Fabrication of the Core-Shell Structured ZSM-5@Mg(Al)O and Its Catalytic Application in Propane Dehydrogenation
Wang Bo
(New Product Development Division of Fine Chemicals Company, SINOPEC Yangzi Petrochemical Company Limited, Nanjing 210048)
1 Introduction
In the past decade, propane dehydrogenation had attracted significant attention because of the growing demand for propylene. Indeed, propylene has a good application prospect in the production of polypropylene, acrolein,polyacrylonitrile, and acrylic acid[1-3]. However, the propane dehydrogenation reaction is an endothermic process that requires a relatively high temperature to obtain a high yield of propylene[4]. Unfortunately, under this severe condition, the unfavorable side reactions are accelerated, leading to the coke formation and even deactivation of the catalysts during the reaction process. In order to overcome the adverse effect, it is important to choose a profitable support for the catalysts. Therefore, as the support for the catalysts, ZSM-5 possesses a well-de fined 10-membered-ring channel system, which can effectively hinder the formation of large hydrocarbon molecules during the reaction process, leading to the restriction of the coke deposits and the improvement of the catalytic stability[5-7]. Moreover, compared with the support of metal oxides, thanks to its large surface area and the particular character of channels, ZSM-5 shows an increased capacity to accommodate the coke. However, the pristine ZSM-5 possesses a large amount of acids and almost half of the acidity is centered in the strong acid sites, which are bene ficial to the undesirable side reactions but harmful to the yield of dehydrogenation reaction[8]. Therefore,ZSM-5 could be considered as a potential catalyst support to be applied in the catalyst reaction, but a lot of work need to be performed on this material.
Many methods have been applied to modify the acidic property of ZSM-5, such as the etching of Al, the introduction of alkali and/or alkali earth metals, the combination with other materials, and so on. Huang, et al.[9]prepared the micro-mesopore hierarchical ZSM-5 material by the alkali treatment method. They found that the modified zeolite showed larger external surface with less acid sites as compared with the pristine ZSM-5, leading to less coke deposits on the metallic surface. But for this method,the pore structure of ZSM-5 was inevitably destroyed and the stability of the catalysts might be in fluenced. Judging
As one of the Mg(Al)O material, MgAl2O4has attracted our attention, which not only has a lot of excellent physiochemical properties such as high melting point (2105ºC), chemical inertness, considerable mechanical strength,favorable chemical stability and good thermal shock resistance[15-17], but also has appropriate properties suitable for being used as a support of metallic catalyst, such as low acidity, high thermal resistance and good interaction with the metallic phase[18]. Virnovskaia, et al.[19]elucidated that PtSn/Mg(Al)O displayed high catalytic stability in the ethane dehydrogenation reaction. But as the support of the catalyst, the small surface area of MgAl2O4is a potential shortcoming for the dispersion of active metals. Moreover, Xu, et al.[20]combined ZSM-5 zeolite and MgAl2O4to obtain a novel bifunctional additive. They found that the solid-state ion exchange would take place between ZSM-5 and MgAl2O4, and this effect could in fluence the catalytic activity of the mixed compounds.
Based on the above reports, in order to modify the acidic property of ZSM-5 and solve the problem of the low surface area in MgAl2O4, we would bring forward a method which could introduce the coating of MgAl2O4phase onto the ZSM-5 support to obtain a core-shell ZSM-5@Mg(Al)O structure. The prepared samples were characterized by several techniques, such as XRD, nitrogen adsorption, SEM, FT-IR, NH3-TPD, XPS and TPO, and the propane dehydrogenation reaction was employed to evaluate the catalytic activity. This strategy can provide us with important information on understanding the effect of support with different surface and porous characteristics of the supported metallic catalyst.
2 Experimental
2.1 Synthesis of the material of MgAl2O4, ZSM-5 and ZSM-5@Mg(Al)O
Mg(NO3)2·6H2O, Al(NO3)3·9H2O and ammonia solution were used as the reagents to prepare MgAl2O4. Firstly, by mixing Mg(NO3)2·6H2O and Al(NO3)3·9H2O in aqueous solution, a 0.5 mol/L solution of nitrates was obtained,in which the Al/Mg mol ratio was equal to 2. Under continued stirring at 40 ºC, a solution containing 35%of NH3·6H2O was dropwise added into the above mixture until the pH value reached up to 9.58. Afterward, a resulting gel was formed and was subject to ageing for 1 h at room temperature. After being filtered and dried at 120 ºC for 12 h, the obtained sample was finally calcined at 800 ºC for 4 h.
The pristine ZSM-5 was prepared according to the descrition in our previous literature[21].
The ZSM-5@Mg(Al)O was prepared according to the following procedure: 2 g of ZSM-5 were added to 80 mL of petroleum ether to obtain the solution A under stirring for 3 h. In addition, different amounts of Mg(NO3)2·6H2O and Al(NO3)3·9H2O were dissolved in aqueous solution with an Al/Mg molar ratio of 2 to obtain the solution B.Then the solution B was added to the solution A. Under stirring, 35% of NH3·6H2O were added to the mixed solution until the pH value was equal to 9.58. The obtained mixed solution was stirred at room temperature for 24 h,and then was filtered and dried at 120 ºC for 12 h. Afterward, the sample was calcined at 800 ºC for 4 h in a tubular furnace at a temperature increase rate of 10oC/min.The resulting material is named as Z@MA-x, in which the word “x” stands for the weight ratio of Mg(Al)O/ZSM-5.
2.2 Preparation of the catalysts
The PtSnNa/ZSM-5 catlyst was prepared by a co-impregnation method. Firstly, the ZSM-5 sample was impregnated in an aqueous solution of 0.427 mol/L NaCl at 80oC for 4 h, and then was impregnated in a solution containing a mixture of 0.033 mol/L H2PtCl6and 0.153 mol/L SnCl4under the same condition. After that,the prepared catalyst was dried at 80oC for 3 h. The nominal composition of each sample covered 0.5% of Pt, 1.0%of Sn and 1.0% of Na. Finally, the catalyst was calcined at 500oC for 5 h, and then was reduced with H2at 500oC for 8 h. The PtSnNa/Z@MA-x catalyst was also prepared according to the same procedure used for preparation of PtSnNa/ZSM-5.
2.3 Catalyst characterization
The powder X-ray diffraction (XRD) patterns of all the products were conducted on a Bruker D-8 Advance X-ray diffractometer producing Cu Kα radiation from a generator operating at 40 kV and a current of 30 mA. An angular range from 5º to 90º was recorded using step scanning and long counting time to determine the position of the peaks.
The nitrogen adsorption-desorption isotherms were measured at -196 ºC on a Micromeritics ASAP 2000 apparatus. The samples were evacuated under a vacuum of 0.667 Pa at 350 ºC for 15 h. The data of pore structure were analyzed by the BJH (Barrett-Joyner-Halenda) method using the Halsey equation for the multilayer thickness.
The inductively coupled plasma (ICP) optical emission spectrometry was used for the determination of the metal content in each sample after the hydrothermal treatment.The measurements were performed with a JA1100 inductively coupled plasma quantometer, and the sample was dissolved in a mixture of HF and HNO3acids before the measurement.
