Bitumen Recovery from Indonesian Oil Sands Using ASP (Alkali, Surfactant and Polymer) Agent
Li Wenshen; Guo Xiaowen; Liu Jie
(College of Chemistry, Chemical Engineering and Environmental Engineering, Liaoning Shihua University,Fushun 113001)
1 Introduction
With the prodigious development of the world economy and the shortage of global petroleum resources, the unconventional petroleum resources, such as oil sands bitumen,extra-heavy oil and shale oil, have attracted more and more attention from researchers. As an important unconventional petroleum resource, abundant oil sands reserves have been found in many countries and regions. The main components of oil sands include sand grains, bitumen residues and water. Especially with respect to the bitumen, it is chemically similar to conventional heavy oil and can be converted into light oil products. The extraction of bitumen from oil sands was commercialized early in Canada, and oil sands are one of the promising resources serving as the long-range alternatives to petroleum.
At present, the methods for separation of bitumen from oil sands involve hot water extraction[1], solvent extraction[2],and retorting[3]. The hot water extraction process is the only commerciallized process for recovering bitumen from oil sands. In the process of separating bitumen from oil sands by hot water extraction, it is necessary to add an alkaline agent and a collector (usually light oil) into the water to maintain the slurry alkaline, which can make bitumen desorbed from the grain sands. Alkaline additives include sodium carbonate, sodium phosphate, sodium hydroxide, etc. It was found that bitumen could be completely liberated from the water-wet model oil sands to form aerated droplets in slurry with water. However, the aerated bitumen droplets were unable to float due to the attachment of sand particles on the surface of the bitumen droplets. It was helpful for sand particles to be separated from the bitumen droplets through a moderate addition of sodium hydroxide, resulting in bitumen floatation[4].
Al-Otoom, et al.[5-6]studied the separation of the Jordanian oil sands by utilizing a fluidized-bed floatation. It was found that the bitumen content in the froth reached 79% under the conditions of adding 0.2 g of NaOH/L at 80 °C for 30 min without any additive. Subsequent study showed that the recovery of bitumen from the Jordanian oil sands reached 86% via the addition of 0.35% of a special cut of light cycle oil used as a collector. The addition of diluent, such as diesel, kerosene, and FCC cycle oil,can reduce the viscosity of bitumen and enhance bitumen separation from sand grains. Schramm, et al.[7]found that the addition of kerosene significantly improved the bitumen recovery from 8% (without kerosene addition) to 79% with the addition of 2% of kerosene at 25 °C for an average-grade ore. Furthermore, the addition of methylisobutyl carbinol could facilitate the bitumen aeration and the bitumen recovery was increased from 79% to 98%.
The addition of alkaline agent can improve bitumen recovery because the surfactant produced by its reaction with some components in oil sands can reduce the interfacial tension, which is similar to the role of alkaline flooding as evidenced by the ASP (alkaline, surfactant and polymer) flooding used in the oil exploration process. In the ASP flooding, three kinds of substances play different roles. The polymer can increase the viscosity of injection water, reduce the ratio of water to oil and expand the flooding sweep[8-9]. The alkaline agent in ASP flooding can react with petroleum acids to produce surface-active substances,resulting in low interfacial tension between phases[10-11]. At the same time the cheap alkali can also reduce greatly the adsorption loss of the expensive chemicals[12]. The addition of surfactant not only can reduce the interfacial tension but can also produce some synergistic effect by compositing a variety of chemical agents, leading to the changing wettability of the rock and the low interfacial tension at very low concentrations of chemical agents.
Although the processing of oil sands is different from the oil exploration, both belong to the separation of oil and solid in essence. Therefore, the application of the ASP agent to the separation of oil sands is worth trying. The oil sands reserve in Indonesia’s Buton Island is rich and has high exploitation value because of its high bitumen content. However, despite the more researches that were focused on the cracking performance of Indonesian oil sands[13-17], the report on the separation of Indonesian oil sands via hot water extraction was limited.
