抗阻运动激活FSTL1-Akt-mTOR信号通路促进心梗大鼠心肌细胞增殖
田振军,郝美丽,席 悦
抗阻运动激活FSTL1-Akt-mTOR信号通路促进心梗大鼠心肌细胞增殖
田振军,郝美丽,席 悦
陕西师范大学 体育学院 暨运动生物学研究所 运动与心血管健康研究室, 陕西 西安 710062.
目的:探讨抗阻运动对心肌梗死(Myocardial Infarction,MI)大鼠卵泡抑素样蛋白1 (Follistatin-like Protein 1,FSTL1)及其受体DIP2A(Disco-interacting Protein 2 homolog A)表达和下游Akt-mTOR信号通路与心肌细胞增殖的影响。方法:雄性SPrague-Dawley大鼠30 只,体重180~220 g,左冠状动脉前降支结扎制备心梗模型,术后随机分为假手术组(S)、心梗安静对照组(MI)、心梗+抗阻运动组(MR),每组10只,其中S组只穿线不结扎。术后1周,MR组先进行1周适应性无负重爬梯运动,再进行4周递增负荷抗阻运动。训练结束后24 h,腹腔麻醉,测定LVSP、LVEDP和±dP/dt max评价心功能。Western blot实验测定心肌FSTL1/DIP2A、PAkt/Akt、P-mTOR/mTOR、CyclinD1、CDK4蛋白表达;免疫荧光实验观察心肌细胞增殖;Masson染色观察并计算心肌胶原容积百分比(CVF%)。结果:与S组比较,MI组心肌FSTL1/DIP2A、CyclinD1和CDK4蛋白表达增加,PAkt/Akt、P-mTOR/mTOR比值显著上升,心肌细胞增殖百分率显著升高,CVF%和LVEDP显著增加,LVSP和±dP/dt max显著降低;与MI组比较,MR组心肌FSTL1/DIP2A、CyclinD1和CDK4蛋白表达显著升高,PAkt/Akt、P-mTOR/mTOR比值显著上升,心肌细胞增殖百分率显著升高,CVF%和LVEDP显著降低,LVSP和±dP/dt max显著升高。结论:抗阻运动上调FSTL1及其受体DIP2A的表达,激活其下游Akt-mTOR信号通路。表明,FSTL1-DIP2A-Akt-mTOR信号通路在抗阻运动促进心梗大鼠心肌细胞增殖、降低心肌纤维化面积和改善心功能中发挥重要作用。
抗阻运动;心肌梗死;卵泡抑素样蛋白1;FSTL1-Akt-mTOR信号通路;心肌细胞增殖
心肌梗死(MyocardialInfarction,MI)后心肌严重缺血缺氧导致心肌细胞大量丢失和心功能恶化是造成心力衰竭的重要原因[27,17],如何预防和治疗MI一直是国内外学者高度关注的问题。研究表明,运动锻炼是预防和治疗心血管疾病,尤其是MI的重要临床干预措施[10],运动可显著减少心肌凋亡和坏死[12],降低心肌纤维化水平[40],促进血管生成[29],诱导心肌细胞增殖[6],改善左心室病理重构并提高心肌收缩力以及左心室功能[11,13]。有文献表明,抗阻运动显著提高MI大鼠心血管的自主调节能力,是MI康复安全有效的方法[14],但其康复靶点与机制需要深入探讨。
