Preparation and Modification of Micro-Mesoporous Carbon Materials for Toluene Adsorption
Liu Fang ; Xue Ming ; Wang Hongxi ; Chai Qingwen ; Li Meng ;Dai Yexin ; Zhao Chaocheng ; Wang Yongqiang
(1. State Key Laboratory of Petroleum Pollution Control, Beijing 102206;2. College of Chemical Engineering, China University of Petroleum, Qingdao 266580)
1 Introduction
Volatile organic compounds (VOCs) from different sources play an important role in the formation of tropospheric ozone and other oxidants that can produce photochemical smog[1], and they also have harmful effects on the environment[2]. Currently, the methods for VOCs treatment cover adsorption, catalytic oxidation, thermal oxidation,catalytic combustion, biological degradation, and condensation. Among all these techniques[3-5], adsorption is one of the most effective methods. Because of the advantages of flexibility, low energy consumption and low operating cost, the adsorption is superior to other methods[6]. Even faced with the low-concentration volatile organic compounds, the adsorption method can also provide better removal effect[7].
The key to the adsorption of VOCs from waste gas is the choice of proper adsorbent. Adsorption can take place in the porous materials, such as zeolites, silica gel, and activated carbon (AC)[8]. Since the successful synthesis of mesoporous molecular sieves (the M41S family) was discovered by Mobil in 1992, numerous studies on their synthesis, characterization, and application have been reported[9-12]. The mesoporous molecular sieve MCM-41 possesses some unique features, such as the adaptability to pore size control, the highly ordered structures, the very high specific surface area[13], and the voids of well-de fined size[14]. Because of these characteristics of the said molecular sieve, macromolecular substances can be easily removed by MCM-41. However, due to its amorphous pore wall and poor thermal stability and hydrothermal stability,the application of MCM-41 in VOC removal is limited[15].Therefore, the preparation of adsorbent with large specific surface area, stable performance, and good adsorption performance is the key to the successful treatment of toluene.
In this paper, the micro-mesopores-structured carbon materials were prepared via synthesis with the template. The synthesis process was performed by using MCM-41 as the template, and sucrose and furfuryl alcohol as the carbon source, respectively. The obtained carbon materials were named MC-1 and MC-2, respectively. The toluene adsorption properties of MC-1 and MC-2 were evaluated under different operation parameters. In order to obtain better adsorption property, MC-1 was modified by nitric acid and its modified parameters were also optimized.
2 Experimental
2.1 Materials
Mesoporous molecular sieve MCM-41 (made by the Nankai University Catslyat Factory), and micro-mesoporous carbon materials (MC-1 and MC-2) (prepared in our laboratory) were used in the experiments. Before use,the samples were dried for 24 hours in a vacuum oven at 110 °C. Toluene (with analytical reagent grade purity provided by the Sinopharm Chemical Reagent Company,)was used as the adsorbate.
2.2 Preparation of MC-1 and MC-2
MC-1 was synthesized by using MCM-41 as the template and sucrose as the carbon source. An aqueous solution(5 mL) was prepared which contained sucrose (1.25 g)and concentrated sulfuric acid (0.14 g). 1 g of activated MCM-41 was put into the aqueous solution under magnetical stirring at 20±2 °C for 24 h. The aqueous solution with MCM-41 was dried at 100 °C for 6 h, and then the temperature was raised to 160 °C and was subject to continuous heating for 6 h to realize the preliminary carbonization of sucrose. After that, 0.8 g of sucrose, 0.09 g of concentrated sulfuric acid and 5 mL of distilled water were mixed to obtain an aqueous solution. The carbonization material was put into the aqueous solution and the previous treatment step was repeated. Then, the resulted material was heated to 900 °C for 2 h under nitrogen atmosphere. Finally, the MCM-41 template was removed by immersing in an ethanol solution of sodium hydroxide(2.5%) at 80 °C. The volume percentage of sodium hydroxide and ethanol was 1:1.
MC-2 was synthesized by using MCM-41 as the template and furfuryl alcohol as the carbon source. 1 g of activated MCM-41 was placed in an ethanol solution containing 0.5 mol/L of p-toluene sulfonic acid under stirring at 20±2 °C for 1 h. The resulted mixed solution was subjected to vacuum filtration prior to addition of ethanol to remove the excess p-toluene sulfonic acid. After having been subject to drying at 80 °C for 3 h, 2.25 mL of furfuryl alcohol solution were added and the mixture was then subject to stirring at 20±2 °C for 18 h. The mixed solution was dried at 100 °C for 6 h and when the temperature was incresed to 160 °C this mixture was furthermore subject to drying for 6 h. After that, the resulted material was heated at 800 °C for 3 h under nitrogen atmosphere. Finally, the MCM-41 template was removed by immersing in the ethanol solution of sodium hydroxide (2.5%) at 80 °C.
2.3 Modification of MC-1
In order to further improve the adsorption capacity of MC-1, nitric acid was used for MC-1 modification. The modified carbon material was named as MC-N. After being subject to high temperature activation, 0.5 g of MC-1 were added to nitric acid solution, which was then placed in an oscillating reactor to be subject to oscillation at different temperatures. After a specified time duration,the modified MC-1 was taken out and washed to neutral reaction in deminiralized water detected by a pH-meter.Then the resulted sample was dried and named as MCN. The mass fraction of nitric acid was kept at 10%, 20%,30%, 40% and 50%, respectively. The time used for modification of MC-1 was equal to 4 h, 8 h, 12 h, 16 h, 20 h,and 24 h, respectively. The modification temperature was set at 30 °C, 45 °C, 60 °C, 75 °C, and 90 °C, respectively.During investigating the toluene adsorption capacity of MC-N, the initial toluene concentration was 1 750 mg/m3,the temperature was controlled at 25 °C, and the bed height was equal to 10 mm.
2.4 Characterization of MC-1, MC-2 and MC-N
The appearance of micro-mesoporous carbon materials were analyzed by a Hitachi S-4800 field-emission scanning electron microscope (SEM) operating under the conditions covering a working voltage of 5.0 kV, a working distance of 8.9 mm, and a magnification of 80.0 k.The samples were observed with a transmission electron microscope (TEM, JEM-2100) operating at an acceleration voltage of 200 kV. Nitrogen adsorption-desorption isotherms were measured with a Micromeritics ASAP 2020 at 77 K. Before measurements, the samples were degassed under vacuum at 523 K for at least 12 h. The specific surface areas were calculated by the Brunauer-Emmett-Teller (BET) method and the mesoporous volume and pore size distributions were measured by menas of the Barrett-Joyneer-Halenda (BJH) model. The microporous surface area and microporous volume were analyzed by the t-plot method and the pore size distributions were estimated through the Horvath-Kawazoe method.
2.5 Adsorption method
The configuration of adsorption system is described in the previous paper[16]. The toluene adsorption on different adsorbents (MCM-41, MC-1, MC-2) was studied. Before adsorption experiment, the adsorbents were degassed at 383 K under vacuum for at least 12 hours. The effects of the temperature, the initial concentration of toluene, and the bed height were investigated. The temperature was set at 25 °C, 35 °C, and 45 °C, respectively. The concentrations of toluene were controlled in the range from 389 mg/m3to 3 869 mg/m3. The bed height was in the range of between 5 mm and 15 mm.
The toluene adsorption capacity of the adsorbent (qe) was determined by Eq.(1):

where m0and m are the mass of initial and saturated adsorption column (mg), respectively.
Gas chromatography (SP-3420A) and the method for analysis of CS2solvent were utilized to measure the toluene concentration.