In this study, the hot water extraction of Indonesian oil sands with the addition of ASP agent was conducted,and special attention was paid to the effect of the mixing temperature, the mass ratio of ASP agent to oil sands, the mixing time, and the floatation time on bitumen recovery.Meanwhile, the research on how to decrease the residual bitumen content in tailings was also carried out in order to reduce the environmental pollution caused by tailing.
2 Experimental
2.1 Experiment reagent and apparatus
Reagents used in experiments: The ASP agent was self-developed. Toluene (AR) and ethyl alcohol (AR) were purchased from the Sinopharm Chemical Reagent Co., Ltd.
Experiment apparatus included a scanning electron microscope (SU8010), a laser particle size analyzer (Mastersizer 2000), and an electronic balance (FA2104N, with a precision of 0.0001g).
2.2 Oil sands
Indonesia’s Buton Island oil sands sample was obtained from the Jinbao Mining Co., Ltd. The sample with a particle diameter of 1—5 mm after being crushed at room temperature was selected as the feedstock. The Dean-Stark toluene extraction method was employed to determine the content of bitumen and water in Indonesian oil sands, and the content of water, bitumen, and residual sand was 1.73%, 30.98%, and 67.29%, respectively. The bitumen after being extracted from the solids was dried for 24 hours under vacuum to remove the solvent prior to being prepared for further analysis.
The hydrocarbon group analysis (ASTM D4124—2009)and distillation range determination (ASTM D2892 and ASTM D5236) of the extracted bitumen were carried out,with the results shown in Table 1 and Figure 1. The initial boiling point was 245 °C, the content of the distillate recovered below 500 °C was 34.74%, and the content of resins and asphaltenes reached up to 62.77%.

Table 1 Group analysis of Indonesian oil sands bitumen
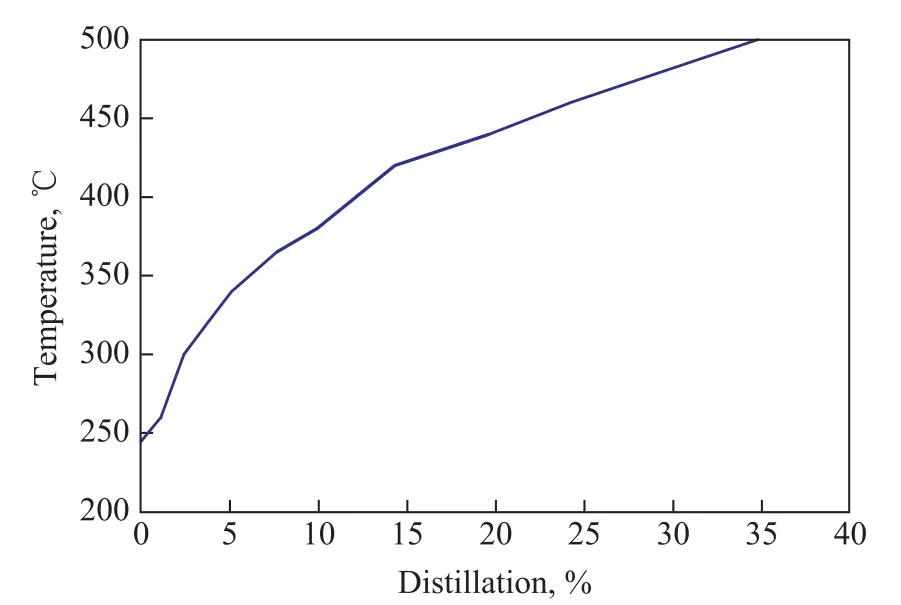
Figure 1 True boiling point distillation curve of bitumen
The composition of the Indonesian oil sands tailings,which was provided by Xi’an Jinshui Mining Science and Technology Development Co., Ltd., is listed in Table 2.Calcium carbonate was the main component of tailings,and its content reached up to 77.67%, while the content of silica dioxide was near 10%. The mineral composition was different from that of the oil sands in the Canada,United States, Jordan and other countries reported in the literatures[5,18].