The platinum dispersion was determined by chemisorption measurements. This experiment was carried out using the dynamic pulse technique with an argon (99.99%) flow at a rate of 50 mL/min and pulses of hydrogen. The experimental process was the same as reported by Dorado,et al.[22], except the sample reduction temperature which was equal to 500oC, and the temperature of the argon gas for removing the hydrogen was by 40oC higher than the reduction temperature.
The crystal morphology of different supports was investigated by a Hitachi S-3400N SEM. The infrared spectra were recorded on a Bruker Tensor 27 FTIR spectrometer using DRIFT techniques, with a scanning range from 4000 cm−1to 400 cm−1. The sample was ground with KBr and pressed into a thin wafer.
The surface acidity was measured by NH3-TPD in a Tp-5000 apparatus at ambient pressure. The sample (150 mg) was preheated at 500 ºC for 1 h and then was cooled down to room temperature in flowing He. At this temperature, suf ficient pulses of NH3were injected until adsorption saturation. TPD was carried out from 100 ºC to 500 ºC at a temperature increase rate of 10 ºC/min using helium (30 mL/min) as the carrier gas.
The X-ray photoelectron spectra (XPS) measurements were carried out in a Thermo Scientific ESCALAB 250 instrument (USA) using non-monochromatic Al Kα 1486.6 radiation. All samples were reduced in-situ in hydrogen at 500oC for 1 h. Then the spectra were recorded at room temperature in a vacuum of lower than 5×10-9mbar. The binding energy values of the XPS signals were calibrated by using the Si 2p peak at 102.9 eV.
Thermogravimetric (TG) analysis was measured in airflow (at a rate of 30 mL/min) with a LCT thermogravimetric analyzer (Beijing Optical Instrument Factory,China) from room temperature to 700 °C at a temperature increase rate of 20 °C/min. 0.02 g of the catalyst were placed in the analyzer.
The temperature-programmed oxidation (TPO) was measured with the same apparatus as that used for NH3-TPD measurement. About 0.15 g of sample were placed in a quartz reactor at room temperature, and then were heated up to 700 °C at a temperature increase rate of 10 °C/min in a 5% O2/He mixture (at a flow rate of 30 ml/min).
2.4 Catalyst reaction
Propane dehydrogenation was carried out in a quartz tubular micro-reactor. The reaction conditions for propane dehydrogenation covered a reaction temperature of 590 ºC,a reaction pressure of 0.1 MPa, a H2/propane molar ratio of 0.25, and a propane weight hourly space velocity(WHSV) of 3.0 h-1. The reaction products were analyzed with an online GC-14C gas chromatograph (Shimadzu Japan).
3 Results and Discussions
3.1 Physicochemical properties of the materials
The XRD patterns of different samples are shown in Figure 1. Basically, the XRD pattern of ZSM-5 had its representative peaks at 8o–9oand 22o–24o, corresponding to that given in reference[23]for pure ZSM-5. By comparison, in Z@MA-x, these typical diffraction peaks of ZSM-5 were still observed, indicating that the structural integrity of ZSM-5 was well preserved in each material after incorporating with Mg(Al)O. Besides, in the patterns of the series Z@MA-x materials, there were multiple new peaks appearing to be located at 2θ = 19.0º, 31.5º, 36.8º,45.4º, 62.3º, and 66.4º. These peaks could perfectly match up to the characteristic peaks specific for MgAl2O4as shown in Figure 1. However, with the Mg(Al)O/ZSM-5 ratio increasing from 1 to 5, the diffraction lines of ZSM-5 became weaker and smaller in each Z@MA-x material,which should be attributed to the increased thickness of the shell structure of Mg(Al)O species in Z@MA-x samples[24].

(1) ZSM-5; (2) Z@MA-1; (3) Z@MA-3; (4) Z@MA-5; and (5)MgAl2O4
The nitrogen adsorption-desorption isotherms of different samples are shown in Figure 2(a). The parent ZSM-5 zeolite represented the type I isotherm with a small N2uptake at higher relative pressure, which was typical of microstructure material without any mesopores[9]. As for the MgAl2O4sample, it exhibited the typical type IV adsorption isotherm with a H1 hysteresis loop as de fined by IUPAC[25]. The isotherm displayed a sharp step with the increase in relative pressure (p/p0), which was characteristic of capillary condensation of nitrogen with mesopores[26].In comparison, after modification, the series Z@MA-x samples turned to be the type IV adsorption isotherms with a H3 hysteresis loop, indicating to a disordered mesostructure[27]. Compared with the former pure materials,we assume that these emerging adsorption isotherms in Z@MA-x were related to the pore structure of MgAl2O4.In this case, due to the interface interaction between the inside ZSM-5 and the outside MgAl2O4shell, the shell structure was correspondingly changed and was different from the MgAl2O4in Figure 2(a). In Figure 2(b), the pore size distribution of different samples is observed. As expected, Z@MA-x displayed two pore size distributions which might be the reason of the coexistence of ZSM-5 and Mg(Al)O in the materials.
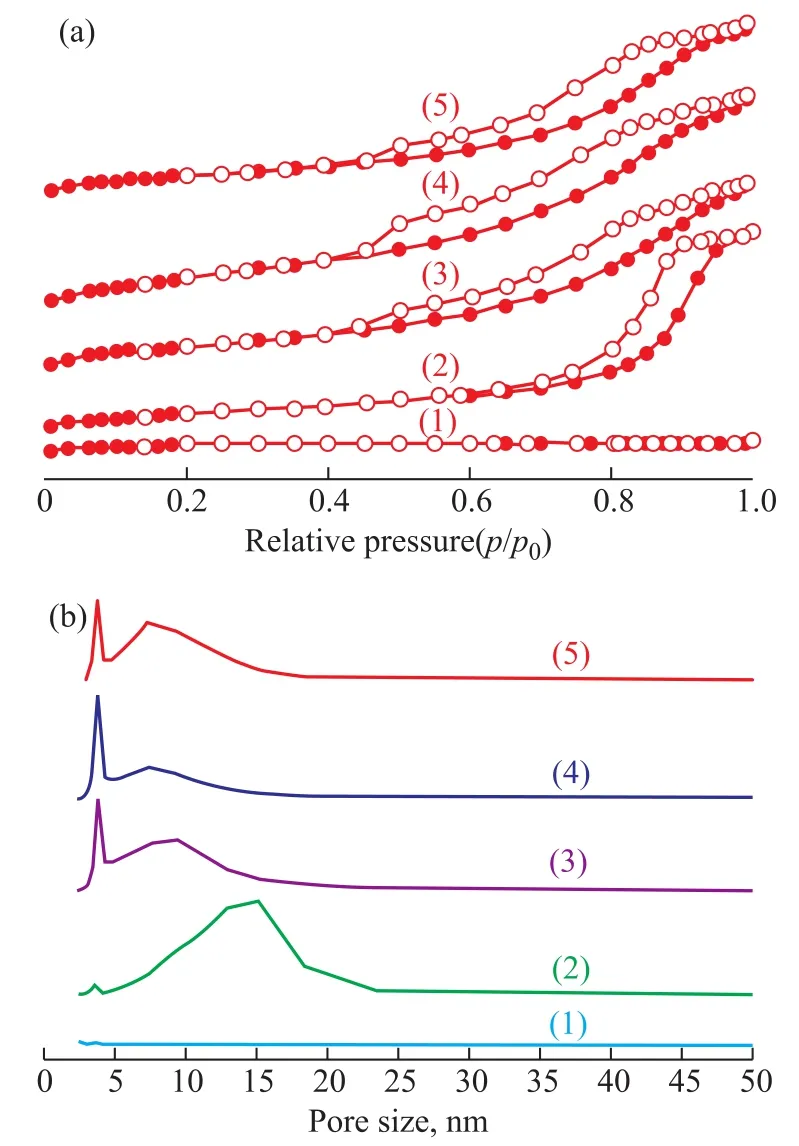
Figure 2 (a) N2 adsorption-desorption isotherms and (b)the corresponding pore size distribution as obtained from the desorption branch of the different samples
The data for characterization of different samples are listed in Table 1. As it can be seen that the surface areas of Z@MA-x were greatly decreased as compared with ZSM-5. This phenomenon could be related to the low surface area of MgAl2O4in the material. It should be noted that when the Mg(Al)O/ZSM-5 ratio reached up to 5, the surface area of Z@MA-5 was reduced significantly to 238 m2/g in distinct contrast with that of Z@MA-1 and Z@MA-3. This phenomenon could be attributed to the high content of Mg(Al)O shell, which would lead to the pore channel block to some extent in the Z@MA-5 material. However, in particular, by the formation of coreshell structure, the Sextof Z@MA-x was significantly increased as compared with that of the pristine ZSM-5, especially for the case of Z@MA-3 (209 m2/g). This apparent improvement of structural properties in Z@MA-3 would be propitious to the dispersion of the metals on the support. Based on this good behavior, it is suggested that Z@MA-3 would show a great potential as a catalyst support.