卵泡抑素样蛋白1(Follistatin-like Protein 1,FSTL1)是分泌型的细胞外基质糖蛋白,最初由小鼠成骨细胞的细胞系MC3T3-E1中克隆得到[30]。研究发现,FSTL1在心、肾和肺等多个器官中均有表达[2],并有可能作为急性冠状动脉综合征预后的生物标志物[36],在细胞的增殖与分化[9]、肥大[33]、抗凋亡和炎症反应[21]、血管再生[23]等方面发挥重要作用。FSTL1与心肌细胞膜上受体DIP2A(Disco-interacting Protein 2 homolog A)结合激活Akt信号发挥心脏保护作用[24]。主动脉狭窄、缺血/再灌注损伤和MI导致心脏FSTL1表达上调。FSTL1过表达或小RNA干扰可通过PI3K-Akt-mTOR和Erk1/2信号通路,影响心肌细胞凋亡[22]。增加心外膜FSTL1表达可减少MI大鼠心肌纤维化,或激活PI3K-Akt通路可诱导心肌细胞增殖[35,37]。本研究前期报道,运动可上调MI心脏FSTL1蛋白表达,诱导血管新生[1],但运动是否能激活MI心脏FSTL1-DIP2A-Akt-mTOR信号通路,诱导心肌细胞增殖,发挥心脏保护作用,目前尚无文献报道。本文采用抗阻运动方式对上述问题进行探讨,为MI心脏运动康复手段和方法筛选提供理论和实验依据。
1 材料与方法
1.1 主要仪器和试剂
主要仪器:小动物呼吸机(ALC-V8)、生物信号采集系统(Power-Lab/8ST)、电泳仪和多色凝胶成像系统(Bio-Rad ChemiDocTMMP)、病理组织包埋机(BM-II)、石蜡切片机(LEICA-RM2126)、生物组织摊烤片机(YT-6C)、光学显微镜(OlymPus BX51)、荧光显微镜(Nikon EcliPse 55i)等。
主要试剂:兔多克隆抗体FSTL1(GeneTex)、DIP2A(Biorbyt)、PAkt、Akt、P-mTOR、mTOR、Cyclin D1和小鼠多克隆抗体PCNA(CST)、CDK4(Snata Cruz)、兔多克隆抗体cTnT(北京博奥森)、山羊抗兔二抗、山羊抗小鼠二抗、Dylight 594标记的山羊抗小鼠二抗、FITC标记的山羊抗兔二抗等。
1.2 实验动物与分组
SPrague-Dawley雄性大鼠购于西安交通大学动物实验管理中心,体重为180~220 g,适应性喂养1周后,左冠状动脉前降支结扎法制备MI模型,术后存活20只,随机分为心梗安静对照组(MI)、心梗+抗阻运动组(MR),每组10只。另选取10只大鼠作为假手术对照组(S),只穿线不结扎。大鼠分组后,分笼饲养,每笼5只,自由进食饮水。S组和MI组不运动,MR组进行负重爬梯训练。
1.3 MI模型制备和抗阻运动方案