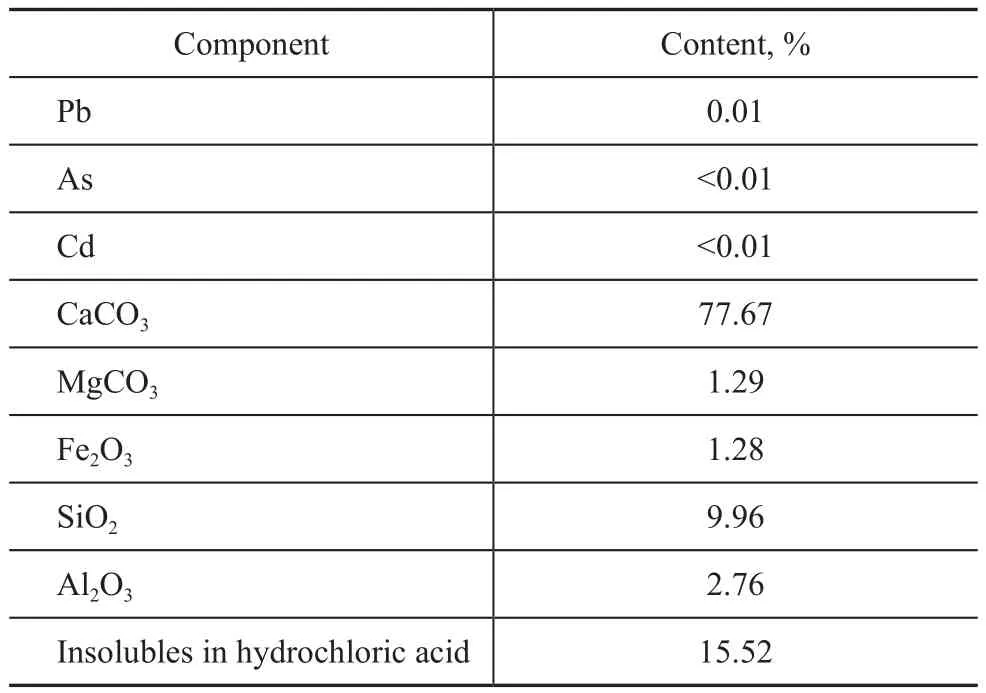
Table 2 Mineral composition of Indonesian oil sands tailings
2.3 Experimental procedure
Alkali (sodium hydroxide), surfactant (sodium dodecyl benzene sulfonate), and polymer (polyacryamide) were dissolved to form an aqueous solution, and the mass fraction of three components was all in the range of 0.05%―2% in the solution. The viscosity and density of the ASP agent at 20 ºC was 62.3 mm2/s and 1.35 g/mL,respectively. A sample of 200 g of oil sands was mixed with a certain proportion of ASP agent in a mixer, and the mixture was mechanically stirred at a constant rate and temperature. After a de finite period of time, the mixture was transferred to a floatation equipment, while a certain proportion of ASP agent serving as a diluent was added into the mixture at a constant temperature, and then the mixture was subject to floatation for a certain time. Bitumen was collected from the top and the tailings were collected from the bottom of the equipment after the floatation. The collected tailings were filtrated under vacuum and then dried in a vacuum oven for 24 h at 80 °C. The bitumen content in the dried tailings sample was determined by the Dean-Stark toluene extraction method. The bitumen recovery was calculated based on the bitumen content in the tailings.
During the experiment, several parameters were investigated to determine the maximum bitumen recovery of Indonesian oil sands. These parameters involved the mass ratio of ASP agent to oil sands, the mixing temperature,the mixing time, and the floatation time.
3 Results and Discussion
3.1 Effect of the mass ratio of ASP agent to oil sands
The effect of the mass ratio of ASP agent to oil sands on bitumen recovery was investigated at a mixing temperature of 80 ºC, a mixing time of 40 min, and a floatation time of 10 min, with the results shown in Figure 2.
