L-1SNP.Percentage germination of embryos treated with 2 mmol L-1SNP was higher than with 0.5 mmol L-1SNP by 7.4%(Fig.2b).Embryos treated with 2 mmol L-1SNP began germinating on day 2,earlier than any other treatment,after which germination began on day 3 or 4(Fig.2b).In summary,these data revealed that,among the concentrations tested,treatment with 2 mmol L-1SNP was the most effective at promoting both the emergence from dormancy and the germination ofS.pohuashanensisembryos.

Fig.1 Eight-day-old seedlings of S.pohuashanensis that developed from a untreated dormant embryos or dormant embryos treated with b SNP,c PTIO,d SNP+PTIO,e cPTIO,or f SNP+cPTIO.Bars=5 mm(a,c,e)and 10 mm(b,d,f)

Fig.2 Effects of SNP pretreatment on S.pohuashanensis embryo germination percentage.Embryos were treated with solutions of a 1–5 mmol L-1SNP or b 0.5–2 mmol L-1SNP.CK is H2O.Values are the mean±SD of three replicates with 30 embryos each.Different letters represent statistically significantly different groups at p < 0.05 using Duncan’s multiple range test
Effects of SNP treatment on mean germination speed of S.pohuashanensis embryos
The effects of SNP treatments on mean germination speed ofS.pohuashanensisembryos are shown in Fig.3.Maximum mean germination speed was achieved by embryos treated with 2 mmol L-1SNP,with values of 0.13(Fig.3a)and 0.12(Fig.3b).At 8 days after treatment,the mean germination speed ofembryos treated with 2 mmol L-1SNP reached 0.08,which was statistically significantly faster than the control.Overall,mean germination speed increased with increasing concentrations of SNP in the range of 0.5–2 mmol L-1(Fig.3b).

Fig.3 Effects of SNP pretreatment on mean germination speed of S.pohuashanensis embryos.Embryos were treated with solutions of a 1–5 mmol L-1SNP or b 0.5–2 mmol L-1SNP.CK is H2O.Values are the ameanverage±SD of three replicates with 30 embryos each.Different letters represent statistically significantly different groups at p < 0.05 using Duncan’s multiple range test
Effects of SNP treatments on embryo germination index of S.pohuashanensis
Eight days following treatment,the embryo germination index was significantly higher than that of the control(p<0.05,Fig.4);the maximum germination index were significantly higher than that of the control,speci fically,5.1(Fig.4a,36.2%more than control)and 4.9(Fig.4b,62.0%morethan control).Theembryo germination index increased with increasing concentrations of SNP(0.5–2 mmol L-1).
Effects of SNP treatments on embryo germination potential of S.pohuashanensis
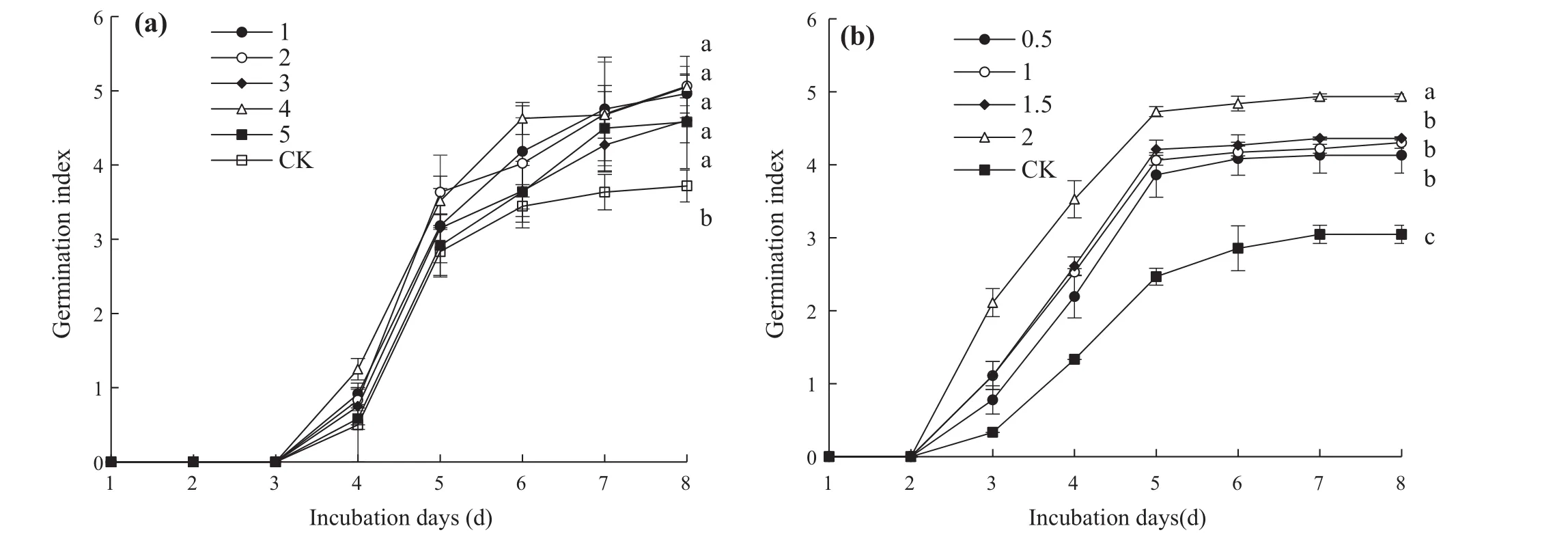