MI模型制备:大鼠腹腔注射戊巴比妥钠(5%,30 mg/kg)麻醉,呼吸面罩辅助呼吸并连接心电图全程记录。常规开胸暴露出心脏,于左心耳和肺动脉圆锥交界以下2 mm处进针,结扎左冠状动脉前降支。以心电图ST段抬高或T波倒置,结扎处以下心肌颜色变浅或变白为手术成功,常规关胸护理。
抗阻运动方案:依据文献和预实验确定抗阻运动方案[4,14]。MR组术后1周开始进行爬梯抗阻运动,爬梯高为1.1 m,间隔为2 cm,倾斜度为85°。第1周为无负重适应性训练,之后进行4周递增负荷爬梯运动,每天递增体重的10%,当负荷递增至体重的75%后,保持75%体重的负荷直到训练结束,5 d/周×4周。
1.4 心功能测定、取材和样品处理
4周训练结束24 h后,腹腔麻醉,常规血流动力学方法测定心功能。导管经右颈总动脉逆行插入至左心室,Power-Lab/8ST生物信号采集系统记录左心室收缩压(left ventricular systolic Pressure,LVSP)、左心室舒张末压(left ventricular end-diastolic Pressure,LVEDP)和左心室压力最大上升和下降速率(±dP/dt max)等。之后即刻开胸摘取心脏,生理盐水清洗残血(0~4 ℃),6只心脏液氮固定24 h后移至-80 ℃冰箱保存,用于Western blot实验,另4只心脏10%中性甲醛固定,用于组织学实验。
1.5 Western blot 实验
取心梗边缘区心肌组织50 mg,加入预冷蛋白抽提试剂500μl,剪碎匀浆,4 °C离心,取上清液,BCA蛋白定量。常规制胶、上样、电泳、转膜,5%BSA室温封闭1 h,孵育一抗FSTL1(1:1 000)、DIP2A(1:200)、PAkt(1:2 000)、Akt(1:1 000)、P-mTOR/mTOR(1:1 000)、CyclinD1(1:400)、CDK4(1:200)、内参为GAPDH(1:8 000),4 ℃过夜。次日室温复温30 min,TBST清洗5 min/次×5次,室温孵育二抗(1:8 000)1 h,TBST清洗二抗5 min/次×5次,ECL发光,凝胶成像系统成像与分析。
1.6 Masson染色
取固定48 h后心脏,流水冲洗5 h,梯度酒精脱水、三氯甲烷透明,石蜡常规浸蜡和包埋,连续切片(5 μm)制片,Masson染色,常规脱水透明,中性树胶封片,OlymPus BX51光学显微镜下观察拍片,Image-Pro Plus 5.1软件测定蓝色胶原面积,计算心肌胶原面积百分比(CVF%),CVF%=胶原面积/心肌组织总面积×100%。
1.7 免疫荧光实验
石蜡切片脱蜡至水,PBS清洗,微波抗原修复,室温冷却,PBS清洗5 min/次×3次,5%BSA室温封闭1 h,甩去BSA,滴加小鼠来源的一抗PCNA(1:100)和兔来源的cTnT(1:100)混合抗体,4 ℃过夜。次日室温复温45 min,PBS清洗,避光滴加Dylight 594标记的山羊抗小鼠(1:100)二抗、FITC标记的山羊抗兔二抗(1:100)和DAPI(1:800)的混合液,37℃避光孵育1 h后,PBS清洗5 min/次×5次(避光),封片,Nikon EcliPse 55i 荧光显微镜观察拍片。同时设置空白对照和阴性对照。