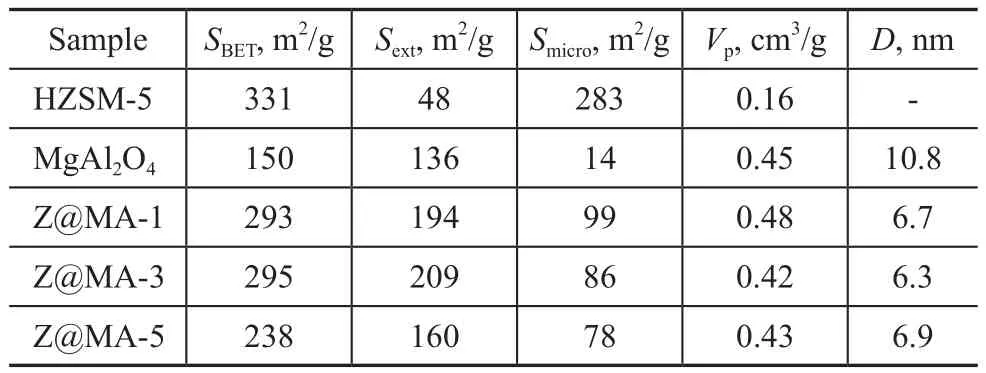
Table 1 Data for characterization of different samples.
For better understanding the core-shell structure in the series of Z@MA materials, Figure 3 represents the scanning electron micrographs of different samples. It can be seen from Figure 3(1) that the HZSM-5 zeolite exhibited a typical MFI structure with the “boat-like”hexagonal-shaped crystal rods, which were quite uniform with a crystal size of 6—8 μm in length. As expected,after being coated with Mg(Al)O, the particle size of Z@MA-x was different. However, for the case of Z@MA-1, the particle size was almost the same as that of ZSM-5, which demonstrated that the content of Mg(Al)O shell was not suf ficient to cover the ZSM-5 to form the structure of core-shell material. In spite of this, when the ratio of Mg(Al)O/ZSM-5 reached up to 3, the particles of Z@MA-3 were enlarged and the shell of Mg(Al)O was well produced. In consequence, the crystal size of Z@MA-3 was between 8 μm and 10 μm in length. This fact meant that the thickness of Mg(Al)O shell in Z@MA-3 was about 2 μm. By increasing the ratio of Mg(Al)O/ZSM-5 to 5, the particle size was continually improved in Figure 3, demonstrating that the core-shell material of Z@MA-5 was over-coated. According to the result of N2adsorption-desorption measurements, this over-thick shell structure might be harmful to the physiochemical properties of the core-shell material.
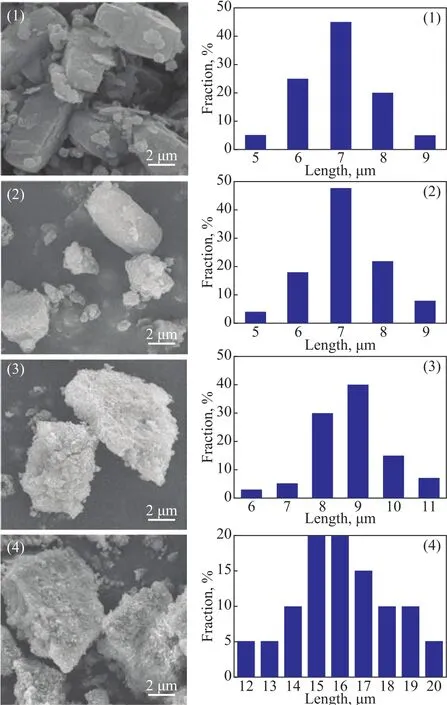
Figure 3 SEM micrographs and the corresponding particle size distributions in length of different samples: (1) ZSM-5,(2) Z@MA-1, (3) Z@MA-3, and (4) Z@MA-5
For further analysis of the physiochemical properties of different zeolite samples, the FT-IR spectra of different samples in the region of 400―4 000 cm-1are shown in Figure 4. The ZSM-5 zeolite exhibited the characteristic peaks of MFI structure at 1 050―1 250 cm-1(external or internal asymmetric stretch), 800 cm-1(external symmetric stretch), 550 cm-1(double rings), and 450 cm-1(T-O bend)[28]. In comparison, these characteristic peaks are still retained and even decreased drastically by covering the Mg(Al)O layer on the ZSM-5 in the series of Z@MA-x materials, indicating that the ZSM-5 structure was well preserved in each catalyst and the layer of Mg(Al)O was gradually thickened after introduction. Moreover, the spectra in the region at 3 000―3 750 cm-1were assigned to O-H stretching, and the absorption band at around 3 600 cm-1was ascribed to the OH groups bridging structural hydroxyl groups of the Si(OH)Al type in the zeolite,which were highly affected by the acidity in the series materials[29]. As shown in Figure 4, since the intensity of IR absorption at between 3000 cm-1and 3 750 cm-1was changed in Z@MA-x, the acidity of the material would be different. Besides, an absorption band at around 3 200 cm-1was increased in Z@MA-x as compared with ZSM-5. The reasonable explanation is that the Mg2+and/or Al3+ions from Mg(Al)O shell were incorporated into the crystal lattice of ZSM-5[30].
