Oxidation of Dibenzothiophene in Model Diesel Using Hydroperoxide Generated via In-Situ Reaction of Octane with Oxygen
Guo Erliang; Li Jianxin; Huang Xiaoqiao; Duan Yongshen; Zhang Lingcong;Yan Lijun; Lu Shuxiang
(1.College of Chemical Engineering and Materials Science, Tianjin University of Science and Technology, Tianjin 300457;2. PetroChina Fuel Oil Company Limited Research Institute, Beijing 100195; 3. Petrochemical Research Institute,PetroChina Company Limited, Beijing 100195)
1 Introduction
Deep desulfurization of diesel has been paid more attention worldwide due to the undesirable sulfur compounds.Hydrodesulfurization (HDS) is regarded as a traditional desulfurization technology that is highly efficient in removing thiols, thioethers and disulfides from diesel[1].Meanwhile, it is dif ficult to remove the refractory aromatic sulfur compounds, because the removal of these compounds requires more severe operating conditions. ODS[2-4]is considered as one of the most promising processes among these techniques because of its mild operating conditions.
During the ODS process, the refractory sulfur compounds can be converted to highly polar sulfones which can be conveniently removed by extraction or adsorption[5-6]. Various oxidants have been used to oxidize sulfur compounds in diesel. Hydrogen peroxide (H2O2) in aqueous solution and oil-soluble alkyl peroxides such as tert-butyl hydroperoxide(BuOOH)[7]and cyclohexanone peroxide (CYHPO)[8]have been the most common oxidants in the ODS systems, while they are expensive and may result in a de finite loss of oil.
Molecular oxygen, which is environmentlly friendly and economical, has long been considered as a desirable oxidant for ODS. Tang, et al.[9]have reported that the sulfur compounds could be effectively oxidized with O2by using heteropoly acid catalyst. Song, et al.[10]explored a novel process for ODS in two steps, in which CuO was used as catalyst for the in-situ generation of hydroperoxides from aromatics in diesel fuels. In the second step, the hydroperoxides formed in the first step were used as the oxidant for the oxidation of sulfur compounds in the presence of MoO3catalyst. If the production of peroxides and the oxidation process of sulfur compounds can be combined in a single unit,the reaction process can be simplified to facilitate the industrial production so that the decomposition of hydroperoxides can be decreased.
Hydrocarbons can be subject to autoxidation reaction to generate hydroperoxides under heating and in the presence of dissolved oxygen[11]. In our study, we utilized the autoxidation of hydrocarbons to produce hydroperoxides in-situ in the absence of catalysts followed by oxidizing DBT to DBTO2.This process can resolve the issues of using expensive oxidants and the mass transfer during the reaction. The composition of diesel is quite complicated which may have a critical impact on the selective oxidation of DBT. However, most of the previous studies were mainly focused on discussing the conversion of DBT and limited literature information was reported about the effects of different hydrocarbons compositions related with diesel fuels. Therefore, in this study we also discussed the effects of different hydrocarbon compositions in diesel on the removal of DBT.
2 Experimental
2.1 Materials
All of the purchased and obtained materials were used without further treatment. n-Octane (99+%), cyclohexane(99+%), n-dodecane (99+%), xylene (99+%) were obtained from the Tianjin Bodi Chemical Company Co., Ltd., China.Dibenzothiophene (DBT, powder, with a purity exceeding 99%), dibenzothiophene sulfone (DBTO2, powder, with a purity of more than 99%) were purchased from the Adamas Reagent Co., Ltd., Shanghai, China. Oxygen (with a purity of more than 99%) was purchased from the Tianjin Sizhi Gas Company, Ltd., China.
2.2 Oxidative desulfurization procedure of model oil
2.2.1 Sulfur analysis
DBT was dissolved in n-octane to form the model diesel with a sulfur concentration of 320 μg/g. In the oxidation experiment, 15 mL of model diesel were added into the autoclave with a Te flon inner sleeve. After air was removed by purging with O2, the autoclave was continuously pressurized with O2until the pressure reached 0.4 MPa.The model diesel was heated from room temperature to 140 °C under continous stirring for 4 h at a speed of 400 r/min with a magnetic stirrer. In the course of the reaction,the solution was periodically collected and analyzed by a GC-HP6890 (Agilent Technologies, Santa Clara, CA). The removal of the DBT was calculated as follows:
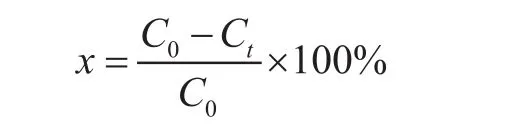
where C0is the initial concentration of DBT in the model fuel and Ctis the concentration of DBT in the oil phase after the reaction proceeded for a specified time duration.