Fig.4 Effects of SNP pretreatment on S.pohuashanensis embryo germination index.Embryos were treated with solutionsof a 1–5 mmol L-1SNP or b 0.5–2 mmol L-1SNP.CK is H2O.Values are the mean±SD of three replicates with 30 embryos each.Different letters represent statistically significantly different groups at p < 0.05 using Duncan’s multiple range test
The effects of SNP treatments on embryo germination potential are shown in Fig.5.The germination potential ofS.pohuashanensisembryos after SNP treatments at concentrations from 1 to 5 mmol L-1initially increased and then decreased.The germination potential of embryos treated with 2 mmol L-1SNP peaked at 46.7,which was significantly higher than the control by 27.3%(Fig.5a).For embryos treated with SNP at concentrations ranging from 0.5 to 2 mmol L-1,the germination potential of embryos treated with 0.5 mmol L-1SNP was significantly higher than the control at 27.8 but not significantly different from the other treatments(p>0.05)(Fig.5b).
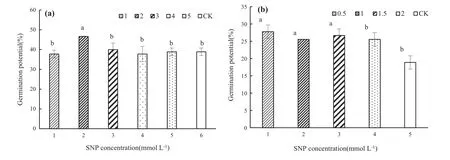
Fig.5 Effects of SNP pretreatment on S.pohuashanensis embryo germination potential.Embryos were treated with solutions ofa 1–5 mmol L-1SNP or b 0.5–2 mmol L-1SNP.CK is H2O. Values are the mean±SD of three replicates with 30 embryos each.Different letters represent statistically significantly different groups at p < 0.05 using Duncan’s multiple range test
Effects of SNP and NO scavengers on S.pohuashanensis embryo germination
The combined effects of SNP and NO scavengers on the percentage germination ofS.pohuashanensisembryos originating from mother tree 2 are shown in Fig.6.Eight days after treatment,germination of embryos treated with only SNP was 75.6%,significantly more than any other treatment(p<0.05),and 79.0%greater than the control.Both NO scavengers PTIO and cPTIO significantly inhibitedS.pohuashanensisembryo germination.The germination percentage 8 days following treatment with cPTIO was lowest at 42.2%.Treatment with PTIO resulted in only a slightly higher germination(43.3%),but not significantly different fom cPTIO,and germination after both treatments was significantly lower than the control(p<0.05).Combined treatment with either SNP and PTIO or SNP and cPTIO resulted in germination percentage statistically similar to the control and significantly higher than those aftertreatmentwith eithercPTIO orPTIO alone(p<0.05).These results demonstrated that NO scavengers can inhibitS.pohuashanensisembryo germination,thereby weakening the germination-promoting effect of low concentrations of SNP.