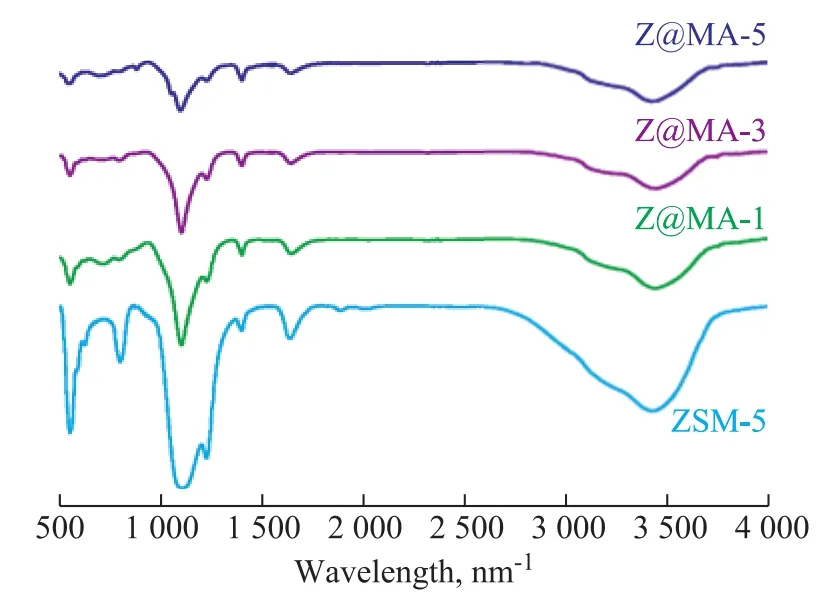
Figure 4 FT-IR spectra of different samples
Upon checking the results of FT-IR spectrometric analysis in order to find out the effect of core-shell structure on the acidity of different samples, the NH3-TPD measurements of different samples were carried out, with the results shown in Fig 5. As shown in Figure 5, two main NH3desorption peaks were observed in the ZSM-5 sample, which could be assigned to the weak (around 234oC)and the strong (around 417oC) acid sites. In contrast, it is worthy of noticing that the amount of ammonia desorption decreased significantly in Z@MA-x, especially without any ammonia desorption at high temperature.This fact indicated that the coverage of shell structure had resulted in serious blockage of strong acidic sites in the Z@MA-x samples. Meanwhile, with an increasing weight ratio of Mg(Al)O/ZSM-5, the maximum value of the low temperature peaks in Z@MA-x shifted toward the lower temperature region as compared with ZSM-5,which meant that weak acid sites of Z@MA-x became continuously weaker after modification. However, to some extent, the acid amount of Z@MA-3 was the least among the Z@MA samples. Furthermore, it is interesting to observe that new ammonia desorption appeared, which corresponded to a temperature of about 340oC. This newly found point implied the emergence of some acid sites with moderate intensity. According to the results of FT-IR spectrometric analysis, this could be attributed to the migration of Al species from MgAl2O4into the framework of ZSM-5[13].

Figure 5 NH3-TPD patterns of different samples
3.2 Characterization of the catalysts
As mentioned above, in our experiments, the as-prepared Z@MA-x materials were used as the support, and then the PtSnNa-catalysts were obtained by the impregnation method. As listed in Table 2, the series of PtSnNa/Z@MA-x catalysts displayed higher value of platinum dispersion (DH2) than the PtSnNa/ZSM-5 catalyst. Possibly, this behavior might be attributed to the fact that the presence of Mg(Al)O species could help the adsorption of platinum chloride anions (PtCl62−) on the support, thus promoting the distribution of metallic particles. Zhang,et al.[12]discussed that a suitable addition of Mg species was beneficial to the platinum dispersion in the PtSnK/Al2O3catalyst, but the excessive Mg species might block the platinum species, leading to the decrease of the metal dispersion. Besides, judging from the data listed in Table 1, the pure ZSM-5 sample demonstrated the higher BET surface area. Thus, this relative improvement of metallic particles distribution would be likely attributed to the existing interactions between the active metal and the support which could affect either the surface characteristics of active metals or the degree of metallic sintering.However, it should be noted that the mesoporous structure of Z@MA-5 had been changed significantly (Table 1), which would be harmful to the interaction between the metal precursors and the support[31]. In this case, a suitable content of Mg(Al)O species would suppress the undesirable reaction and thus affect the catalytic performance in propane dehydrogenation. Further discussions will be presented in the following section.
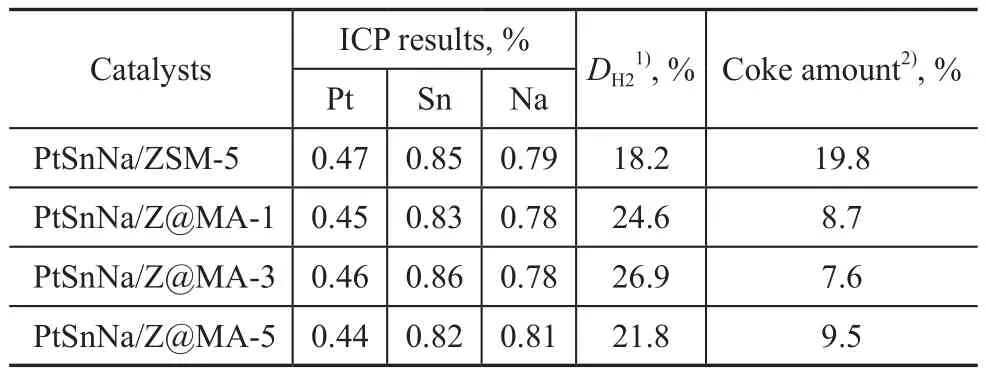
Table 2 Characterization data for different catalysts
As referred to in the reports of our previous publications[32-33], the loading of additional Mg and/or Al species on the catalysts could strengthen the interaction between Sn and the support to stabilize the oxidized tin species in the catalysts. In order to obtain the information about the oxidation state of the metal phase (Sn) on the surface of catalysts, the characterization of different catalysts by XPS was carried out. Figure 6 shows the Sn3d5/2XPS region for the different catalysts, while the measured binding energy of Si2p, Al2p, Sn3d5/2levels is summarized in Table 3. By taking PtSnNa/ZSM-5 as the representative sample, based on the deconvolution of the spectrum, three peaks were obtained at 485.2 eV, 486.5 eV, and 487.4 eV,which were assigned to different tin species. The component at lower binding energy was attributed to the metallic state of Sn (Sn0), while the others were ascribed to the different types of tin oxides (SnO and/or SnO2)[34-35]. But among these oxides of tin, their binding energy was very close, since it was difficult to distinguish between Sn4+and Sn2+by the XPS analysis[36]. Therefore, the single conclusion from these experiments is that most amount of tin in each catalyst existed in the oxidized form. Judging from the data compiled in Table 3, the percentage of zero-valent Sn in PtSnNa/ZSM-5, PtSnNa/Z@MA-1, PtSn-Na/Z@MA-3, and PtSnNa/Z@MA-5 was 29.1%, 28.9%,26.4%, and 32.1%, respectively. These results suggested that a suitable amount of Mg(Al)O in the core-shell structure could strengthen the interaction between Sn and the support, whereas an excess of Mg(Al)O would weaken this interaction, resulting in the increase of metallic tin. Futhermore, the metallic Sn could be alloyed with Pt, leading to the permanent deactivation of PtSn catalysts[37]. Thus, it seems that the formation of coreshell structure has an important effect on the oxide status of tin species.