2.2.2 Hydroperoxide analysis
Hydroperoxide in the fuel was determined by iodometric titration[12]. In a typical analysis hydroperoxide dissolved in carbon tetrachloride could react with aqueous potassium iodide solution under the acidic condition to form iodine. Then the iodine was titrated with sodium thiosulfate solution.
2.2.3 Characterization of the oxidation products
The oxidation products of DBT were characterized by Fourier infrared spectroscopy and nuclear magnetic resonance (1H-NMR). The FT-IR spectra were recorded over a range of 4 000—400 cm-1on a Thermo Nicolet Nexus 470 spectrometer (Bruker, Germany) using a KBr disc. The NMR spectra were recorded with the help of a Bruker Avance Ш spectrometer with a 5 mm probe and chloroform that was used as the solvent.
3 Results and Discussion
3.1 Effect of reaction temperature on DBT removal
As shown in Figure 1, the removal of DBT increased remarkably from 40% to 98.4% as the temperature increased from 130 °C to 140 °C. When the temperature was higher than 140 °C, the solution turned yellow and there was a pungent odor after the reaction. At the bottom of the autoclave a small amount of sandybrown liquid and brown deposit appeared. The oxidation reaction of n-octane is a free radical reaction, when the reaction temperature is too low to provide enough energy to generate active free radicals, the concentration of hydroperoxides is insuf ficient to completely oxidize DBT. And the hydroperoxides are thermally unstable so that they may decompose to form other oxygenated by-products at high temperature. With the proceeding of oxidation reaction, the oxygenated by-products can form deposits via condensation and polymerization reactions[13]. The FT-IR spectra of the original model diesel and the O2-oxidized samples are displayed in Figure 2.
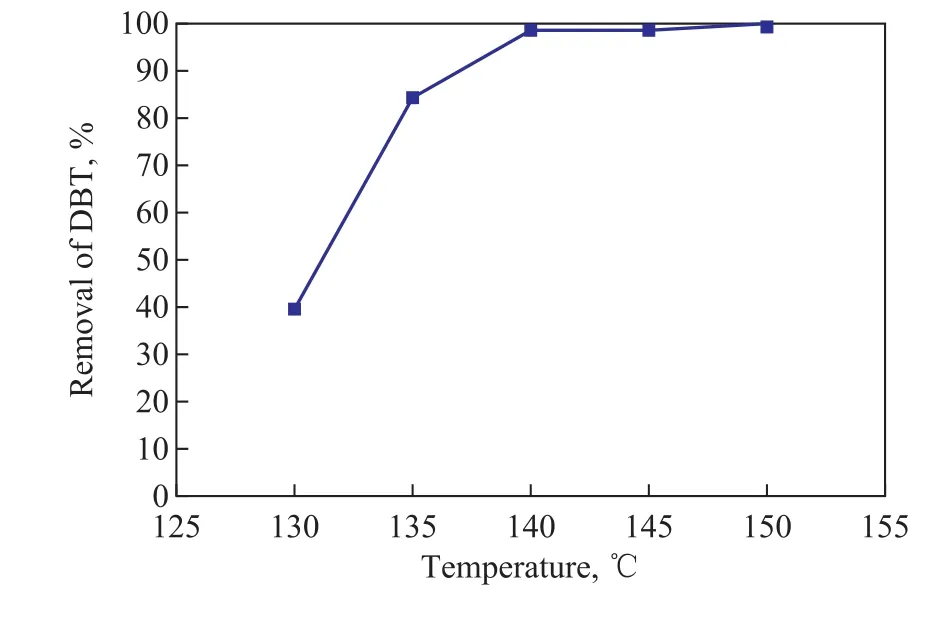
Figure 1 Effect of reaction temperature on DBT removal
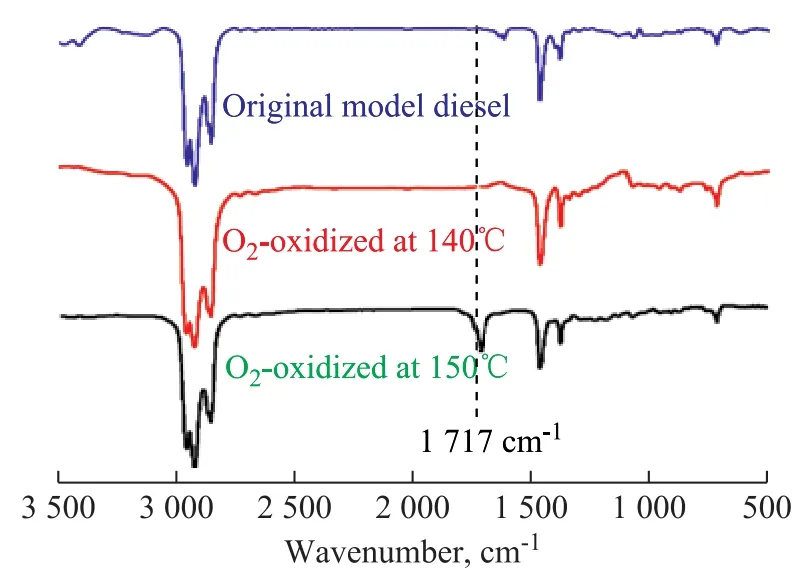
Figure 2 FT-IR spectra of the solvent after reaction
From the FT-IR spectra we could find a new absorption peak at 1 717 cm-1in the O2-oxidized model diesel at 150 °C. The new peak was attributed to the stretching vibration of C=O. But the same peak did not appear in the O2-oxidized model diesel at 140 °C. The results con firmed that the oxygenated by-products were not generated at 140 °C. The C=O might belong to the oxygenated species(e.g.: aldehyde, ketone, carboxylic acid, and ester)[14]that were generated by a series of oxidation reactions without any radicals being decomposed from the hydroperoxides.