3 Results and Discussion
3.1 Characterization analysis
The appearances of MCM-41, MC-1, and MC-2 observed by SEM and TEM are presented in Figure 1. Figure 1a shows that MCM-41 has the characteristics of high crystallinity, uniform size and regular structure. As shown by TEM image (Figure 1b), MCM-41 has a regular pore structure and obvious hexagonal array of uniform channels[9,17]. The distribution of the pore size is uniform,which is about 2—3 nm. Figure 1c shows that MC-1 has a layered structure composed of macropores, mesopores and micropores. Figure 1d and Figure 1f reveal that the pore distributions of MC-1 and MC-2 are disordered and they constitute non-uniform pore sizes.
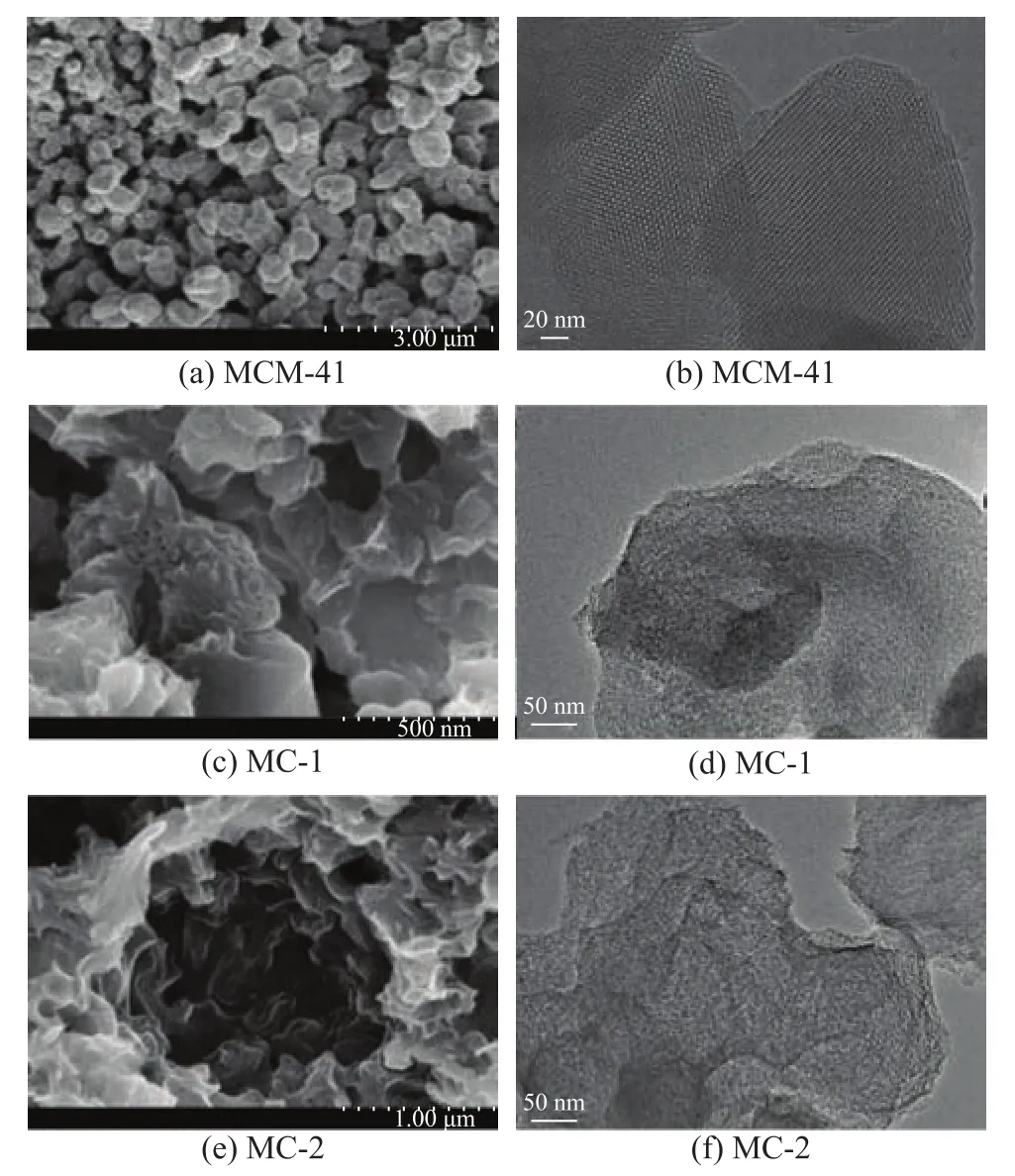
Figure 1 SEM and TEM images of adsorbents
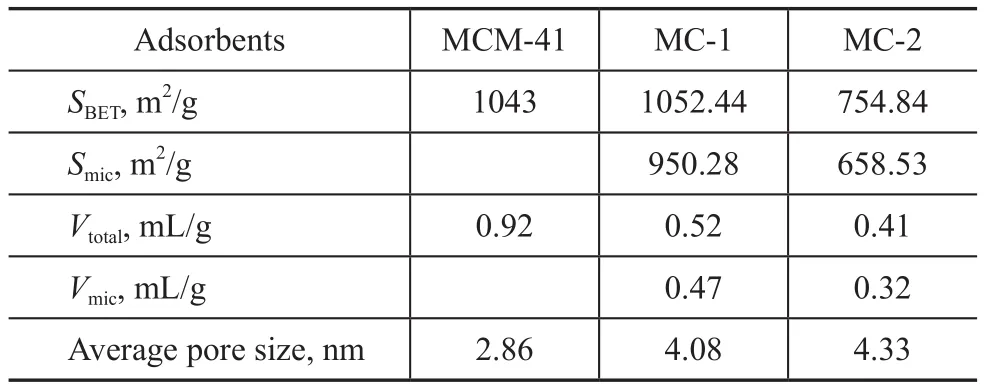
Table 1 Textural parameters of MCM-41, MC-1 and MC-2
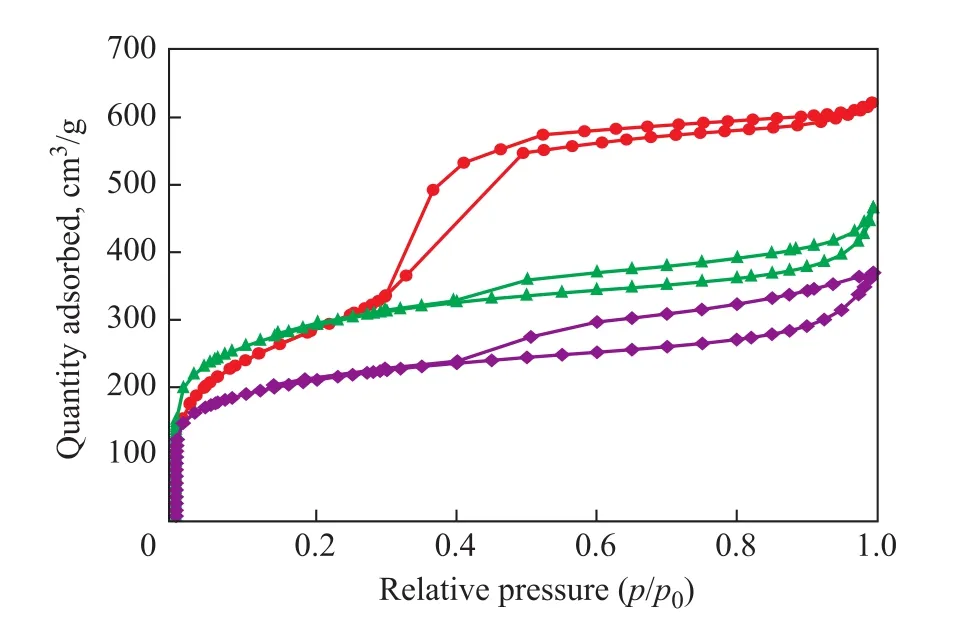
Figure 2 Nitrogen adsorption desorption isotherms of adsorbents
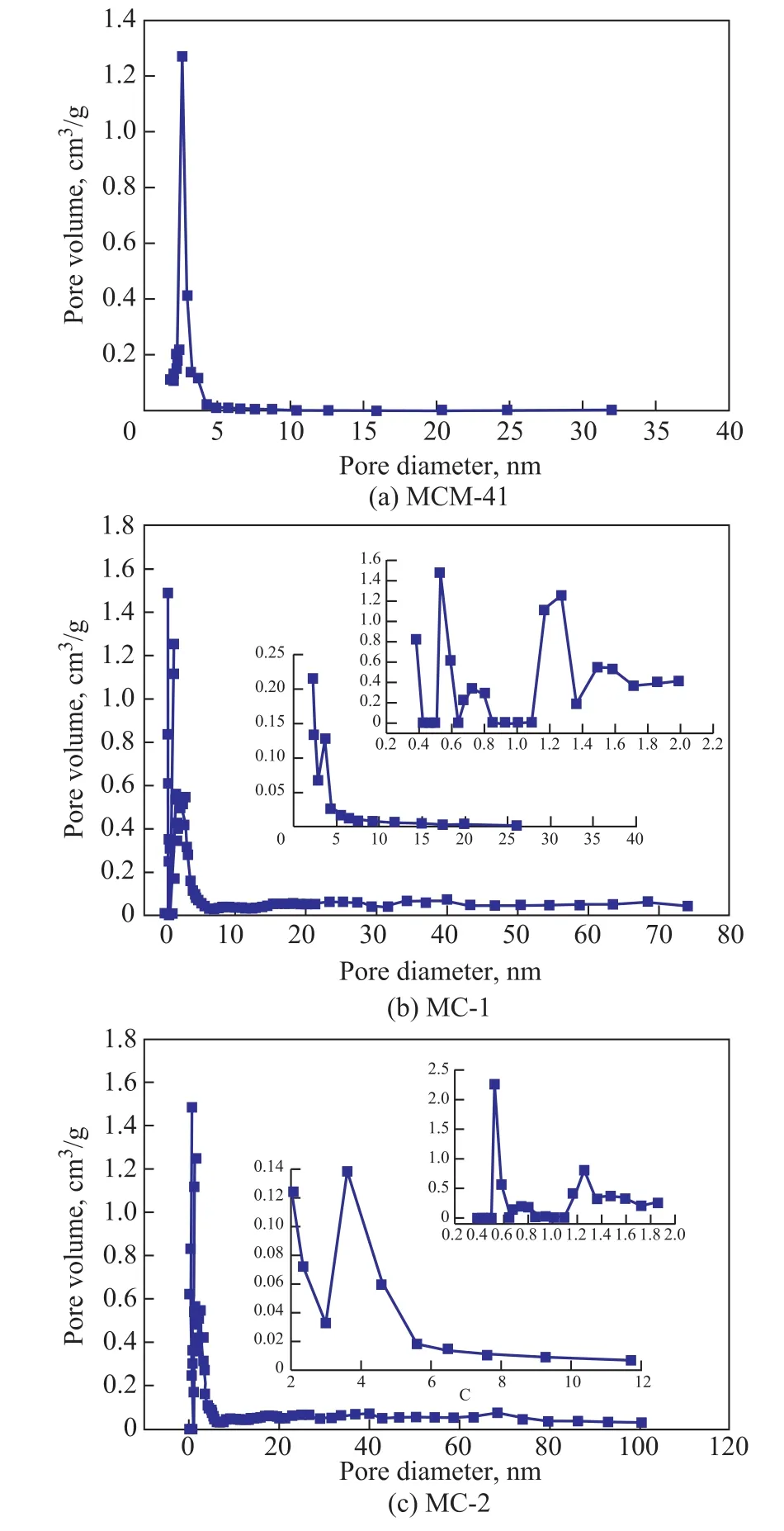
Figure 3 Pore size distributions of absorbents(a) MCM-41; (b)MC-1; and (c)MC-2
Figure 2 and Figure 3 show the N2adsorption-desorption isotherms and pore size distribution of MCM-41, MC-1,and MC-2, respectively. According to International Union of Pure and Applied Chemistry (IUPAC) classification[18],MC-1 and MC-2 are the typical type IV isotherms with well-de fined H4 hysteresis loops. Yet, MCM-41 is a type IV isotherm with well-de fined H2 hysteresis loop, which is characteristic of mesoporous structure (Figure 2). The textural parameters of MCM-41, MC-1, and MC-2 are given in Table 1. The microporous surface areas of MC-1 and MC-2 are 950.28 m2/g and 658.53 m2/g, respectively.MCM-41 shows a larger pore volume, while MC-1 possesses the largest microporous volume.