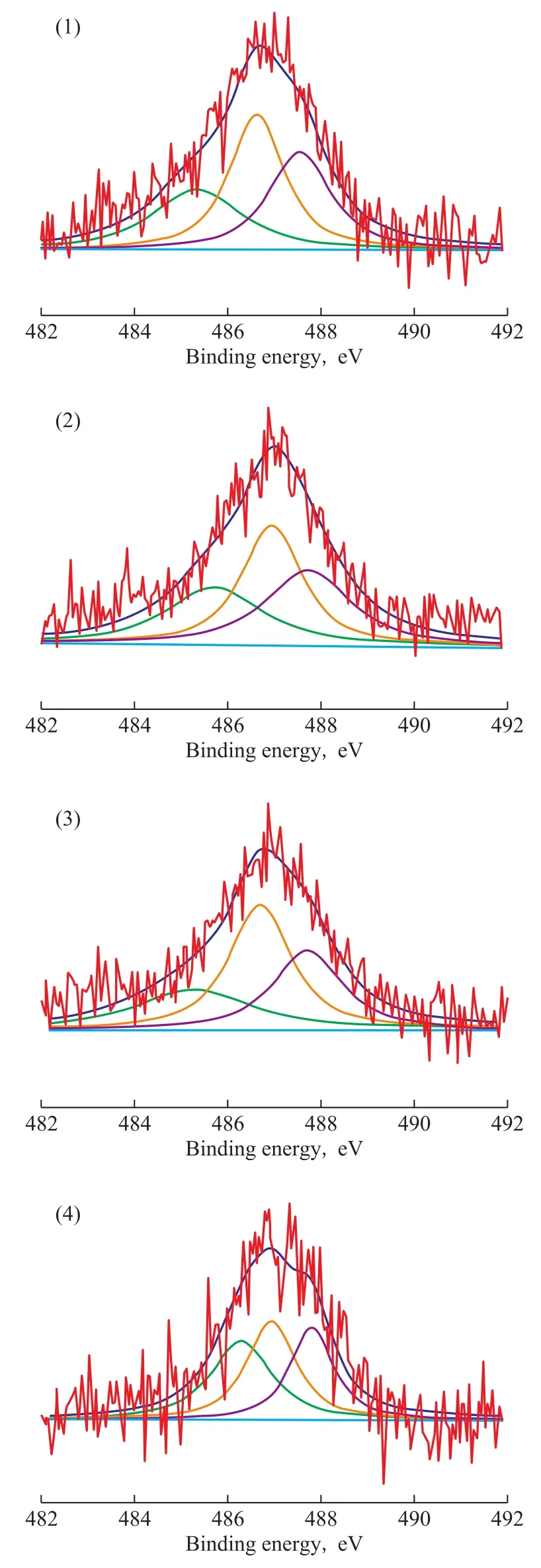
Figure 6 XPS spectra corresponding to Sn 3d5/2 signals on reduced samples: (1) PtSnNa/ZSM-5, (2) PtSnNa/Z@MA-1,(3) PtSnNa/Z@MA-3, and (4) PtSnNa/Z@MA-5
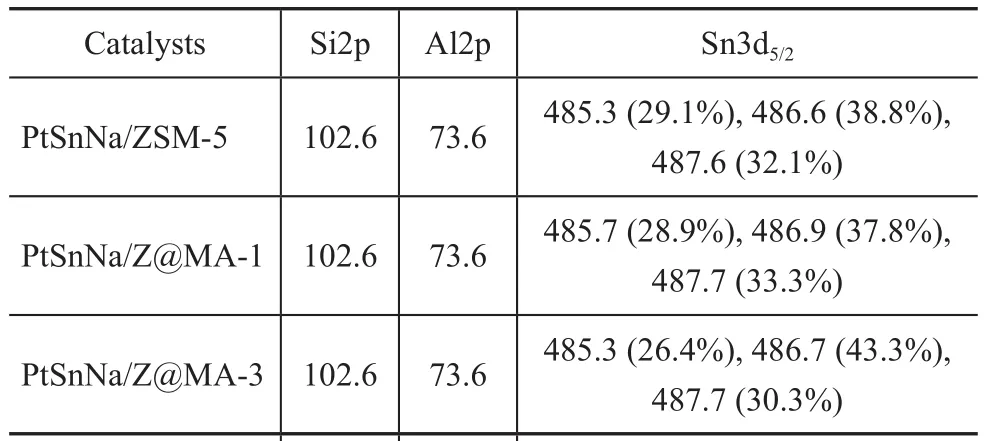
Table 3 XPS binding energy for the Si2p, Al2p and Sn3d5/2 measured for different catalysts
3.3 Catalytic performance
Since the physicochemical properties of the series of Z@MA-x materials were greatly modified in comparison with pure ZSM-5, it is reasonable to believe that the catalytic performance of different catalysts should be different. Figure 7 shows the catalytic behaviors of different catalysts, with the corresponding data listed in Table 4. It can be seen from Figure 7(a) that the PtSn-Na/ZSM-5 catalyst exhibited a lowest initial reaction activity, and the propane conversion decreased from 35.6 % to 29.4 % after reaction for 8 h. This decrease of conversion rate was related to the carbon deposits on the catalyst, which prohibited the active metal from keeping in contact with the reactor molecules. After the introduction of Mg(Al)O, the catalytic activity of the series of PtSnNa/Z@MA catalysts increased drastically,especially for the PtSnNa/Z@MA-3 catalyst. To explain these findings, it should be noted that the catalysts were bifunctional and the two active centers of PtSnNa/ZSM-5 (the metal particles and the acid sites) might work collaboratively: (de)hydrogenation and ring opening could occur on the metal particles, whereas isomerization through C3+carbenium ions could occur on the acid sites[38]. Besides, on the bimetallic PtSn catalyst, platinum was the only active metal and propylene could be only formed on the metal by dehydrogenation; the main cracking product (ethene) was mainly formed through cracking on the carrier and ethane was formed by hydrogenolysis of propane and also by hydrogenation of ethene, with both reactions taking place on the metal[39].Judging from the results of XPS analysis, more amount of Sn existed in the oxidized form in the PtSnNa/Z@MA-3, which was favorable to the anchoring of Pt on Sn oxide. The Pt sites anchored on Sn oxide surface with a“sandwich structure” were the main reaction active sites of PtSn-catalysts. In this case, the propane conversion over PtSnNa/Z@MA-3 in 8 h was much higher than that achieved by other catalysts. Besides, in view of NH3-TPD analysis, the Z@MA-x catalysts had the weakened acid sites than ZSM-5. According to this mechanism, a much lower propylene selectivity was observed for the PtSnNa/ZSM-5 catalyst. In combination with the results depicted in Table 3, the deactivation parameter of PtSn-Na/Z@MA-3 was the lowest (13.7%) among these catalysts, demonstrating the admirable catalytic stability of PtSnNa/Z@MA-3 catalyst.