3.2 Effect of reaction time on DBT removal
As presented in Figure 3, with the reaction time being prolonged the removal of DBT increased from 5% in 1 h to 50% in 2 h and then reached a maximum value of 98.4% in 4 h. The removal of DBT obtained in the range from 2 to 4 hours was much faster than the value achieved in the first one hour, which might be attributed to the presence of a long induction period for the oxidation of octane to generate hydroperoxides[15]. During the induction period, the oxidation of octane was slow, which resulted in a low rate for production of hydroperoxides and accordingly a limited removal of DBT.
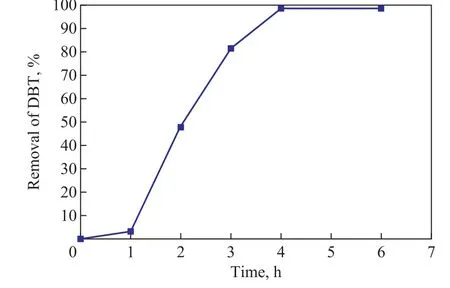
Figure 3 Effect of reaction time on DBT removal
3.3 Effect of oxygen partial pressure on DBT removal
Oxygen is the oxidant necessary for the in-situ production of hydroperoxides, and the oxygen partial pressure may in fluence the removal of DBT. As shown in Figure 4, the removal of DBT could reach 82.46% at an oxygen partial pressure of 0.2 MPa. The variations in conversion were not obvious when the oxygen partial pressure exceeded 0.4 MPa. It demonstrated that an oxygen partial pressure of 0.4 MPa was suf ficient to completely oxidize DBT.
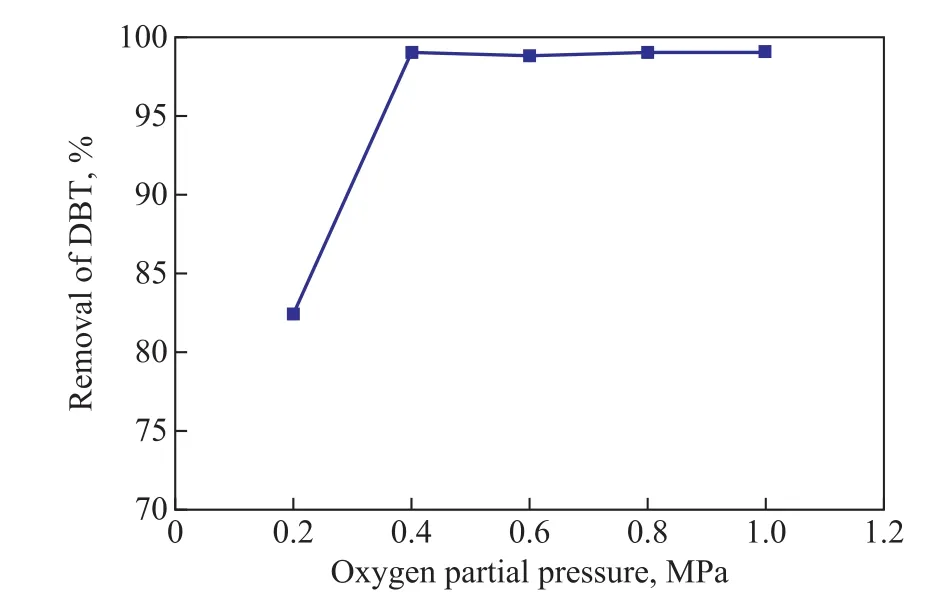
Figure 4 Effect of oxygen partial pressure on DBT removal
3.4 Effects of fuel composition in model diesel on DBT removal
A high DBT removal rate has been achieved in model diesel consisting of single sulfur compound by the oxidant hydroperoxides generated in-situ. However, the model diesel samples are generally prepared with a certain amount of dibenzothiophene dissolved in n-octane, which is far from the actual complicated composition of diesel. Diesel is a mixture of various types of hydrocarbons, such as straight chains, cyclane compounds and aromatics, etc. The selective oxidation of DBT may be affected by the nature of fuel composition to some extent. To further understand the effects of different types of hydrocarbons on ODS, a certain amount of cyclohexane, xylene, and n-dodecane serving as models of cyclanes, aromatics, and straight-chain alkanes were added into the model diesel to investigate their effects on the removal of DBT, respectively.