During the experiment, we attempted the hot water extraction of Indonesian oil sands using only NaOH, and the bitumen recovery was only 46.2%, while the bitumen foam quality was very poor. However, it can be seen from Figure 2 that the bitumen recovery significantly increased by using the ASP agent method, and the bitumen recovery reached up to 86%, which was attributed to the comprehensive action of each component of the ASP agent. On one hand, the presence of sodium dodecyl benzene sulfonate in ASP agent, as a surfactant, could further reduce the interfacial tension between phases. On the other hand, polyacryamide could increase the viscosity of water and expand the swept volume. The bitumen recovery increased from 75.6% to 86.4% with the mass ratio of ASP agent to oil sands increasing from 2:10 to 4:10. Less bitumen would be separated from sands at a mass ratio of ASP agent to oil sands equating to lower than 2:10,because of no formation of slurry in the mixture of ASP agent and oil sands. In addition, the bitumen recovery decreased slightly when the mass ratio of ASP agent to oil sands increased to above 5:10, which could be attributed to the reduction of shearing force resulted from a too thin mixture at a large dosage of ASP agent, leading to the poor slurry effect. Therefore, a suitable mass ratio of ASP agent to oil sands was 4:10.
3.2 Effect of mixing temperature
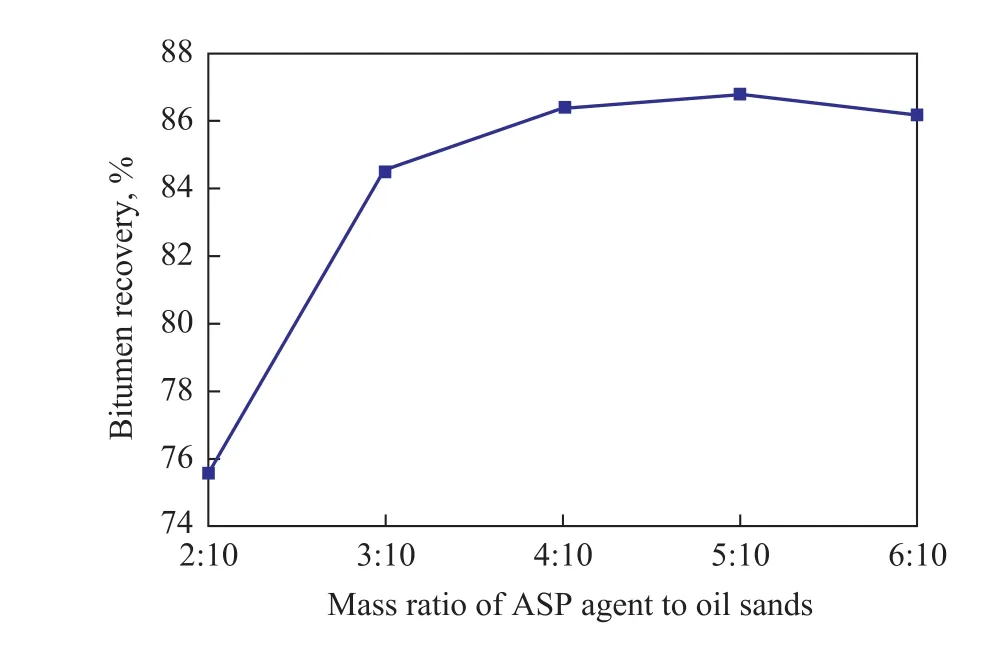
Figure 2 Effect of mass ratio of ASP agent to oil sands on bitumen recovery
The effect of mixing temperature on bitumen recovery was studied at a mass ratio of ASP agent to oil sands of 4:10, a mixing time of 40 min, and a floatation time of 10 min. The bitumen was very viscous at below 40 °C,leading to insuf ficient mixing of oil sands and ASP agent.Figure 3 shows the effect of temperature on the bitumen recovery. It was obvious that the temperature had a significant effect on bitumen recovery. Bitumen recovery increased from 20.9% at 50 °C to 86.4% at 80 °C.Moreover, the bitumen recovery varied slightly at a temperature exceeding 80 °C. This phenomenon could be explained by the fact that the bitumen viscosity decreased with an increasing temperature, which would facilitate the bitumen separation and subsequently further increase the bitumen recovery. The suitable mixing temperature was specified as 80 °C.