Figure 3 depicts the pore size distribution of MCM-41, MC-1,and MC-2. MCM-41 possesses rich amount of mesopores with a pore diamater of around 2.85 nm. The pore size distribution of MC-1 and MC-2 focuses on micropores and mesopores. MC-1 possesses micropores (with peaks identified at around 0.5—0.6 nm, 0.6—0.8 nm, 1.1—1.4 nm, and 1.4—1.7 nm) and mesopores (identified at around 2—5 nm).MC-2 owns two peaks of micropores identified at around 0.5—0.6 nm and 1.2—1.4 nm, and mesopores identified at at around 3—5 nm. Obviously, MC-1 has the larger specific surface area of micropores (950.28 m2/g), the ratio of specific surface of micropore area to total specific surface area is higher (90.33%). So, compared with the other two adsorbents, MC-1 has good pore structure, making itself a promising candidate for VOCs adsorption.
3.2 Adsorption of toluene by adsorbents
Figure 4 shows the breakthrough curves of toluene adsorbed by MCM-41, MC-1, and MC-2, respectively, with the corresponding data listed in Table 2. The adsorption capacity of MC-1 is the largest, while that of MCM-41 is the smallest. It is reported that the adsorption capacity is not controlled by the apparent BET surface area of the adsorbent[19], since it is mainly controlled by its narrow micropore volume[20-21]and also by its oxygen surface group content[17]. The adsorption capacity of three adsorbents decreases in the following order: MC-1>MC-2>MCM-41. MC-1 has the highest toluene adsorption capacity because of its maximum micropore volume. This result is consistent with the microporosity, which plays a key role in the adsorption of toluene[22].