It can be seen from Figure 5 that the presence of n-dodecane in the model diesel had no negative in fluence on the removal of DBT. However, at the beginning the removal of DBT increased by 30% in the presence of various mass fractions of n-dodecane in comparison with the n-dodecane free case. The results might be attributed to the effect of carbon chain length. With the carbon chain length increasing, the ability of anti-oxidation of straight-chain alkane decreased. So the time required for formation of free radicals was shortened and the conversion of DBT was enhanced within 1 h.
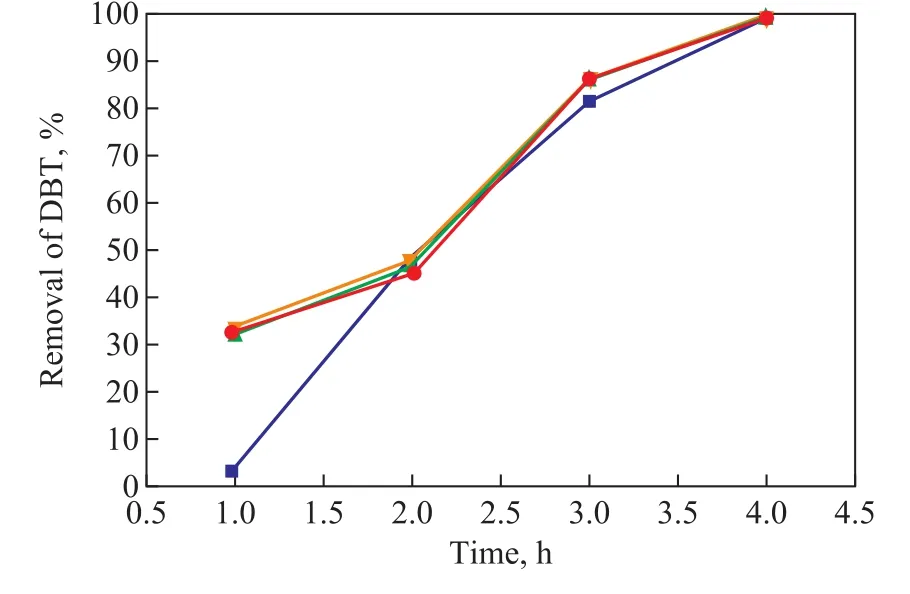
Figure 5 Effect of n-dodecane on the removal of DBT(Reaction conditions: T=140 °C; t = 4 h; p(O2)=0.4 MPa;
Figure 6 shows the effect of adding various mass fractions of cyclohexane in the model diesel on removal of DBT.It can be seen that the removal of DBT almost did not change with the addition of cyclohexane in a reaction duration of 4 h. The results suggested that the presence of cyclohexane in the model diesel had no in fluence on the removal of DBT.