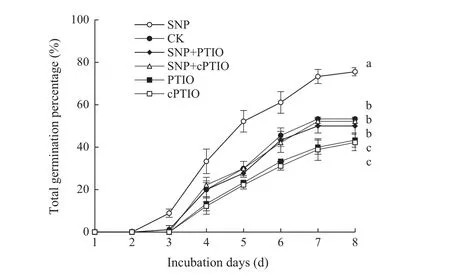
Fig.6 Effects of combined pretreatment with SNP and NO scavengers on S.pohuashanensis embryo germination.Embryos were treated with solutions of 2 mmol L-1SNP,SNP+300 μmol L-1 PTIO,SNP+300 μmol L-1cPTIO,PTIO,cPTIO,or water(control).CK is H2O.Values are the mean±SD of three replicates with 30 embryos each.Different letters represent statistically significantly different groups at p < 0.05 using Duncan’s multiple range test
Effects of SNP and NO scavengers on mean germination speed of S.pohuashanensis embryos
As shown in Fig.7,after the second day of the germination test,mean germination of embryos from mother tree 2 after treatment of SNP gradually increased and was significantly higher than other treatments.On day 5,mean germination speed of embryos from mother tree 2 reached the maximum(0.10).Finally,germination had significantly decreased by day 8 of the test;the final value was 0.09.The mean germinationspeedofothertreatmentsreachedthemaximumon day 6 and 7 of culture;however,it decreased by day 8.On day 8,the mean embryo germination speed in the combined treatment of SNP and NO scavenger was significantly higher than in the NO scavengertreatment,but the speed did differ significantly from the control(p>0.05).

Fig.7 Effects of combined pretreatment with SNP and NO scavengers on mean germination speed of S.pohuashanensis embryos.Embryoswere treated with solutionsof2 mmol L-1SNP,SNP+300 μmol L-1PTIO,SNP+300 μmol L-1cPTIO,PTIO,cPTIO,or water(control).CK is H2O.Values are the mean±SD of three replicates with 30 embryos each.Different letters represent statistically significantly different groups at p < 0.05 using Duncan’s Multiple Range Test
Effects of SNP and NO scavengers on S.pohuashanensis embryo germination index

Fig.8 Effects of combined pretreatment with SNP and NO scavengers on S.pohuashanensis embryo germination index.Embryos were treated with solutions of 2 mmol L-1 SNP,SNP+300 μmol L-1PTIO,SNP+300 μmol L-1cPTIO,PTIO,cPTIO,or water(control).CK is H2O.Values are the mean±SD of three replicates with 30 embryos each.Different letters represent statistically significantly different groups at p < 0.05 using Duncan’s multiple range test
As shown in Fig.8,the embryo germination index ofS.pohuashanensisembryos following treatment with 2 mmol L-1SNP was significantly improved over that of the control and other treatments(p<0.05).Eight days after treatment with only SNP,the embryo germination index reached a maximum value of 4.9,or 51.6%higher than the control.Eight days after treatment with either cPTIO or PTIO,the index reached the lowest values of 2.4 and 2.5,respectively(p<0.05),or 25%lower than the control.
Effects of SNP and NO scavengers on embryo germination potential of Sorbus pohuashanensis
As shown in Fig.9,the highest potential of 24.4 was reached after treatment with SNP.This value was signi ficantly greater than any other treatment and 29.4%higher than the control(p<0.05).Treatment with either NO scavenger,PTIO or cPTIO,significantly reduced embryo germination potential.The lowest potential of 12.2 resulted from treatment with cPTIO,which was 35.3%lower than the control.Germination potential resulting from combined treatment with SNP and either NO scavenger(18.9 with PTIO,and 22.2 with cPTIO)was significantly higher than treatment with either scavenger alone.However,the potential after combined treatment with SNP and cPTIO,but not PTIO,was significantly higher than the control by 17.7%(p<0.05).
Discussion and conclusion

Fig.9 Effects of combined pretreatment with SNP and NO scavengers on S.pohuashanensis embryo germination potential.Embryos were treated with solutions of 2 mmol L-1 SNP,SNP+300 μmol L-1PTIO,SNP+300 μmol L-1cPTIO,PTIO,cPTIO,or water(control).CK is H2O.Values are the mean±SD of three replicates with 30 embryos each.Different letters represent statistically significantly different groups at p < 0.05 using Duncan’s multiple range test
Plant seed germination is facilitated by a series of complex in vivo chemical reactions.NO is one of the smallest molecules found in nature,and a number of reports have indicated that it acts as a signaling molecule in the response of plants to biotic and abiotic stresses,programmed cell death,respiration,fruit maturation,leaf extension,stomata closure,senescence,seed germination,bloom regulation,root development,and hormone responses(Lamotte et al.2005;Sanz et al.2015;Chang et al.2016).Compared with other NO donors,the NO donor SNP has two advantages:(1)it can continuously produce NO over a relatively long period and(2)it is less expensive and more stable than other NO donors.The above results,which revealed that the germination ofS.pohuashanensisembryos increased remarkably after exogenous treatment with SNP,are consistent with reports that SNP breaks dormancy and facilitates germination inArabidopsisandM.pumila(Libourel et al.2006;Yang 2013).