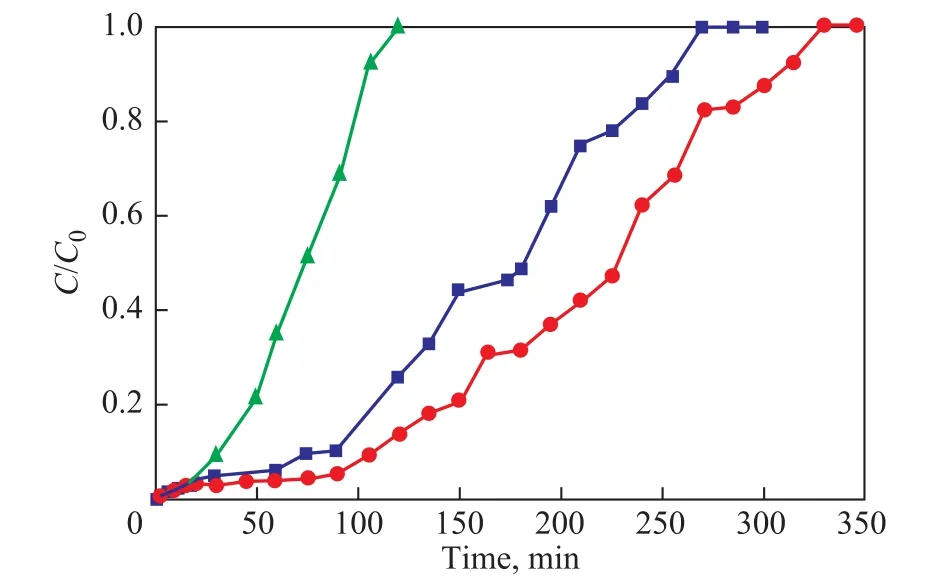
Figure 4 Breakthrough curves of toluene adsorbed by adsorbents (with the toluene initial concentration equating to 1750 mg/m3, the temperature controlled at 25°C, and the bed height being equal to 10 nm)

Table 2 Comparison of adsorption parameters for toluene on MCM-41, MC-1 and MC-2
Table 2 lists the breakthrough time (time corresponding to a C/C0ratio of 0.1) and the saturation time (time corresponding to a C/C0ratio of 0.95) of three adsorbents. The saturation time decreases in the following order: MC-2> MC-1 > MCM-41, which is in accordance with the average pore size of three adsorbents. The saturation time of MCM-41 is the shortest and the adsorption rate is the largest, which may be ascribed to its uniform mesoporous structure and the smallest average pore size. The adsorbent with small average pore size has the strong superposition effect and the adsorption behavior is more readily to occur at large rate[23].
The above results indicate that the toluene adsorption capacity of MC-1 and MC-2 is greater than that of MCM-41, so in the following experiments only MC-1 and MC-2 are used to investigate the factors influencing the adsorption capacity. As shown in Table 3, with the increase of toluene initial concentration from 389 mg/m3to 3 869 mg/m3, the adsorption capacity of MC-1 and MC-2 increases and the breakthrough time and saturation time all are reduced. This is mainly due to the increase of initial concentration and the increase of mass transfer resistance, resulting in insuf ficient contact between toluene and the adsorbent[24-25]. Table 4 shows that the toluene adsorption capacity, the corresponding breakthrough time, and the saturation time all increase with the increase of bed height.The effect of temperature on the toluene adsorption by MC-1 and MC-2 is examined at temperatures of 25 °C,35 °C, and 45 °C, respectively, with the results illustrated in Table 5. The toluene adsorption capacity, the breakthrough time, and the saturation time all decline with the increase of temperature. The reason may be attributed to the intraparticle resistance of toluene molecules[26]. An increase in temperature generally improves the diffusion within the pores of adsorbent materials[27]. The decrease in adsorption capacity with the increase of temperature is ascribed to the enhancement of desorption step in the adsorption mechanism. It is also due to the decrease of adsorption force between the active sites on the prepared adsorbent and toluene, and also between adjacent toluene molecules on the adsorbed surface[28].

Table 3 Toluene adsorption parameters at different initial toluene concentrations
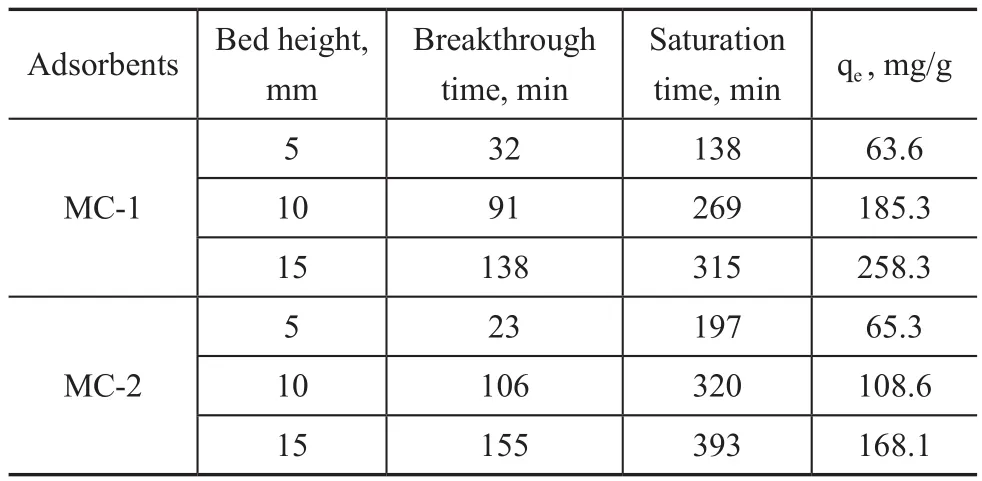
Table 4 Toluene adsorption parameters at different bed heights
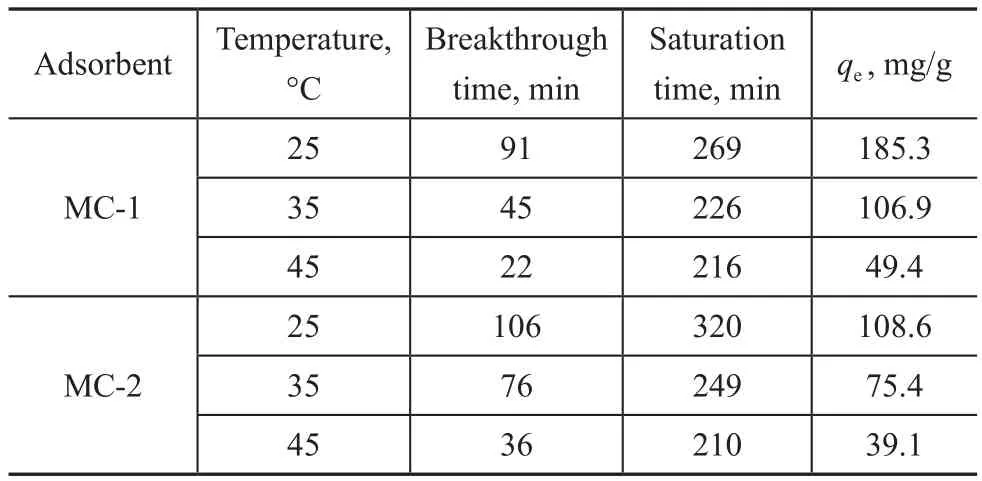
Table 5 Toluene adsorption parameters at different temperatures
3.3 Toluene adsorption isotherms
The Langmuir model is widely used in the field of both physical and chemical adsorption. It is represented as Eq. (2):

where qmand qeare the maximum adsorption capacity(mg/g) and the adsorption capacity (mg/g), respectively.KLis the Langmuir constant and C0is the equilibrium concentration (mg/m3).