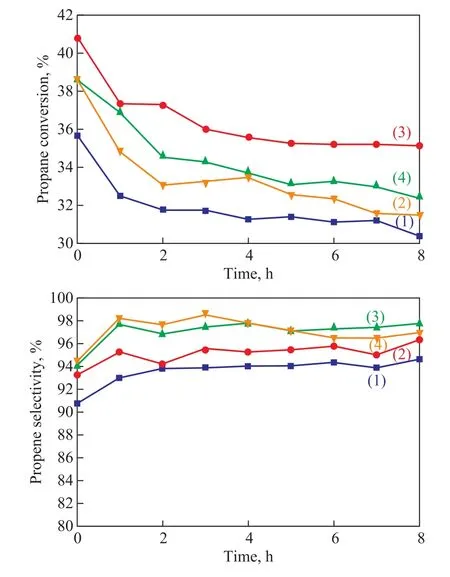
Figure 7 (a) Propane conversion and (b) propylene selectivity of different catalysts for propane dehydrogenation:(1)―PtSnNa/ZSM-5; (2)―PtSnNa/Z@MA-1;(3)―PtSnNa/Z@MA-3; (4)―PtSnNa/Z@MA-5
3.4 Coke analysis
To analyze the coke deposits on the catalyst, the TPO pro files of different catalysts are presented in Figure 8.In the TPO profiles, there were two successive peaksrepresenting two different carbon deposits. The carbon deposits correponding to the first peak at low temperature were those located on the active centers of metals,while the second peak at high temperature represented those located on the external surface of the support[40]. It can be easily seen from Figure 8 that there was a large amount of coke deposited on PtSnNa/ZSM-5, especially for the one located on the acitive centers. Our previous reports[13]showed that the coke deposited on the external surface of the support had little impact on the catalytic acitivity. This might be the main reason that the deactivation rate of PtSnNa/ZSM-5 was relatively high as shown in Figure 7. Compared with PtSnNa/ZSM-5, the coke deposition peaks of other PtSnNa/Z@MA-x catalysts on the active metal shifted towards the lower temperature region, suggesting that the deposited carbon became more reactive. Moreover, the quantitative analysis of coke in Table 2 clearly showed that the amount of coke formed over the series of PtSnNa/Z@MA-x catalysts was much lower than that formed over the PtSnNa/ZSM-5 catalyst (19.8 %). In addition, the content of Mg(Al)O species could also influence the amount of coke formed over the PtSnNa/Z@MA-x catalysts. As summarized in Table 2, the PtSnNa/Z@MA-3 catalyst possessed the lowest coke amount (7.6 %). This outcome could be attributed to the weakened acidity in these catalysts after the introduction of Mg(Al)O shell in Z@MA-x materials. The intrinsic acidity of the support could affect the undesirable reaction such as cracking/isomerization, and ole fins had turned out to be the primary precursors of the mechanism of coke formation[41].Therefore, based on the results of NH3-TPD analysis,a lower amount of acid sites in Z@MA-3 could be the main reason of less coke deposits on the catalyst. In addition, the relatively large Sextof Z@MA would facilitate the transportation of coke precursors, leading to a lower carbon amount and weaker carbon intensity in the catalyst[42]. Moreover, the presence of Sn oxides in Pt catalyst would weaken the binding between hydrocarbons and metal in the catalyst[43-44]. Thus, a much more amount of Sn oxides in the catalyst would promote the migration of coke precursor from the metal surface to the external surface of support, making more metallic active sites exposed. Combining with the results of XPS analysis, in the series of Z@MA catalysts, the coke precursor formed on the metal might migrate to the support and the acid sites[45]. As a conclusion, in the PtSnNa/Z@MA-3, the coke precursor formed on the metal was highly suppressed and could be even migrated to the external surface of support, making the catalytic activity and stability of PtSnNa/Z@MA-3 the best.
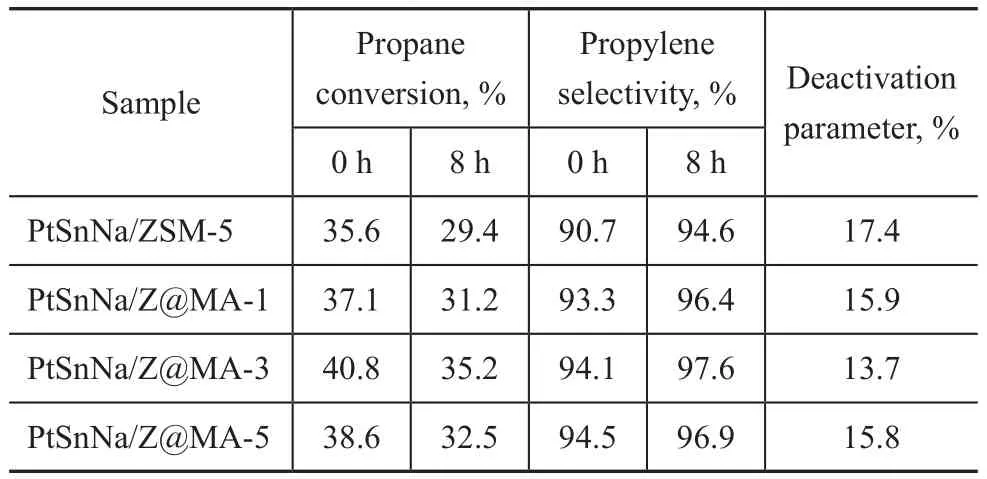
Table 4 Catalytic properties of different catalysts after conducting reaction for 8 h
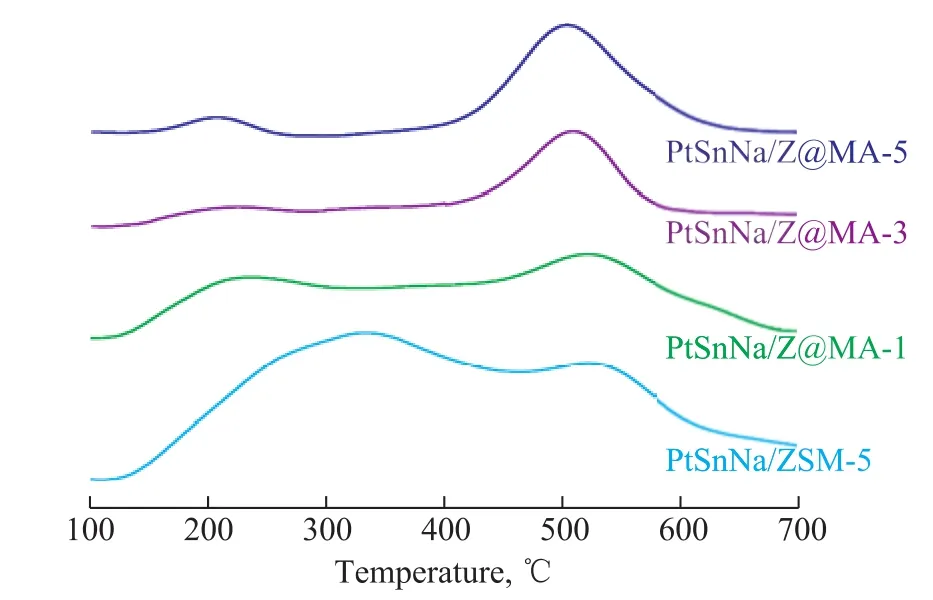
Figure 8 TPO pro files of different catalysts
3.5 Catalytic Stability
Judging from the results of the catalytic performance, the reaction activity and stability of the PtSnNa/Z@MA-3 catalyst were superior to those of other catalysts. Consequently, this catalyst was chosen for the stability test at a reaction temperature of 590oC. The propane conversion and selectivity to propylene as a function of reaction time are shown in Figure 9. The result showed that the selectivity to propylene was higher than 97%, and the propane activity was still over 32% after the reaction had continued for 150 h. In conclusion, these results have demonstrated that the PtSnNa/Z@MA-3 catalyst would be a better dehydrogenation catalyst, which possesses high catalytic performance and stability.