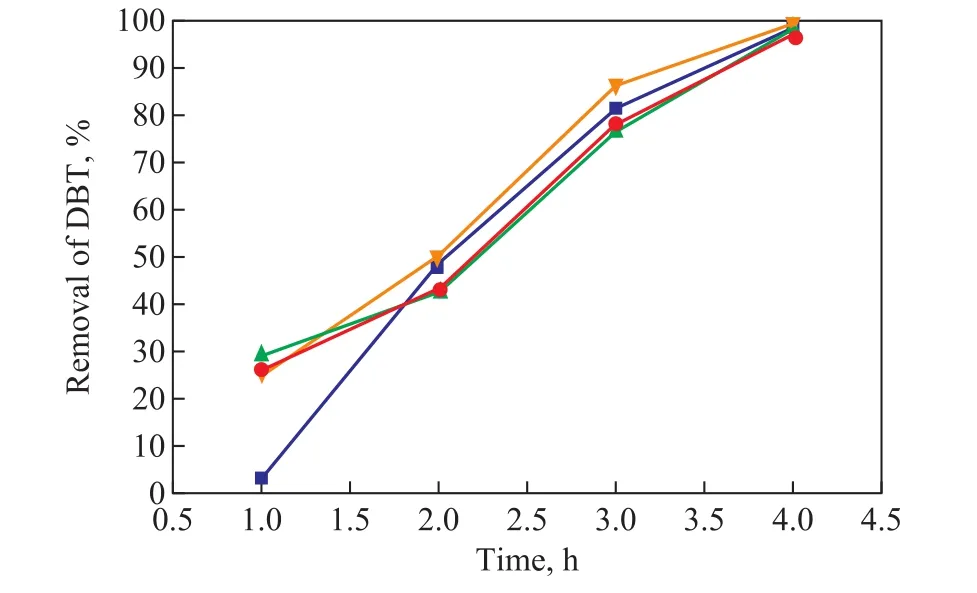
Figure 6 Effect of cyclohexane on the removal of DBT(Reaction conditions: T=140 °C; t = 4 h; p(O2)=0.4 MPa;C0=320 μg/g)
As demonstrated in Figure 7, the removal of DBT decreased from 98.4% without addition of xylene in the model diesel to 91.3%, 82.5% and 75.4% with the addition of 5%, 10% and 15% of xylene in the model diesel, respectively. The results exhibited that the presence of xylene in the model diesel had a negative in fluence to some extent on the oxidation of DBT. The presence of aromatics inhibited the oxidation of DBT, which could be attributed to the competitive oxidation between xylene and DBT. The alkyl groups attached to the aromatic ring are susceptible to oxidation due to the presence of the active α-hydrogen on the side-chain alkyl groups[16]. The xylene consumed a certain amount of hydroperoxides,so the amount of hydroperoxides required for oxidizing DBT decreased.
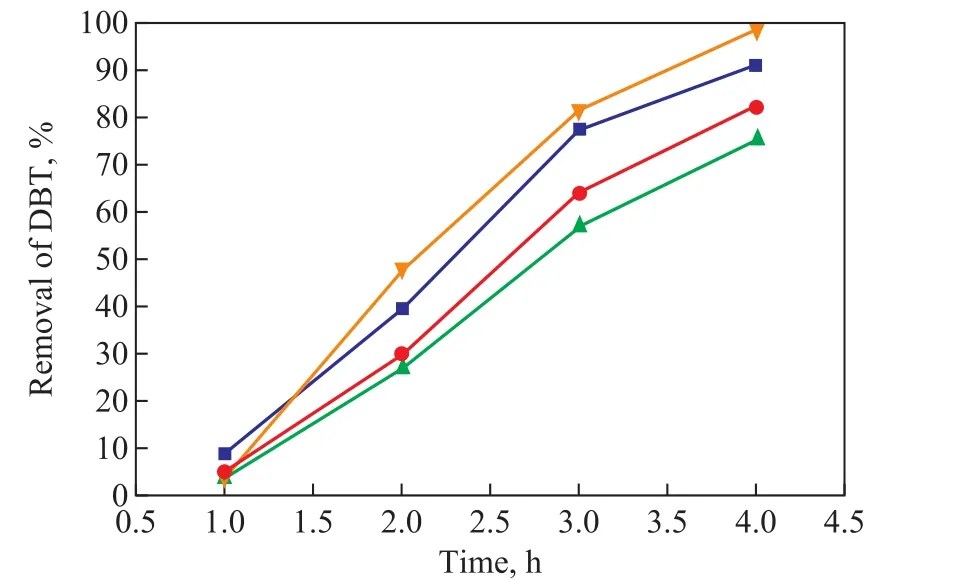
Figure 7. Effect of xylene on the removal of DBT(Reaction conditions: T=140 °C; t = 4 h; p(O2)=0.4 MPa;C0=320 μg/g)
3.5 DBTO2 analysis
A comparison of FT-IR spectra between the white crystal washed off by methanol and pure DBTO2is exhibited in Figure 8. The characteristic peaks at 1 289 cm-1and 1 165 cm-1could be assigned to the asymmetrical and symmetrical stretching vibration modes of O-S-O,respectively[17-18].1H-NMR was further used to confirm the chemical structure of the white crystal. The data of1H-NMR (Figure 9) were consistent with the structure of DBTO2that had been proved by Zhou[19]. Therefore, we could con firm that the white crystal was DBTO2.