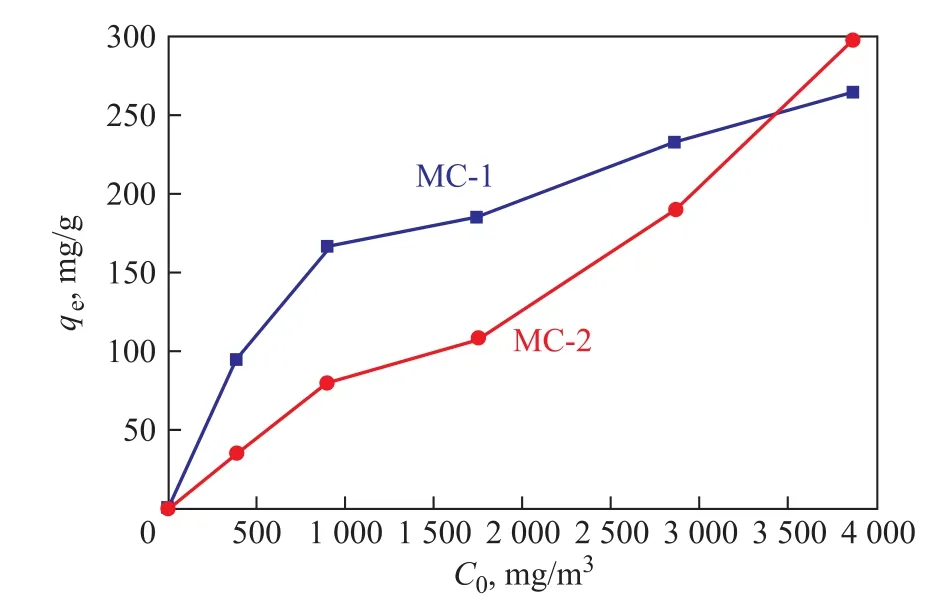
Figure 5 Adsorption isotherms of MC-1 and MC-2(with the temperature being kept at 25°C and bed height being controlled at 10 mm)
The Freundlich model is an empirical equation utilized to describe adsorption, which is usually written as Eq. (3):

where KFand n are empirical constants, and C0is the equilibrium concentration (mg/m3).
The fitting curves of two models are depicted in Figure 6.The corresponding parameters are listed in Table 6. The Langmuir model is suitable for describing the adsorption isotherms due to high R2values (>0.98). The n value from the Freundlich model represents a favorable adsorption condition. A higher n value of MC-1 indicates that MC-1 is easier to adsorb toluene as compared with MC-2.
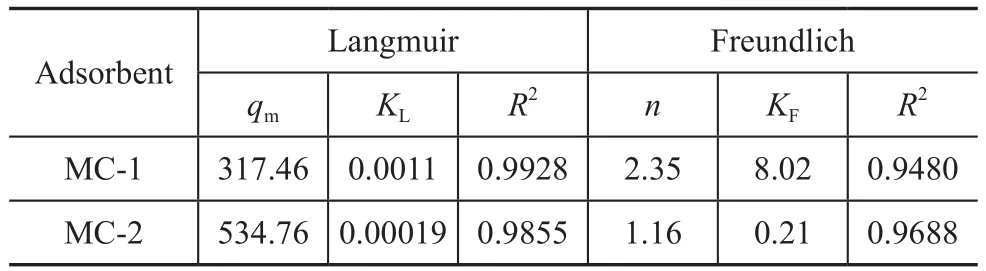
Table 6 Related parameters of Langmuir model and Freundlich model

Figure 6 Langmuir model fitting curves (a) and Freundlich model fitting curves (b)
In general, it can be concluded that the toluene adsorption on MC-1 and MC-2 largely depended on the micropore volume because the molecule size of toluene is suitable for adsorption in micropores[29]. So, the adsorbent with large specific surface area and suitable micropore volume is considered to have higher toluene adsorption capacity[30]. According to the previous experimental data, the adsorption capacity of MC-1 is greater than MC-2. At the same time, MC-1 has the advantages in terms of easy preparation and low cost. Therefore, in the next experiment, MC-1 was modified by nitric acid to yield a new carbon material for improving the adsorption capacity.
3.4 Optimization of MC-N modification parameters
Table 7 and Figure 7 reveal the effects of the nitric acid concentration, the modification time and the temperature on the adsorption capacity and the breakthrough curves of MC-N. The MC-N adsorption capacity reached a maximum value when the mass fraction of nitric acid was 20%(with the modification time equating to 12 h at 60 °C).The adsorption capacity was improved because the acidic groups increased the af finity between MC-N and toluene molecules at low concentration of nitric acid[31]. Figure 7a shows that the adsorption saturation time was shortened and the adsorption rate increased with an increasing mass concentration of nitric acid.
Table 7 presents that the adsorption capacity of MC-N reached a maximum value of 416 mg/g when the modification time was 12 h, with the nitric acid mass fraction reaching 20% at 60°C. Figure 7b re flects that the saturation time was shortened and the saturation rate increased with an increasing modification time. The free π electrons on the graphite layer of MC-N were affected by nitric acid and the density of π electron cloud decreased, which reduced the dispersion force between MC-N surface and the toluene molecule and accelerated the adsorption rate[32]. In addition, the amount of macropores, which did not have adsorption ability, increased because of modification, which also led to the acceleration of adsorption rate.
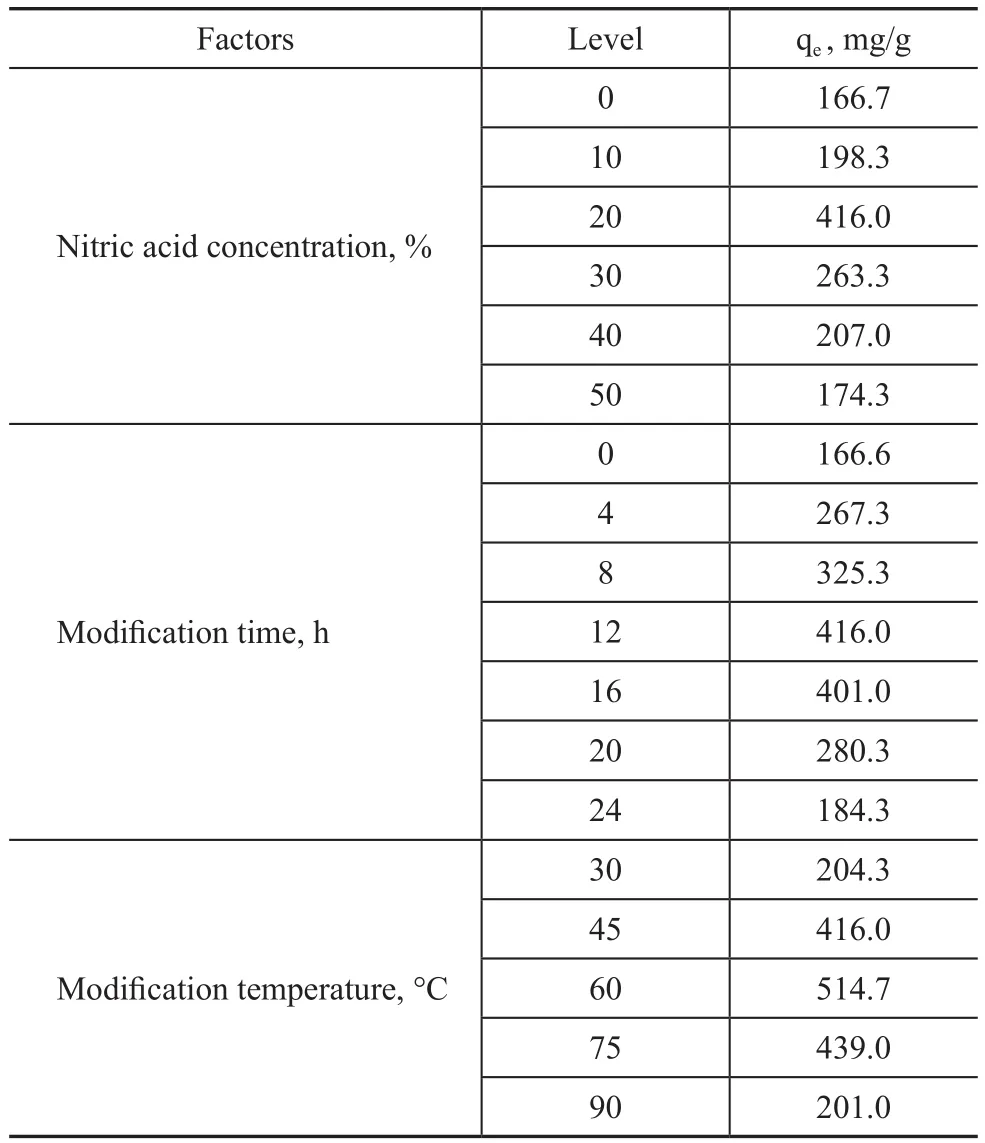
Table 7 Effects of modification parameters on MC-N adsorption capacity
A maximum adsorption capacity (514.7 mg/g) was obtained at 60 °C, when MC-N was treated by nitric acid with a mass fraction of 20% for 12 h (Table 7). The acidity and oxidation properties of nitric acid were brought into full play at an appropriate temperature so that the micropore volume of MC-N increased while the mesopores volume was not in fluenced obviously (with the characterization data presented in Section 3.5). It can be seen from Figure7c that when the modification temperature was higher than 75 °C, the adsorption rate became fast and the adsorption capacity dropped.