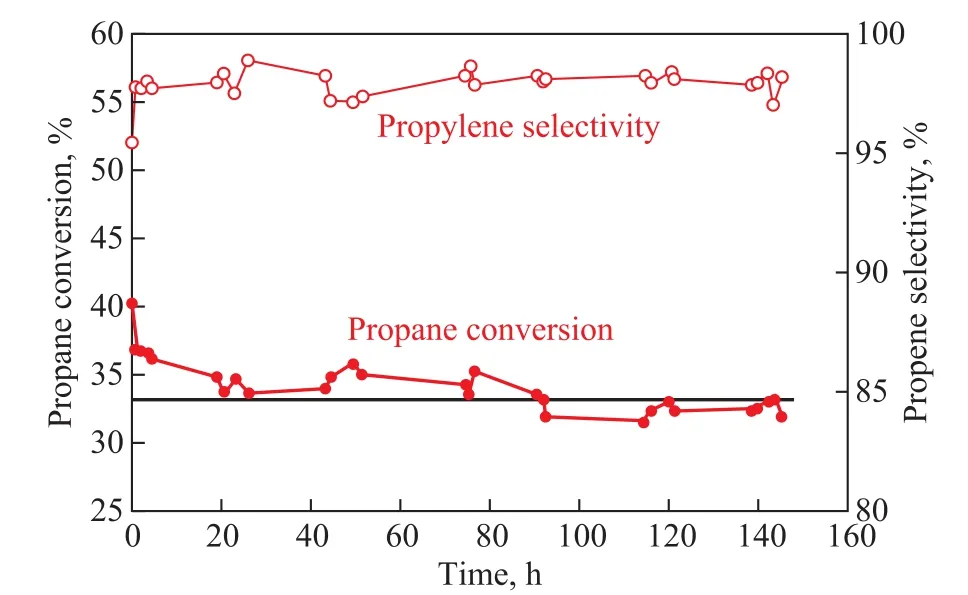
Figure 9 Catalytic stability of PtSnNa/Z@MA-3 catalyst for propane dehydrogenation at 590 oC
4 Conclusions
The present work manifests that the fabrication of coreshell structure of ZSM-5@Mg(Al)O could effectively modify the structural characteristics of ZSM-5 especially in terms of the acid property, and thus the catalytic activity and stability were significantly improved. Notably,when the weight ratio of Mg(Al)O/ZSM-5 was equal to 3, the catalytic activity and stability for propane dehydrogenation over the PtSnNa/Z@MA-3 catalyst were the best among these catalysts, which could be attributed to the improved effect of Pt-Sn interaction coupled with the modified acid property. Moreover, the weakened acid sites in the Z@MA-3 sample could suppress the formation of coke precursor on the metal and even help the coke precursor migrate to the external surface of the support.Based on these effects, it is reasonable to believe that the PtSnNa/Z@MA-3 catalyst would be a promising potential catalyst for propane dehydrogenation reaction.
[1] Ates A, Hardacre C, Goguet A. Oxidative dehydrogenation of propane with N2O over Fe-ZSM-5 and Fe–SiO2:In fluence of the iron species and acid sites[J]. Appl Catal A,2012, 441–442: 30-41
[2] Michorczyk P, Pietrzyk P, Ogonowski J. Preparation and characterization of SBA-1–supported chromium oxide catalysts for CO2assisted dehydrogenation of propane[J].Microporous Mesoporous Mater, 2012, 161(2): 56-66
[3] Zhang Y, Zhou Y, Tang M, Liu X, et al. Effect of La calcination temperature on catalytic performance of PtSnNaLa/ZSM-5 catalyst for propane dehydrogenation[J].Chem Eng J, 2012, 181–182: 530-537
[4] Gao L, Wang Y, Wang J, et al. A Novel Zn II-Sensitive Fluorescent Chemosensor Assembled within Aminopropyl-Functionalized Mesoporous SBA-15[J]. Inorg Chem, 2006,45(17): 6844-6850
[5] Narbeshuber T F, Brait A, Seshan K, et al. Dehydrogenation of light alkanes over zeolites[J]. J Catal, 1997, 172(1):127-136
[6] Kissin Y V. Primary products in hydrocarbon cracking over solid acidic catalysts under very mild conditions: relation to cracking mechanism[J]. J Catal, 1998, 180(1): 101-105
[7] Milas I, Nascimento M A C. The dehydrogenation and cracking reactions of isobutane over the ZSM-5 zeolite[J].Chem Phys Lett, 2003, 373(3): 379-384
[8] Zhang Y, Zhou Y, Shi J, et al. Comparative study of bimetallic Pt-Sn catalysts supported on different supports for propane dehydrogenation[J]. J Mol Catal A: Chem,2014, 381(1): 138-147
[9] Huang L, Zhou S, Zhou Y, et al. Influence of the alkali treatment of HZSM-5 zeolite on catalytic performance of PtSn-based catalyst for propane dehydrogenation[J]. China Petroleum Processing & Petrochemical Technology, 2013,15(2): 11-18
[10] Bai L, Zhou Y, Zhang Y, et al. Effect of magnesium addition to PtSnNa/ZSM-5 on the catalytic properties in the dehydrogenation of propane[J]. Ind Eng Chem Res,2009, 48(22): 9885-9891
[11] Xue M, Zhou Y, Zhang Y, et al. Effects of Mg addition on catalytic performance of PtNa/Sn-ZSM-5 in propane dehydrogenation[J]. Acta Physico-Chimica Sinica, 2012,28(4): 928-934
[12] Zhang Y, Zhou Y, Wan L, et al. Effect of magnesium addition on catalytic performance of PtSnK/γ-Al2O3catalyst for isobutane dehydrogenation[J]. Fuel Process Technol, 2011, 92(8): 1632-1638
[13] Zhang Y, Zhou Y, Qiu A, et al. Effect of alumina binder on catalytic performance of PtSnNa/ZSM-5 catalyst for propane dehydrogenation[J]. Ind Eng Chem Res, 2006,45(7): 2213-2219
[14] Lu J, Fu B, Kung M C, et al. Coking- and sinteringresistant palladium catalysts achieved through atomic layer deposition[J]. Science, 2012, 335(6037): 1205-1208
[15] Naghizadeh R, Rezaie H R, Golestani-Fard F. Effect of TiO2on phase evolution and microstructure of MgAl2O4spinel in different atmospheres[J]. Ceram Int, 2011, 37(1): 349-354
[16] Lavat A E, Grasselli M C, Lovecchio E G. Effect of α and γ polymorphs of alumina on the preparation of MgAl2O4-spinel-containing refractory cements[J]. Ceram Int, 2010,36(1): 15-21
[17] Păcurariu C, Lazău I, Ecsedi Z, et al. New synthesis methods of MgAl2O4spinel[J]. J Eur Ceram Soc, 2007,27(2/3): 707-710
[18] Bocanegra S A, Zgolicz P D, Scelza O A, et al. New trimetallic catalysts supported on coprecipitated MgAl2O4for n-paraffins selective dehydrogenation processes[J].Catal Commun, 2009, 10(10): 1463-1466
[19] Virnovskaia A, Rytter E, Olsbye U. Kinetic and isotopic study of ethane dehydrogenation over a semicommercial Pt,Sn/Mg(Al)O catalyst[J]. Ind Eng Chem Res, 2008,47(19): 7167-7177
[20] Xu X, Ran X, Cui Q, et al. ZSM-5- and MgAl2O4-based bifunctional additives for enhancing the production of propene and removal of SO2in the fluid catalytic cracking(FCC) process[J]. Energy Fuels, 2010, 24(7): 3754-3759
[21] Zhang Y, Zhou Y, Huang L, et al. Sn-modified ZSM-5 as support for platinum catalyst in propane dehydrogenation[J]. Ind Eng Chem Res, 2011, 50(13):7896-7902