Dormant seeds have higher levels of abscisic acid,and Yang Ling et al.found that pretreatment with SNP could weaken the inhibitory effect of abscisic acid on embryo germination(Yang 2013).We speculated that by producing the important signaling molecule NO,SNP is involved in the metabolic activity of abscisic acid.The function of NO and ethylene appears to be the mutual promotion and facilitation of seed germination(Gniazdowska et al.2007).Germination of most seeds is accompanied by an increase in ethylene,and indeed low concentrations of ethylene promote seed germination(Petruzzelli et al.2000).Gniazdowska et al.(2007)reported that NO stimulates ethylene synthesis after breaking embryo dormancy inM.pumila.Extending from these results,we wanted to further explain how NO promotes embryo germination.
Both PTIO and cPTIO have been used as NO scavengers(Cao and Reith 2002).Treatment with either NO scavenger significantly inhibited embryo germination compared with the control.However,germination following combined treatment with SNP and an NO scavenger was significantly higher than after treatment with either NO scavenger alone.These results are consistent with those reported by Yang(2013),which demonstrated a role for NO concentration in the maintenance and eventual releaseS.pohuashanensisembryos from dormancy.
Application of SNP had a concentration-dependent effect onS.pohuashanensisembryo germination.At low concentrations up to 2 mmol L-1,embryo germination increased; above this concentration, germination decreased.In addition,because not enough embryos were available from mother tree 1 embryos for further experiments,we used embryos from mother tree 2.The same results were obtained in two mother tree embryos,which indicates that the positive effects ofS.pohuashanensisembryo under the treatment of 2 mmol L-1were universal.It is likely that different concentrations of exogenous NO donor produced different concentrations of free NO.Additionally,exogenous NO concentration may have in fluenced the synthesis and catabolism of endogenous NO.This in fluence may result in changes in NO concentration inS.pohuashanensisembryos,thereby affecting endogenous hormones and enzymes involved in germination(Jia and Zhang 2014).Therefore,NO might initiate a series of changes that promote embryo germination.Exogenous NO in fluences endogenous NO synthesis and decomposition within embryos,but it is not known how exogenous NO regulates metabolism in the embryo,via signal transduction,to effect changes in the intracellular concentrations of endogenous hormones or enzymes.Further studies will thus increase our understanding of the mechanism of exogenous NO regulation ofS.pohuashanensisembryo germination.
In conclusion,this is a comprehensive report on effects of nitric oxide donor and nitric oxide scavengers onS.pohuashanensisembryo germination.The exogenous NO concentration plays an important role in releasing and maintaining embryo dormancy inS.pohuashanensis.Treatment with 2 mmol L-1SNP had the greatest effect on embryo germination and was the most effective at increasing the uniformity and speed of embryo germination among SNP treatments.Treatment with excessive concentrations of SNP did not appear to enhance embryo germination,and low concentrations of exogenous NO promoted germination.Treatment ofS.pohuashanensisembryos with SNP and either of the NO scavengers counteracted the germination-promoting effects of SNP acting alone.
AcknowledgementsWe thank all the colleagues in our lab for constructive discussion and technical support.