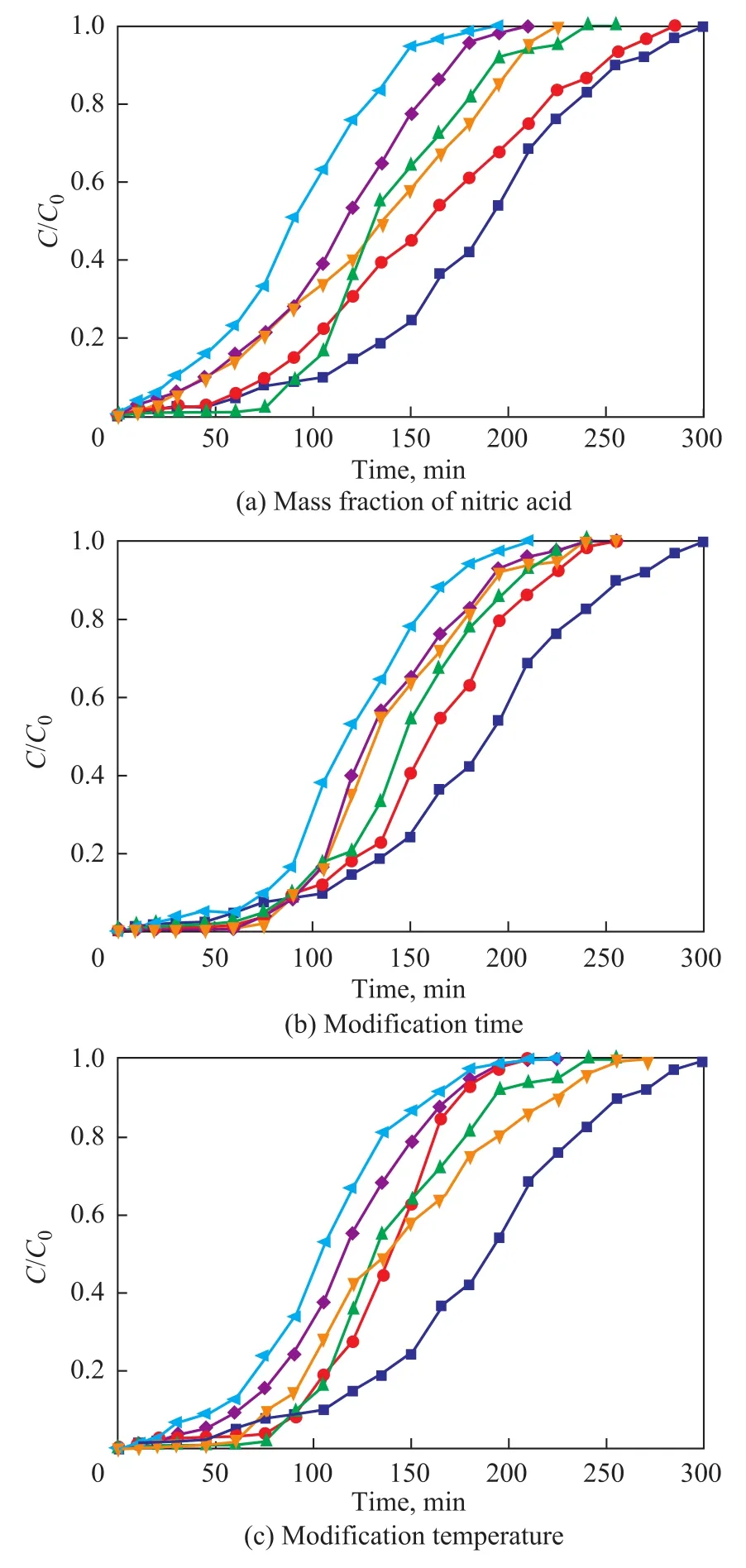
Figure 7 Effects of modification parameters on MC-N breakthrough curves
3.5 Characterization of MC-N
Figure 8a shows that MC-N was still comprised of a layered structure. Compared with MC-1, there was no obvious change in the appearance of MC-N. Figure 8 b shows that MC-N was still out of order.
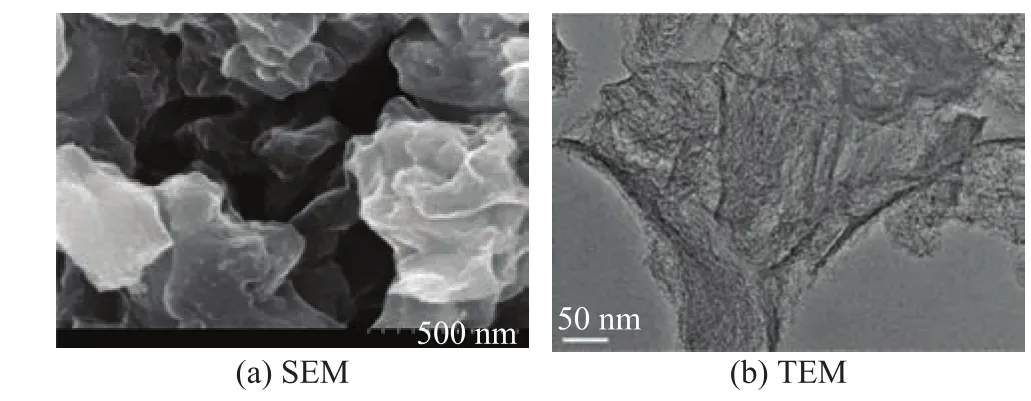
Figure 8 SEM and TEM of MC-N images
Figure 9 shows the N2adsorption-desorption isotherms at 77 K for MC-1 and MC-N. MC-1 and MC-N are typical type IV isotherms with well-defined H4 hysteresis loops.The textural parameters of MC-1 and MC-N are given in Table 8. The specific surface area of MC-N (1 085.76 m2/g)is slightly higher than that of MC-1 (1 052.44 m2/g).