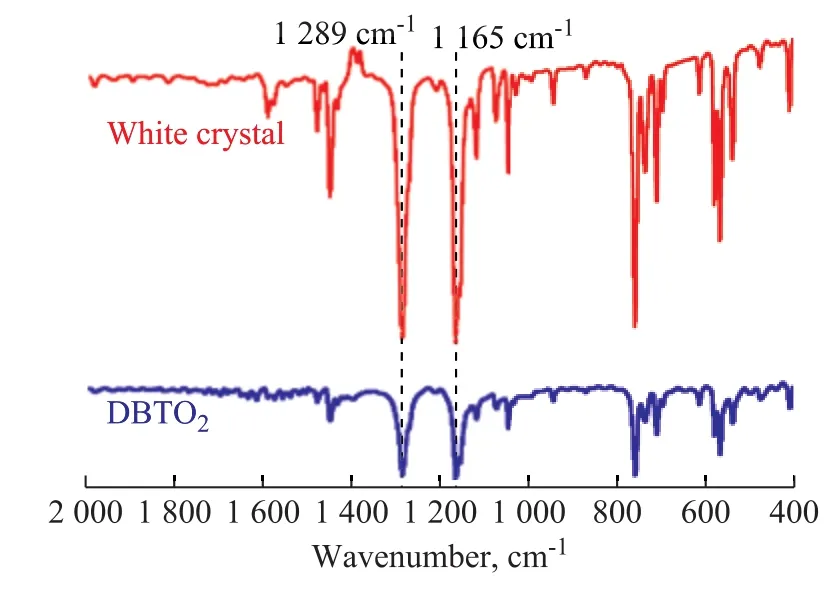
Figure 8 FT-IR spectra of the white crystal and pure DBTO2
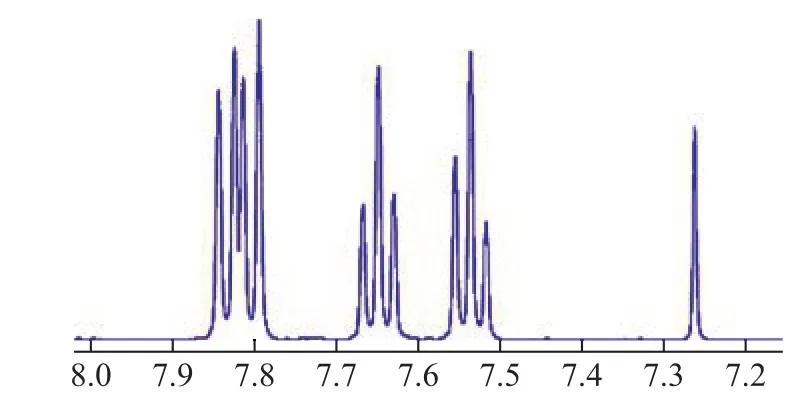
Figure 9 1H-NMR spectra of white crystal
3.6 Mechanistic interpretation
Phenolic species contain highly labile hydrogen,which can be rapidly donated to peroxyl radicals that would interfere with the oxidation process[20]. Phenolic species are recognized as free radical terminators, so m-dihydroxybenzene as a kind of phenolic species was chosen as the free radical terminator in our reaction. In order to observe if the oxidation reaction was a free radical chain reaction, a certain amount of m-dihydroxybenzene was added to the oxidation reaction system. The removal of DBT was almost zero in the presence of m-dihydroxybenzene. The result implied that m-dihydroxybenzene inhibited the formation of active free radicals, and the oxidation reaction was terminated.A series of results indicated that the possible oxidation reaction was a free radical chain reaction. The reaction mechanism may be expressed as Scheme 1[12,21]:
The reaction mechanism for the DBT oxidation in the absence of catalyst system can be recognized that the reaction may proceed through these steps: the first step is the chain initiation reaction, in which the octane molecular is attacked by oxygen to produce the active alkane radical(Eq. A1). In the chain propagation step the alkane radical reacts with oxygen to form the alkylperoxy radical (ROO•)(Eq. A2), and then the alkylperoxy radical absorbs a hydrogen atom from octane to form hydroperoxides(Eq. A3). The chain initiation and the propagation steps represent the formation of hydroperoxides, and then the concentration of hydroperoxides increases gradually with the reaction time. In the chain termination step the free radicals begin to form other oxidized non-radical products(Eq. A4). The hydroperoxides as intermediates of the O2oxidation process are not thermally stable and are prone to decomposition (Eq. A5). The DBT is oxidized to DBTO2by hydroperoxides, while the hydroperoxides are converted to the corresponding alcohols (Eq. A6).
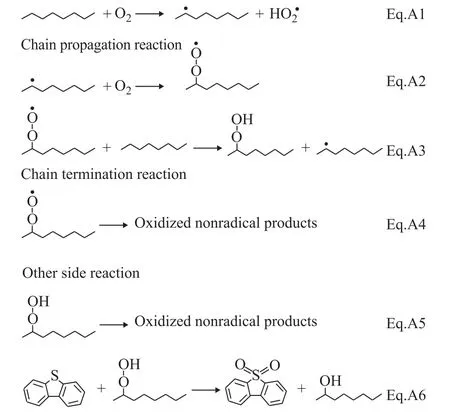
Scheme 1 Reaction pathway of oxygen-initiated oxidative desulfurization system
The concentration of hydroperoxides contained in the O2-oxidized octane and model diesel was titrated with sodium thiosulfate solution, with the results presented in Figure 10. The hydroperoxides concentration in n-octane gradually increased with the reaction time and then decreased after reaching a maximum value. This might be due to the thermal instability of the hydroperoxides since the hydroperoxides can decompose to form other oxygenated products.