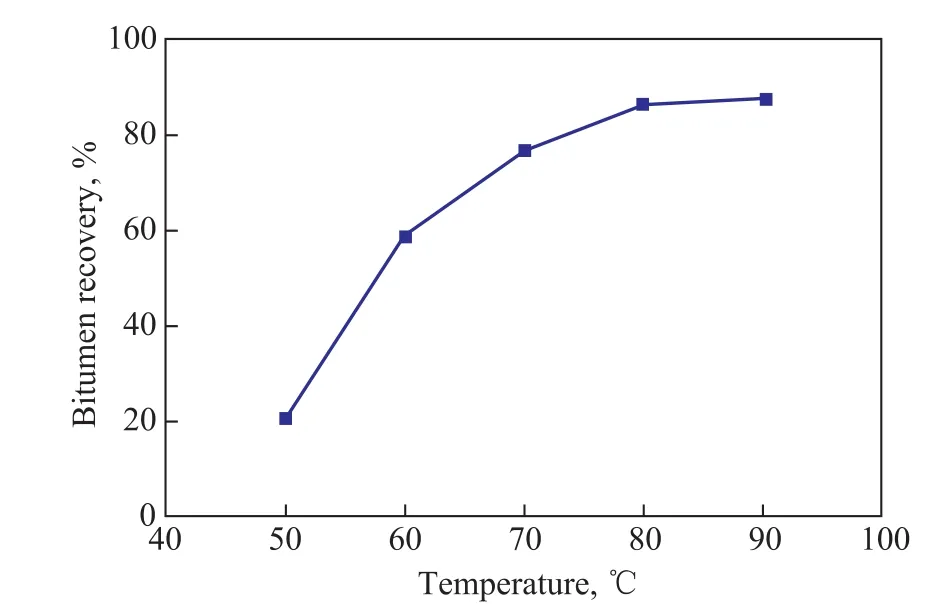
Figure 3 Effect of temperature on bitumen recovery
3.3 Effect of mixing time
The effect of mixing time on bitumen recovery was investigated at a mass ratio of ASP agent to oil sands of 4:10, a mixing temperature of 80 ºC, and a floatation time of 10 min. The results are shown in Figure 4.
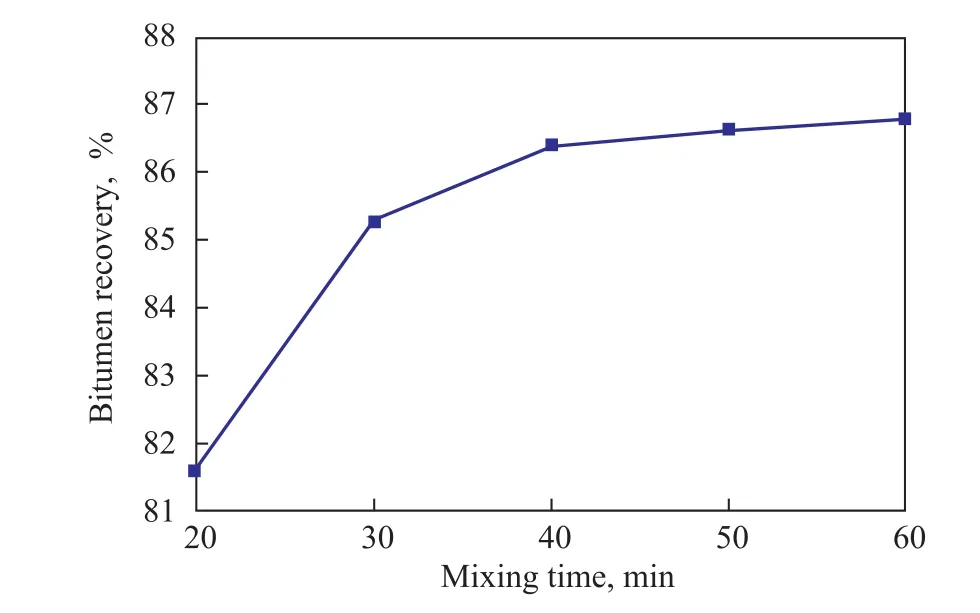
Figure 4 Effect of mixing time on bitumen recovery
As shown in Figure 4, the bitumen recovery increased from 81.6% to 86.4% with the mixing time extending from 20 min to 40 min. Further extension of the mixing time would not obviously contribute to an improved bitumen recovery, and it increased slightly when mixing time was greater than 40 min. A short mixing time would result in an insuf ficient contact of ASP agent with oil sands,which was not favorable to a better separation effect.However, a too long mixing time was not economic for the separation of oil sands. The optimal mixing time was determined as 40 min.