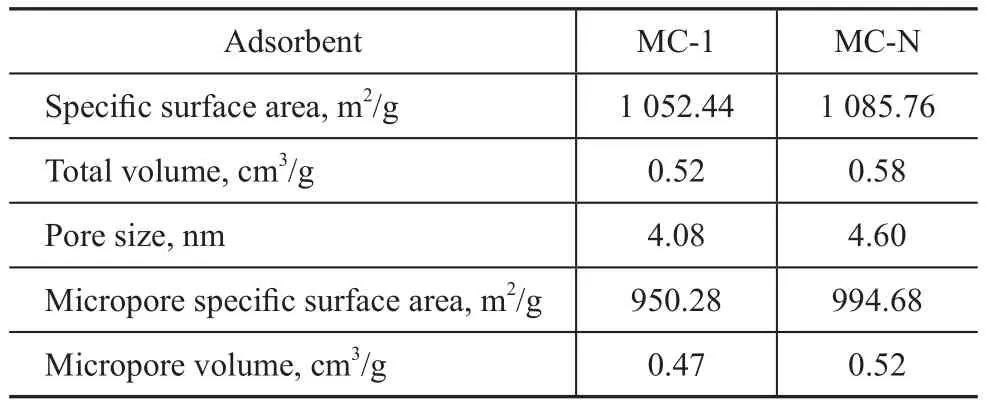
Table 8 Textural parameters of MC-1 and MC-N
3.6 Correlation analysis between toluene adsorption capacity and pore structure parameters of adsorbents
In order to investigate the effects of pore structure of adsorbents on toluene adsorption capacity, the correlation analysis between toluene adsorption capacity and specific surface area, pore volume of adsorbents was carried out.The experimental conditions covered a toluene initial concentration of 1 750 mg/m3, a temperature of 25 °C, and an adsorption bed height of 10 mm. The adsorbents consisted of MC-1, MC-N1, and MC-N2. MC-N1 was prepared at a temperature of 60 °C for 12 h with a nitric acid mass fraction of 20%. MC-N2 was prepared at a temperature of 60 °C for 24 h while being treated with a 20% nitric acid solution. Figure 10a shows the relationship between the specific surface area of adsorbent and its toluene adsorption capacity. The equilibrium adsorption capacity of toluene was improved with the increase in total specific surface area and specific surface area of micropores.The linear correlation coef ficients of simulation lines are 0.8479 and 0.8626, respectively. This fact indicates that the specific surface area is the important parameter that can affect the toluene adsorption capacity. The increase of specific surface area can improve toluene adsorption capacity. Figure10b reveals the relationship between pore volume and toluene adsorption capacity. The linear correlation coefficients of simulation lines reach 0.999 and 0.9389, respectively. The high correlation indicates that toluene adsorption capacity was obviously in fluenced by the pore volume, especially the total pore volume. The results may be ascribed to two reasons. Besides the main adsorption by micropores, small size mesopores were also helpful to toluene adsorption. In addition, the adsorbent with higher total pore volume was easily to be modified by nitric acid and more surface oxygen groups were introduced. In general, the adsorption capacity of adsorbent was mainly dependent on its surface area and surface chemical properties[32-33].
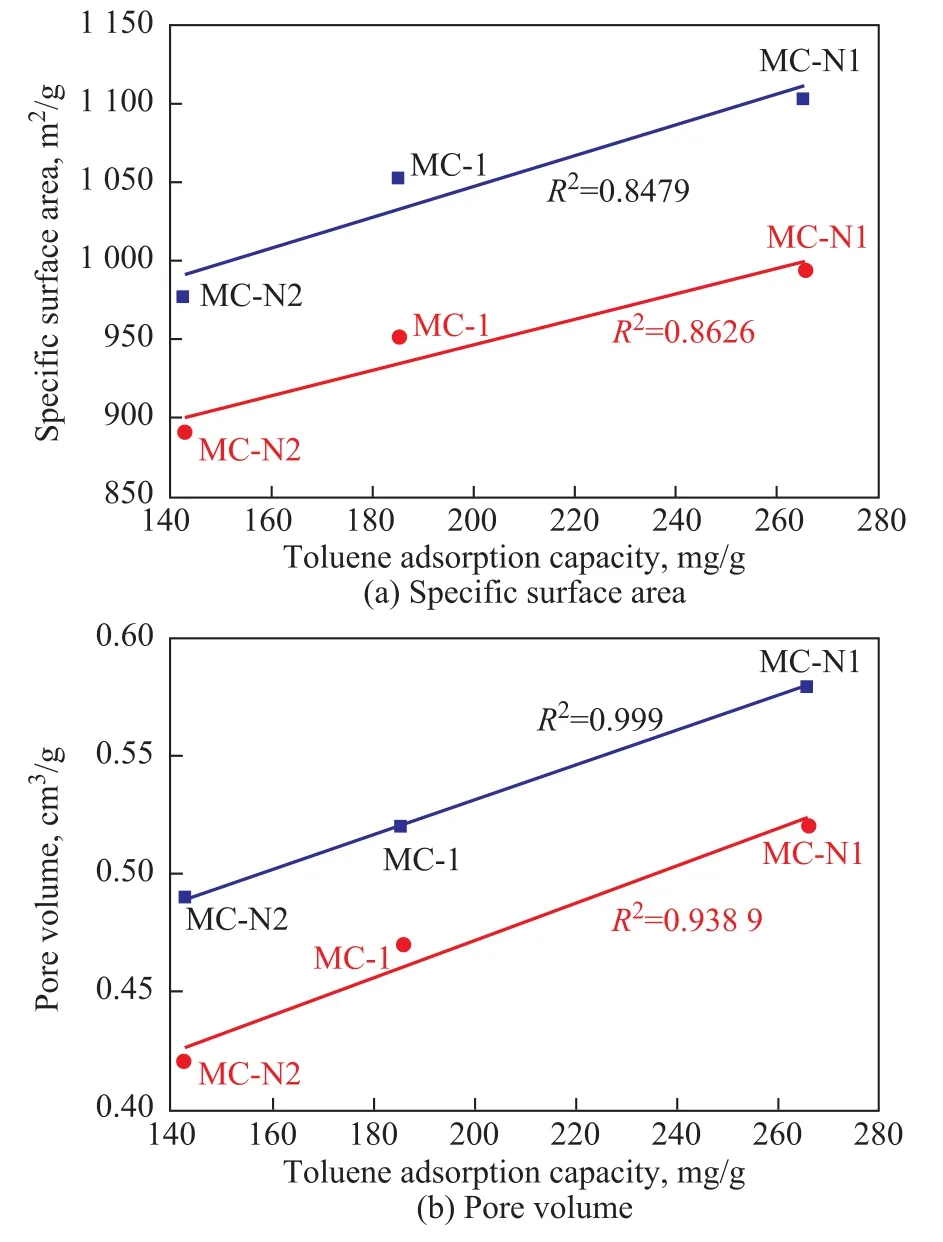
Figure 10 Relationships between specific surface area, pore volume and adsorption capacity
4 Conclusions
In this research, the micro-mesoporous structured carbon materials were prepared by using MCM-41 as the template, and sucrose and furfuryl alcohol as the carbon source. The obtained carbon materials were named MC-1 and MC-2. The toluene adsorption capacity decreased in the following order: MC-1 > MC-2 > MCM-41. The toluene adsorption capacity, the breakthrough time, and the saturation time of MC-1 and MC-2 were all affected by the initial concentration of toluene, the bed height, and the temperature. Upon considering that MC-1 possessed the largest adsorption capacity, so it was modified with nitric acid to further improve its property. The optimal modification conditions covered a temperature of 60 °C,and a modification duration of 12 h upon treating with nitric acid with a mass fraction of 20 %. The adsorption capacity of the adsorbent was improved from an original value of 185.3 mg/g to a maximum value of 514.7 mg/g after modification. The adsorption capacity of adsorbent was mainly depende on its surface area and surface oxygen groups.