References
Cao BJ,Reith MEA(2002)Nitric oxide scavenger carboxy-PTIO potentiates the inhibition of dopamine uptake by nitric oxide donors.Eur J Pharmacol 448(1):27–30
Chang QS,Lx Zhang,Yang W,Ss Zhou,Huang QZ,Lu¨FJ,Huang Y,Ge SH,Zhang TM(2016)Effects of exogenous nitric oxide on antioxidant activity and photosynthetic characteristics of Prunella vulgaris seedlings under NaCl stress.Acta Pratacult Sinica 25(7):121–130
Deng W,Qing XG,Yang Y(2010)Effects of applying organic fertilizer on rice lodging resistance and yield.J Agric Sci Technol 11(2):98–101
Gniazdowska A,Dobrzyn´ska U,Baban´czyk T,Bogatek R(2007)Breaking the apple embryo dormancy by nitric oxide involves the stimulation of ethylene production.Planta 225(4):1051–1057
Gniazdowska A,Krasuska U,Czajkowska K,Bogatek R(2010)Nitric oxide,hydrogen cyanide and ethylene are required in the control of germination and undisturbed development of young apple seedlings.Plant Growth Regul 61(1):75–84
Jia HF,Zhang HY(2014)Effects of exogenous nitric oxide on seed germination and seedling physiological characteristics of Isatis indigotica underNaClstress.Chin TraditHerb Drugs 45(1):118–124
Krasuska U,Ciacka K,Bogatek R,Gniazdowska A(2014)Polyamines and nitric oxide link in regulation of dormancy removal and germination of apple(Malus domestica,Borkh.)embryos.J Plant Growth Regul 33(3):590–601
Lamotte O,Courtois C,Barnavon L,Pugin A,Wendehenne D(2005)Nitric oxide in plants:the biosynthesis and cell signalling properties of a fascinating molecule.Planta 221(1):1–4
Libourel IGL,Bethke PC,Michele RD,Jones RL(2006)Nitric oxide gas stimulates germination of dormant Arabidopsis seeds:use of a flow-through apparatus for delivery of nitric oxide.Planta 223(4):813–820
Ma Z,Marsolais F,Bykova NV,Igamberdiev AU(2016)Nitric oxide and reactive oxygen species mediate metabolic changes in barley seed embryo during Germination.Front Plant Sci 7(126):1–13
Petruzzelli L,Coraggio I,Leubner-Metzger G(2000)Ethylene promotes ethylene biosynthesis during pea seed germination by positive feedback regulation of 1-aminocyclo-propane-1-carboxylic acid oxidase.Planta 211(1):144–149
Sanz L,Albertos P,Mateos I,Sa´nchez-Vicente I,Lecho´n T,Ferna´ndez-Marcos M,Lorenzo O(2015)Nitric oxide(NO)and phytohormones crosstalk during early plant development.J Exp Bot 66(10):2857–2868
Shen H(2006)In fluencing factors to seed dormancy and germination characteristics ofSorbus pohuashanensis.Scientia Silvae Sinicae 42(10):133–138
Tang WQ,Zhang YX,Liu BH(2014)Grafting survival rate in different strains ofSorbusspp.Prot For Sci Technol 7:14–15
Wang P,Zhu JK,Lang Z(2015)Nitric oxide suppresses the inhibitory effect of abscisic acid on seed germination by S-nitrosylation of SnRK2 proteins.Plant Signal Behav 10(6):1–3
Xu JW,Shen HL,Zhang XL,Zhang P,Huang J(2010)Sorbus pohuashanensisseed dispersal and germination and their relationships with population natural regeneration.Chin J Appl Ecol 21(10):2536–2544
Yang L (2013)Effects of exogenous nitric oxide on embryo germination and ROS accumulation in seedling growth initial stage ofSorbuspohuashanensis.Scientia Silvae Sinicae 49(6):60–67
Yang L,Shen HL(2011)Effect of electrostatic field on seed germination and seedling growth ofSorbus pohuashanesis.J For Res 22(1):27–34
Yang L,Zhang P(2011)Experimental course of tree seeds.Northeast Forestry University Press,Harbin,pp 85–87
Yang L,Liu CP,Shen HL(2008)Effect of cold strati fication durations and germination temperatures on seed germination ofSorbus Pohuashanensis.Seed 27(10):20–22
Yang L,Shen HL,Liu CP,Zhai XJ(2010)Somatic embryogenesis from mature zygotic embryo explants ofSorbus pohuashanensisHedl.Bull Bot Res 30(2):174–179
Yin H,Liu Q(2004)Advances in studies on molecular biology of seed dormancy and germination.Chin Bull Bot 21(2):156–163
Zhang YY(2015)Study on the Technology of Microcutting and Rooting inSorbus aucupariaL.Planting in Harbin.Journal of Anhui.Agric Sci 43(22):128–131
 Journal of Forestry Research2018年3期
Journal of Forestry Research2018年3期
- Journal of Forestry Research的其它文章
- In vitro propagation of conifers using mature shoots
- ‘Relationships between relationships’in forest stands:intercepts and exponents analyses
- Effects of application date and rate of foliar-applied glyphosate on pine seedlings in Turkey
- Assaying the allelopathic effects of Eucalyptus camaldulensis in a nursery bed incorporated with leaf litter
- Effects of soil compaction on growth variables in Cappadocian maple(Acer cappadocicum)seedlings
- Variation and selection analysis of Pinus koraiensis clones in northeast China