3.4 Effect of floatation time
Floatation is very important to realize the separation of bitumen foams at the top of the separation device. During the floatation process, air was injected and dispersed throughout the slurry. The effect of floatation time on bitumen recovery was investigated at a mass ratio of ASP agent to oil sands of 4:10, a mixing temperature of 80 ºC, and a mixing time of 40 min, with the results presented in Figure 5.
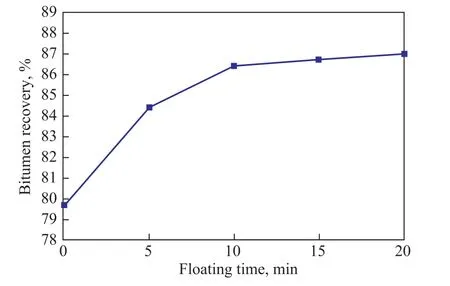
Figure 5 Effect of floatation time on bitumen recovery
It can be seen from Figure 5 that the bitumen recovery increased from 79.7% (without floatation) to 86.4% at a floatation time of 10 min. In addition, the bitumen recovery increased slightly when the floatation time was longer than 10 min, indicating that most bitumen could be separated from tailings within 10 min, so the optimal floatation time was set at 10 min.
Judging from the above results and discussion, the optimal process conditions for the hot water extraction using ASP agent covered a mass ratio of ASP agent to oil sands of 4:10, a mixing temperature of 80 °C, a mixing time of 40 min, and a floatation time of 10 min.
3.5 Reprocessing of tailings
Most bitumen can be separated by hot water extraction using the ASP agent, but the tailings cannot meet the disposal requirements because of its high bitumen content,so it is necessary to reprocess the tailings. The relationship between the bitumen content in tailings and different particle sizes was determined, with the results presented in Figure 6.
It can be seen from Figure 6 that the bitumen content reduced with a decreasing particle size of tailings. The bitumen content was 13.32% at a particle size of above 150 μm, while the bitumen content was only 1.68% at a particle size of less than 75 μm. So it will be an effective way to reduce bitumen content in tailings by reducing the particle sizes of tailings.