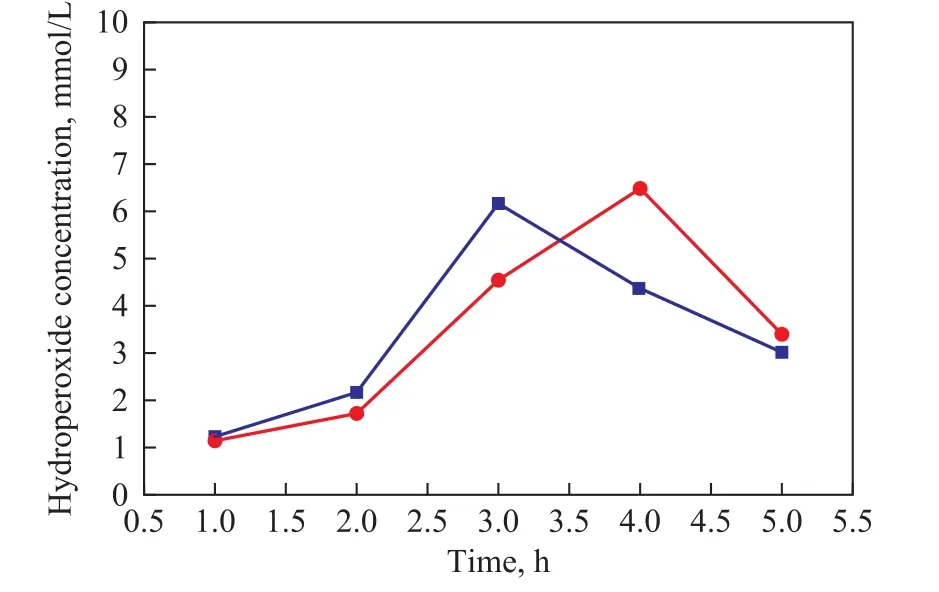
Figure 10 Hydroperoxide concentration in O2-oxidized n-octane and model diesel
The change of hydroperoxides concentration in O2-oxidized model diesel exhibited a similar trend as the case with the O2-oxidized n-octane. But the concentration of hydroperoxides was slightly lower than that experienced during the oxidation of n-octane for 3 h. The reason might be assumed that a certain amount of hydroperoxides serving as the oxidant in the oxidation process was consumed on oxidizing DBT. Since DBT already has been subject to complete oxidation for 4 h, it does not need to consume hydroperoxides. So the concentration of hydroperoxides demonstrated an obvious growth and reached a maximum value in 4 h.
4 Conclusions
In this work, n-octane was subject to autoxidation reaction to generate hydroperoxide via the in-situ reaction with oxygen. The hydroperoxides were effective in oxidizing DBT to form the corresponding sulfones which were con firmed by FT-IR and1H-NMR. The removal of DBT reached 98.4% in the model diesel at 140 °C. The presence of cyclohexane and n-dodecane had no impact on the removal of DBT, while the presence of xylene had a slight negative in fluence on the removal of DBT. This study paved the way for the ODS process using O2as an oxidant.
Acknowledgements:The authors are grateful to the Undergraduate Innovation and Entrepreneurship Training Project (201710057009) for providing funding and support for this research. Special thanks are extended to the Research Centre of Modern Analytical Technology, Tianjin University of Science and Technology for H1-NMR analyses.