[1] Atkinson R. Atmospheric chemistry of VOCs and NOx [J].Atmospheric Environment, 2013, 34(12/14): 2063–2101
[2] Cal M P, Larson S M, Rood M J. Experimental and modeled results describing the adsorption of acetone and benzene onto activated carbon fibers [J]. Environmental Progress & Sustainable Energy, 2010, 13(1): 26–30
[3] Beld L V D, Bijl M P G, Reinders A, et al. The catalytic oxidation of organic contaminants in a packed bed reactor[J]. Chemical Engineering Science,1994, 49(24): 4361–4373
[4] Bouazza N, Lillo-Ródenas M A, Linares-Solano A.Photocatalytic activity of TiO2-based materials for the oxidation of propene and benzene at low concentration in the presence of humidity [J]. Applied Catalysis B Environmental, 2008, 84(3): 691–698
[5] Hort C, Gracy S, Platel V, et al. Evaluation of sewage sludge and yard waste compost as a biofilter media for the removal of ammonia and volatile organic sulfur compounds (VOSCs) [J]. Chemical Engineering Journal,2009, 152(1): 44–53
[6] Wang H N, Tang M, Zhang K, et al. Functionalized hollow siliceous spheres for VOCs removal with high ef ficiency and stability [J]. Journal of Hazardous Materials, 2014,268: 115–123
[7] Ruddy E N, Carroll L A. Select the best VOC control strategy [J]. Chemical Engineering Progress, 1993, 89(7):28–35
[8] Hu Q, Li J J, Hao Z P, et al. Dynamic adsorption of volatile organic compounds on organofunctionalized SBA-15 materials [J]. Chemical Engineering Journal, 2009,149(1/3): 281–288
[9] Builes S, Vega L F. Understanding CO2capture in aminefunctionalized MCM-41 by molecular simulation [J].Journal of Physical Chemistry C, 2012, 116(4): 3017-3024
[10] Beck J S, Vartuli J C, Roth W J, et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates [J]. J A C S, 2016, 114(27): 10834–10843
[11] Hui K S, Chao C Y H. Synthesis of MCM-41 from coal fly ash by a green approach: In fluence of synthesis pH [J].Journal of Hazardous Materials, 2006, 137(2): 1135–1148
[12] Kresge C T, Leonowicz M E, Roth W J, et al. Ordered mesoporous molecular sieves synthesized by a liquidcrystal template mechanism [J]. Nature 1992, 359: 710–712
[13] Marzouqa D M, Zughul M B, Taha M O, et al. Effect of particle morphology and pore size on the release kinetics of ephedrine from mesoporous MCM-41 materials [J].Journal of Porous Materials, 2012, 19(5): 825–833
[14] Horcajada P, Rámila A, Pérez-Pariente J, et al. Influence of pore size of MCM-41 matrices on drug delivery rate[J]. Microporous & Mesoporous Materials, 2004, 68(1):105–109
[15] Mody H M, Kannan S, Bajaj H C, et al. A simple room temperature synthesis of MCM-41 with enhanced thermal and hydrothermal stability [J]. Journal of Porous Materials,2008, 15(5): 571–579
[16] Liu F, Yan X, Fan F T, et al. Application of micro-meso hierarchical porous carbon for toluene adsorption treatment[J]. Micro & Nano Letters, 2016, 11(7): 372–377
[17] ÁgnesSzegedi, Popova M, Lázár K. In fluence of the acid/base and redox properties of catalysts in the gas-phase dehydration-dehydrogenation of cyclohexanol on iron and titania containing mesoporous materials [J]. Reaction Kinetics, Mechanisms and Catalysis, 2011, 104(2): 291–301
[18] Sing K S W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity [J]. Pure and Applied Chemistry,1985, 57(4): 603–619
[19] Lillo-Ro´denas MA, Cazorla-Amoro's D, Linares-Solano A. Behaviour of activated carbons with different pore size distributions and surface oxygen groups for benzene and toluene adsorption at low concentrations [J]. Carbon, 2005,43(8): 1758–1767
[20] Carrataláabril J, Lilloródenas M A, Linaressolano A, et al.Activated carbons for the removal of low-concentration gaseous toluene at the semipilot scale [J]. Industrial &Engineering Chemistry Research, 2009, 48(4): 2066–2075[21] Maldonado-Hódar F J, Moreno-Castilla C, Carrasco-Marín F, et al. Reversible toluene adsorption on monolithic carbon aerogels [J]. Journal of Hazardous Materials, 2007,148(3): 548–552
[22] Qi J W, Li J S, Li Y, et al. Synthesis of porous carbon beads with controllable pore structure for volatile organic compounds removal [J]. Chemical Engineering Journal,2011, 307: 989–998
[23] Kosuge K, Kubo S, Kikukawa N A, et al. Effect of pore structure in mesoporous silicas on VOC dynamic adsorption/desorption performance [J]. Langmuir, 2007,23(6): 3095–3102
[24] Vivekanand G, Ashutosh S, Nishith V. Preparation and characterization of ACF for the adsorption of BTX and SO2[J]. Chemical Engineering and Processing: Process Intensification, 2006, 45(1): 1–13
[25] Mohan N, Kannan G K, Upendra S, et al. Breakthrough of toluene vapours in granular activated carbon filled packed bed reactor [J]. Journal of Hazardous Materials, 2009,168(2-3): 777–81
[26] Huang Z H, Kang F, Liang K M, et al. Breakthrough of methylethylketone and benzene vapors in activated carbon fiber beds [J]. Journal of Hazardous Materials, 2003, 98(1-3): 107–115
[27] Cotoruelo L M, Marqués M D, Díaz F J, et al. Adsorbent ability of lignin-based activated carbons for the removal of p-nitrophenol from aqueous solutions [J]. Chemical Engineering Journal, 2012, 184(3): 176–183
[28] Tan I A, Ahmad A L, Hameed B H. Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies [J]. Journal of Hazardous Materials, 2008, 154(1/3):337–346
[29] Bartorelli C, Cavalca L. Quantitative study of the adsorption of aromatic hydrocarbons (benzene, toluene,and p-xylene) on dealuminated clinoptilolites [J]. Industrial& Engineering Chemistry Research, 2005, 44(9): 2908–2916
[30] Jiang T, Zhong W, Jafari T, et al. Siloxane D4 adsorption by mesoporous aluminosilicates [J]. Chemical Engineering Journal, 2016, 289: 356–364
[31] Sarkar M, Majumdar P. Application of response surface methodology for optimization of heavy metal biosorption using surfactant modified chitosan bead [J]. Chemical Engineering Journal, 2011, 175(1): 376–387
[32] Huang G, Shi J X, Langrish T A G. Removal of Cr(VI)from aqueous solution using activated carbon modified with nitric acid [J]. Chemical Engineering Journal, 2009,152(2/3): 434–439
[32] Ansón A, Jagiello J, Parra J B, et al. Porosity, surface area,surface energy, and hydrogen adsorption in nanostructured carbons [J]. Journal of Physical Chemistry B, 2009,108(40): 15820–15826
[33] Roach P, Farrar D, Perry C C. Surface tailoring for controlled protein adsorption: effect of topography at the nanometer scale and chemistry [J]. J A C S, 2006, 128(12):3939–3945
- 中国炼油与石油化工的其它文章
- Synthesis of Core-Shell HZSM-5@SBA-15 Composite and Its Performance in the Conversion of Methanol to Aromatics
- Effects of Gasoline with Ester Additives on the Swelling Behavior of Rubbers
- Fabrication of the Core-Shell Structured ZSM-5@Mg(Al)O and Its Catalytic Application in Propane Dehydrogenation
- ZSM-5/MAPO Composite Catalyst for Converting Methanol to Ole fins in a Two-Stage Unit with a Dimethyl Ether Pre-Reactor
- Oxidation of Dibenzothiophene in Model Diesel Using Hydroperoxide Generated via In-Situ Reaction of Octane with Oxygen
- Controllable Synthesis of Mixed-Phase TiO2 with Small Anatase and Rutile Particle and Its Enhanced Photocatalytic Activity