[22] Dorado F, Romero R, Cañizares P. Hydroisomerization of n-butane over Pd/HZSM-5 and Pd/Hβ with and without binder[J]. Appl Catal A, 2002, 236(1/2): 235-243
[23] Kulkarni S J, Srinivasu P, Narender N, et al. Fast and ef ficient synthesis of ZSM-5 under high pressure[J]. Catal Commun, 2002, 3(3): 113-117
[24] Nie R, Lei H, Pan S, et al. Core-shell structured CuOZnO@H-ZSM-5 catalysts for CO hydrogenation to dimethyl ether[J]. Fuel, 2012, 96(7): 419-425
[25] Gregg S J, Sing K S W. Adsorption, Surface Area and Porosity[M]. London: Academic Press, 1982
[26] Hartmann M. Hierarchical zeolites: A proven strategy to combine shape selectivity with ef ficient mass transport[J].Angewandte Chemie International Edition, 2004, 43(44):5880-5882
[27] Vu X H, Steinfeldt N, Armbruster U, et al. Improved hydrothermal stability and acidic properties of ordered mesoporous SBA-15 analogs assembled from nanosized ZSM-5 precursors[J]. Microporous Mesoporous Mater,2012, 164: 120-126
[28] Jansen J C, van der Gaag F J, van Bekkum H. Identification of ZSM-type and other 5-ring containing zeolites by IR.spectroscopy[J]. Zeolites, 1984, 4(4): 369-372
[29] Bordiga S, Escalona Platero E, Otero Areán C, et al. Low temperature CO adsorption on Na-ZSM-5 zeolites: An FTIR investigation[J]. J Catal, 1992, 137(1): 179-185
[30] Wu W, Weitz E. Modification of acid sites in ZSM-5 by ion-exchange: An in-situ FTIR study[J]. Appl Surf Sci,2014, 316(1): 405-415
[31] Zhang Y, Zhou Y, Sheng X, et al. Effect of the competitive adsorbates on the catalytic performance of PtSnK/γ-Al2O3catalyst for isobutane dehydrogenation[J]. Fuel Process Technol, 2012, 104: 23-30
[32] Duan Y, Zhou Y, Zhang Y, et al. Effect of aluminum modification on catalytic properties of PtSn-based catalysts supported on SBA-15 for propane dehydrogenation[J]. J Nat Gas Chem, 2012, 21(2): 207-214
[33] Duan Y, Zhou Y, Sheng X, et al. Influence of alumina binder content on catalytic properties of PtSnNa/AlSBA-15 catalysts[J]. Microporous Mesoporous Mater, 2012, 161:33-39
[34] Scelza O A, Bocanegra S A, de Miguel S R,et al. n-Butane dehydrogenation on PtSn supported on MAl2O4(M : Mg or Zn) catalysts[J]. Catal Lett, 2004, 96(3/4): 129-140
[35] Ballarini A D, Ricci C G, de Miguel S R, et al.Use of Al2O3–SnO2as a support of Pt for selective dehydrogenation of light paraffins[J]. Catal Today, 2008,133–135: 28-34
[36] Morales R, Melo L, Llanos A, et al. Characterization of bifunctional PtSn/H[Al]ZSM-5 catalysts: a comparison between two impregnation strategies[J]. J Mol Catal A:Chem, 2005, 228(1/2): 227-232
[37] Bacaud R, Bussière P, Figueras F. Mössbauer spectra investigation of the role of tin in platinum-tin reforming catalysts[J]. J Catal, 1981, 69(2): 399-409
[38] Kuhlmann A, Roessner F, Schwieger W, et al. New bifunctional catalyst based on Pt containing layered silicate Na-ilerite[J]. Catal Today, 2004, 97(4): 303-306
[39] Larsson M, Hultén M, Blekkan E A, et al. The effect of reaction conditions and time on stream on the coke formed during propane dehydrogenation[J]. J Catal, 1996, 164(1): 44-53
[40] Zhang Y, Zhou Y, Qiu A, et al. Effect of Na addition on catalytic performance of PtSn/ZSM-5 catalyst for propane dehydrogenation[J]. Acta Physico-Chimica Sinica, 2006,22(4): 672-678
[41] Afonso J C, Schmal M, Fréty R. The chemistry of coke deposits formed on a Pt-Sn catalyst during dehydrogenation of n-alkanes to mono-olefins[J]. Fuel Process Technol,1994, 41(1): 13-25
[42] Hao K, Shen B, Wang Y, et al. Influence of combined alkaline treatment and Fe–Ti-loading modification on ZSM-5 zeolite and its catalytic performance in light ole fin production[J]. J Ind Eng Chem, 2012, 18(5): 1736-1740
[43] Lieske H, Sárkány A, Völter J. Hydrocarbon adsorption and coke formation on Pt/Al2O3and Pt-Sn/Al2O3catalysts[J]. Appl Catal, 1987, 30(1): 69-80
[44] Lin L, Zhang T, Zang J, et al. Dynamic process of carbon deposition on Pt and Pt–Sn catalysts for alkane dehydrogenation[J]. Appl Catal, 1990, 67(1): 11-23
[45] Li Q, Sui Z, Zhou X,et al. Coke formation on Pt–Sn/Al2O3catalyst in propane dehydrogenation: Coke characterization and kinetic study[J]. Top Catal, 2011, 54(13/15): 888-896
- 中国炼油与石油化工的其它文章
- Oxidation of Dibenzothiophene in Model Diesel Using Hydroperoxide Generated via In-Situ Reaction of Octane with Oxygen
- Bitumen Recovery from Indonesian Oil Sands Using ASP (Alkali, Surfactant and Polymer) Agent
- Study on Mechanical Performance and Wear Resistance of Halloysite Nanotubes/PTFE Nanocomposites Prepared by Employing Solution Mixing Method
- Study on a New Type of Lubricating Oil for Miniature Bearing Operating at Ultra-low Temperature
- Lubricating Performance of Rapeseed Oil Under Electromagnetic Field
- Preparation and Modification of Micro-Mesoporous Carbon Materials for Toluene Adsorption