[1] Tang N, Zhao X, Jiang Z, et al. Oxidation of dibenzothiophene using oxygen and a vanadophosphate catalyst for ultra-deep desulfurization of diesels[J]. Chinese Journal of Catalysis, 2014, 35(9): 1433–1437
[2] Lu S, Zhong H, Mo D, et al. A H-titanate nanotube with superior oxidative desulfurization selectivity[J]. Green Chemistry, 2017, 19(5): 1371–1377
[3] Jiang B, Yang H, Zhang L, et al. Efficient oxidative desulfurization of diesel fuel using amide-based ionic liquids[J]. Chemical Engineering Journal, 2016, 283:89–96
[4] Tian Y, Yao Y, Zhi Y, et al. Combined extraction–oxidation system for oxidative desulfurization (ODS) of a model fuel[J]. Energy & Fuels, 2015, 29(2): 618–625
[5] Lu Z, Guo E, Zhong H, et al. Kinetic modeling of the extraction–oxidation coupling process for the removal of dibenzothiophene[J]. Energy & Fuels, 2016, 30(9): 7214–7220
[6] Lü H, Zhang Y, Jiang Z, et al. Aerobic oxidative desulfurization of benzothiophene, dibenzothiophene and 4,6-dimethyldibenzothiophene using an Anderson-type catalyst [(C18H37)2N(CH3)2]5[IMo6O24][J]. Green Chemistry,2010, 12(11): 1954–1958
[7] Bazyari A, Khodadadi A A, Mamaghani A H, et al.Microporous titania–silica nanocomposite catalystadsorbent for ultra-deep oxidative desulfurization[J].Applied Catalysis B: Environmental, 2016, 180: 65–77
[8] Long Z, Yang C, Zeng G, et al. Catalytic oxidative desulfurization of dibenzothiophene using catalyst of tungsten supported on resin D152[J]. Fuel, 2014, 130: 19–24[9] Tang N, Zhang Y, Lin F, et al. Oxidation of dibenzothiophene catalyzed by [C8H17N(CH3)3]3H3V10O28using molecular oxygen as oxidant[J]. Chemical Communications,2012, 48(95): 11647–11649
[10] Sundararaman R, Song C. Catalytic oxidative desulfurization of diesel fuels using air in a two-step approach[J].Industrial & Engineering Chemistry Research, 2014, 53(5):1890–1899
[11] Zhang W, Xiao J, Wang X, et al. Oxidative desulfurization using in-situ-generated peroxides in diesel by light irradiation[J]. Energy & Fuels, 2014, 28(8): 5339–5344
[12] Sundararaman R, Ma X, Song C. Oxidative desulfurization of jet and diesel fuels using hydroperoxide generated in situ by catalytic air oxidation[J]. Industrial & Engineering Chemistry Research, 2010, 49(12): 5561–5568
[13] Li D, Fang W, Xing Y, et al. Spectroscopic studies on thermal-oxidation stability of hydrocarbon fuels[J]. Fuel,2008, 87(15): 3286–3291
[14] Herbinet O, Husson B, Serinyel Z, et al. Experimental and modeling investigation of the low-temperature oxidation of n-heptane[J]. Combustion and Flame, 2012, 159(12):3455–3471
[15] Zhang H X, Gao J J, Meng H, et al. Catalytic oxidative desulfurization of fuel by H2O2in situ produced via oxidation of 2-propanol[J]. Industrial & Engineering Chemistry Research, 2012, 51(13): 4868–4874
[16] Xiao J, Wu L, Wu Y, et al. Effect of gasoline composition on oxidative desulfurization using a phosphotungstic acid/activated carbon catalyst with hydrogen peroxide[J].Applied Energy, 2014, 113: 78–85
[17] Bhasarkar J B, Chakma S, Moholkar V S. Investigations in physical mechanism of the oxidative desulfurization process assisted simultaneously by phase transfer agent and ultrasound[J]. Ultrasonics Sonochemistry, 2015, 24:98–106
[18] Chamack M, Mahjoub A R, Aghayan H. Cesium salts of tungsten-substituted molybdophosphoric acid immobilized onto platelet mesoporous silica: Efficient catalysts for oxidative desulfurization of dibenzothiophene[J]. Chemical Engineering Journal, 2014, 255: 686–694
[19] Xinrui Zhou, Juan Li, Xiuna Wang, et al. Oxidative desulfurization of dibenzothiophene based on molecular oxygen and iron phthalocyanine[J]. Fuel processing technology, 2009, 90(2): 317–323
[20] Fattah I M R, Masjuki H H, Kalam M A, et al. Effect of antioxidants on oxidation stability of biodiesel derived from vegetable and animal based feedstocks[J]. Renewable and Sustainable Energy Reviews, 2014, 30: 356–370
[21] Balster L M, Balster W J, Jones E G. Thermal stability of jet-fuel/paraffin blends[J]. Energy & fuels, 1996, 10(6):1176–1180
- 中国炼油与石油化工的其它文章
- Synthesis of Core-Shell HZSM-5@SBA-15 Composite and Its Performance in the Conversion of Methanol to Aromatics
- Effects of Gasoline with Ester Additives on the Swelling Behavior of Rubbers
- Fabrication of the Core-Shell Structured ZSM-5@Mg(Al)O and Its Catalytic Application in Propane Dehydrogenation
- ZSM-5/MAPO Composite Catalyst for Converting Methanol to Ole fins in a Two-Stage Unit with a Dimethyl Ether Pre-Reactor
- Bitumen Recovery from Indonesian Oil Sands Using ASP (Alkali, Surfactant and Polymer) Agent
- Controllable Synthesis of Mixed-Phase TiO2 with Small Anatase and Rutile Particle and Its Enhanced Photocatalytic Activity