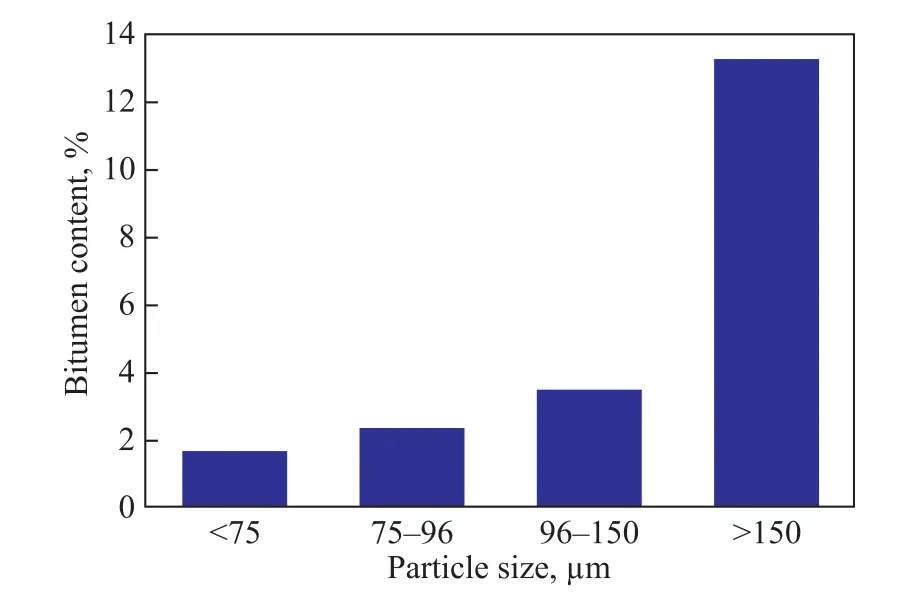
Figure 6 Bitumen content in tailings depending upon different particle size
On the basis of the above finding, the dried tailings sample was milled by a mortar, and the separation of the milled tailings was carried out using the ASP agent-assisted hot water method under the above optimal operating conditions. The particle size distribution of the milled tailings and the bitumen content in tailings depending on the milling time are illustrated in Figure 7.
The particle diameter became smaller with the increase of milling time. For example, tailings with particle diameter larger than 150 μm decreased from 26.37% to 0.88%,and tailings particle smaller than 75 μm increased from 18.34% to 87.63% when the milling time was extended from 0 min to 30 min. Accordingly, the residual bitumen content in tailings decreased from 5.47% at zero milling time to 1.25% at a milling time of 30 min.
Figure 8 shows the view of the tailings before and after reprocessing. It can be seen from the SEM image that the particle size of tailings before milling was much bigger than that after milling. The color of tailings before reprocessing was darker than that after reprocessing since there was more dark bitumen contained in the sand particles.Milling of tailings could significantly reduce the residual bitumen content in tailings.
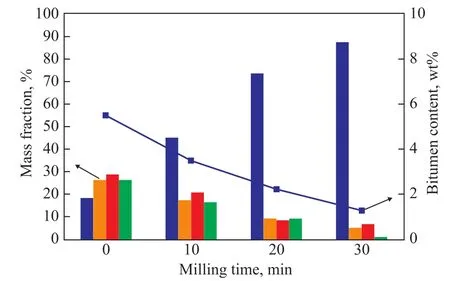
Figure 7 Particle size distribution and bitumen content of milled tailings treated at different milling time

Figure 8 View of the tailings: (A) tailings before milling,(B) tailings after milling, (C) SEM image of photo A and (D)SEM image of photo B
4 Conclusions
The hot water extraction using the ASP agent was proposed to recover bitumen from Indonesian oil sands. Experimental results showed that the use of ASP agent could separate bitumen from the Indonesian oil sands effectively, achieving more than 86% of bitumen recovery. The optimal recovery conditions covered: a mass ratio of ASP agent to oil sands of 4:10, a mixing temperature of 80 °C,a mixing time of 40 min, and a floatation time of 10 min.The residual bitumen content of tailings decreased with a decreasing particle size. Reducing the particle size of tailings by milling was an effective way to reduce the bitumen content in the tailings.
[1] Luo M, Geng A S, Liao Z W. Technology status of extracting bitumen from oil sand using hot alkaline water[J].Bulletin of Mineralogy, Petrology and Geochemist, 2011,30(1): 113–118 (in Chinese)
[2] Zhang K H, Bin Y F. Study on the process conditions of extracting bitumen from Indonesia oil sand with organic solvent [J]. Petroleum Processing and Petrochemicals,2008, 39(6): 63–66 (in Chinese)
[3] Pakdel H, Roy C. Recovery of bitumen by vacuum pyrolysis of Alberta tar sands[J]. Energy & Fuels, 2003, 17(5):1145–1152
[4] Qi D, Chung K H. Hot water extraction process mechanism using model oil sands[J]. Fuel, 1996, 75(2): 220–226
[5] Al-Otoom A, Allawzi M, Al-Harahsheh A M, et al. A parametric study on the factors affecting the froth floatation of Jordanian tar sand utilizing a fluidized bed floatator[J].Energy, 2009, 34 (9): 1310–1314
[6] Al-Otoom A, Allawzi M, Al-Omari N, et al. Bitumen recovery from Jordanian oil sand by froth flotation using petroleum cycles oil cuts[J]. Energy, 2010, 35(10): 4217–4225
[7] Schramm L L, Stasiuk E N, Yarranton H, et al. Temperature effects from the conditioning and flotation of bitumen from oil sands in terms of oil recovery and physical properties[J]. Journal of Canadian Petroleum Technology, 2002,42 (8): 55–61
[8] Liu S. Alkaline surfactant polymer enhanced oil recovery process[D]. Gradworks 2008
[9] Hirasaki G J, Miller C A, Puerto M. Recent advances in surfactant EOR[J]. SPE Journal, 2008, 16 (4): 889–907
[10] Al-Sahhaf T, Suttar A, Elkamel A. Producing ultralow interfacial tension at the oil/water interface[J]. Petroleum Science & Technology, 2002, 20(7/8): 773–788
[11] Gao S, Li H, Li H. Laboratory investigation of combination of alkali/ surfactant/ polymer technology for Daqing EOR[J]. SPE Reservoir Engineering, 1995, 10 (3): 194–197
[12] Krumrine P H, Falcone J S, Campbell T C. Surfactant flooding 1: the effect of alkaline additives on IFT, surfactant adsorption, and recovery ef ficiency[J]. Society of Petroleum Engineers Journal, 1982, 22 (4): 503–513.
[13] Wang Q, Jia C, Ge J, et al.1H NMR and13C NMR studies of oil from pyrolysis of Indonesian oil sands [J]. Energy &Fuel, 2016, 30 (3): 2478–2491
[14] Ma Y, Li S. The pyrolysis, extraction and kinetics of Buton oil sand bitumen[J]. Fuel Processing Technology, 2012,100: 11–15
[15] Ma Y, Li S. Study of the characteristics and kinetics of oil sand pyrolysis[J]. Energy & Fuel, 2010, 24 (3): 1844–1847
[16] Liu P, Zhu M, Zhang Z, et al. Thermogravimetric studies of characteristics and kinetics of pyrolysis of Buton oil sand[J]. Energy Procedia, 2014, 61: 2741–2744
[17] Liu P, Zhu M, Zhang Z, et al. Pyrolysis of an Indonesian oil sand in a thermogravimetric analyser and a fixed-bed reactor[J]. Journal of Analytical & Applied Pyrolysis,2015, 117: 191–198
[18] Miller J D, Misra M. Hot water process development for Utah tar sands[J]. Fuel Processing Technology, 1982, 6(1):27–59
- 中国炼油与石油化工的其它文章
- Oxidation of Dibenzothiophene in Model Diesel Using Hydroperoxide Generated via In-Situ Reaction of Octane with Oxygen
- Study on Mechanical Performance and Wear Resistance of Halloysite Nanotubes/PTFE Nanocomposites Prepared by Employing Solution Mixing Method
- Study on a New Type of Lubricating Oil for Miniature Bearing Operating at Ultra-low Temperature
- Lubricating Performance of Rapeseed Oil Under Electromagnetic Field
- Preparation and Modification of Micro-Mesoporous Carbon Materials for Toluene Adsorption
- Preparation and SO2 Adsorption Behavior of Coconut Shell-Based Activated Carbon via Microwave-Assisted Oxidant Activation