1.8 图像与数据处理

2 实验结果
2.1 抗阻运动上调MI心脏FSTL1及其受体DIP2A蛋白表达
Western Blot结果显示,与S组比较,MI组FSTL1蛋白表达显著上调(<0.01),DIP2A蛋白表达升高,但无显著差异。与MI组比较,MR组FSTL1及其受体DIP2A蛋白表达均显著增加(<0.01)。表明,MI心脏FSTL1及其受体DIP2A表达出现代偿性升高,而抗阻运动可显著增加MI心脏FSTL1及其受体DIP2A 表达(图1)。

图1 MI心脏FSTLI及其受体DIP2A蛋白表达结果
Figure 1 The Protein Expression Result of FSTL1 and Its Receptor DIP2A in MI Heart
2.2 抗阻运动激活MI心脏PI3K/Akt信号通路
Western Blot结果显示,与S组比较,MI组PAkt/Akt、P-mTOR/mTOR的比值均显著增加(<0.05),CyclinD1和CDK4蛋白表达均显著增加(<0.01,<0.05);与MI组比较,MR组PAkt/Akt、P-mTOR/mTOR的比值均显著升高(<0.01,<0.05),CyclinD1和CDK4蛋白表达均显著升高(<0.05)。表明,MI后PI3K/Akt通路的磷酸化水平代偿性上调,抗阻运动可有效激活PI3K/Akt通路,启动细胞增殖周期(图2)。
2.3 抗阻运动诱导MI大鼠心肌细胞增殖
免疫荧光结果显示,PCNA为细胞增殖核抗原,呈红色荧光(图3,A~C);cTnT为心肌肌钙蛋白,标记心肌细胞,呈绿色荧光(图3,D~F);DAPI标记细胞核,呈蓝色荧光(图3,G~I)。PCNA+/cTnT+双阳性,表示新生的心肌细胞。与S组比较,MI组PCNA+/cTnT+心肌细胞数目增多,心肌细胞增殖百分率显著增加(<0.01);与MI组比较,MR组PCNA+/cTnT+心肌细胞数目增多,心肌细胞增殖百分率显著升高(<0.05)。表明,MI后心肌细胞增殖出现代偿性增加,抗阻运动可显著诱导MI大鼠心肌细胞增殖(图3,J~P)。
2.4 抗阻运动降低MI心脏的心肌纤维化面积,改善心功能
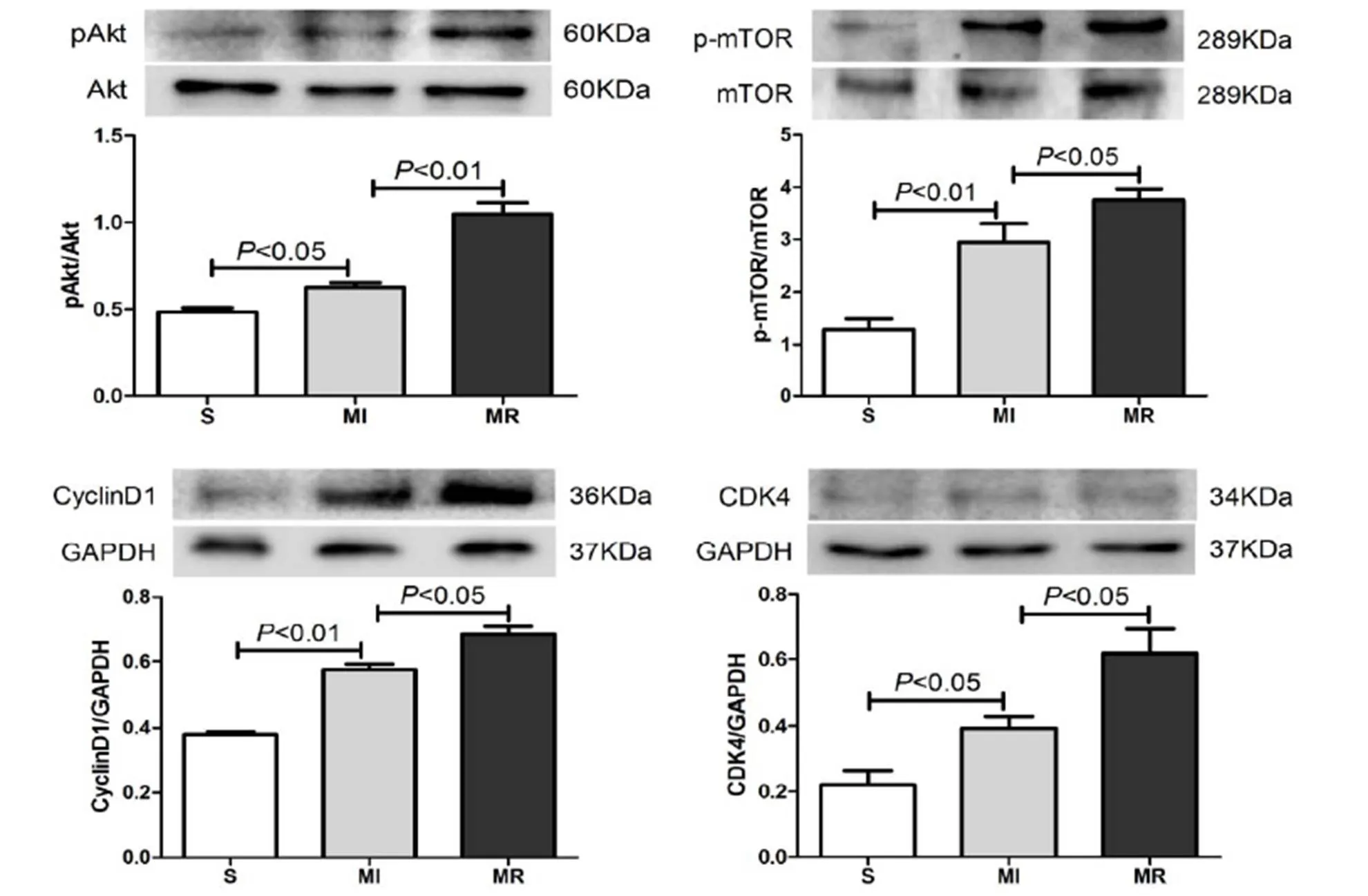
图2 MI心脏PAkt/Akt、P-mTOR/mTOR、CyclinD1和CDK4蛋白表达结果
Figure 2. The Protein Expression Result of PAkt/Akt、P-mTOR/mTOR、CyclinD1 and CDK4 in MI Heart
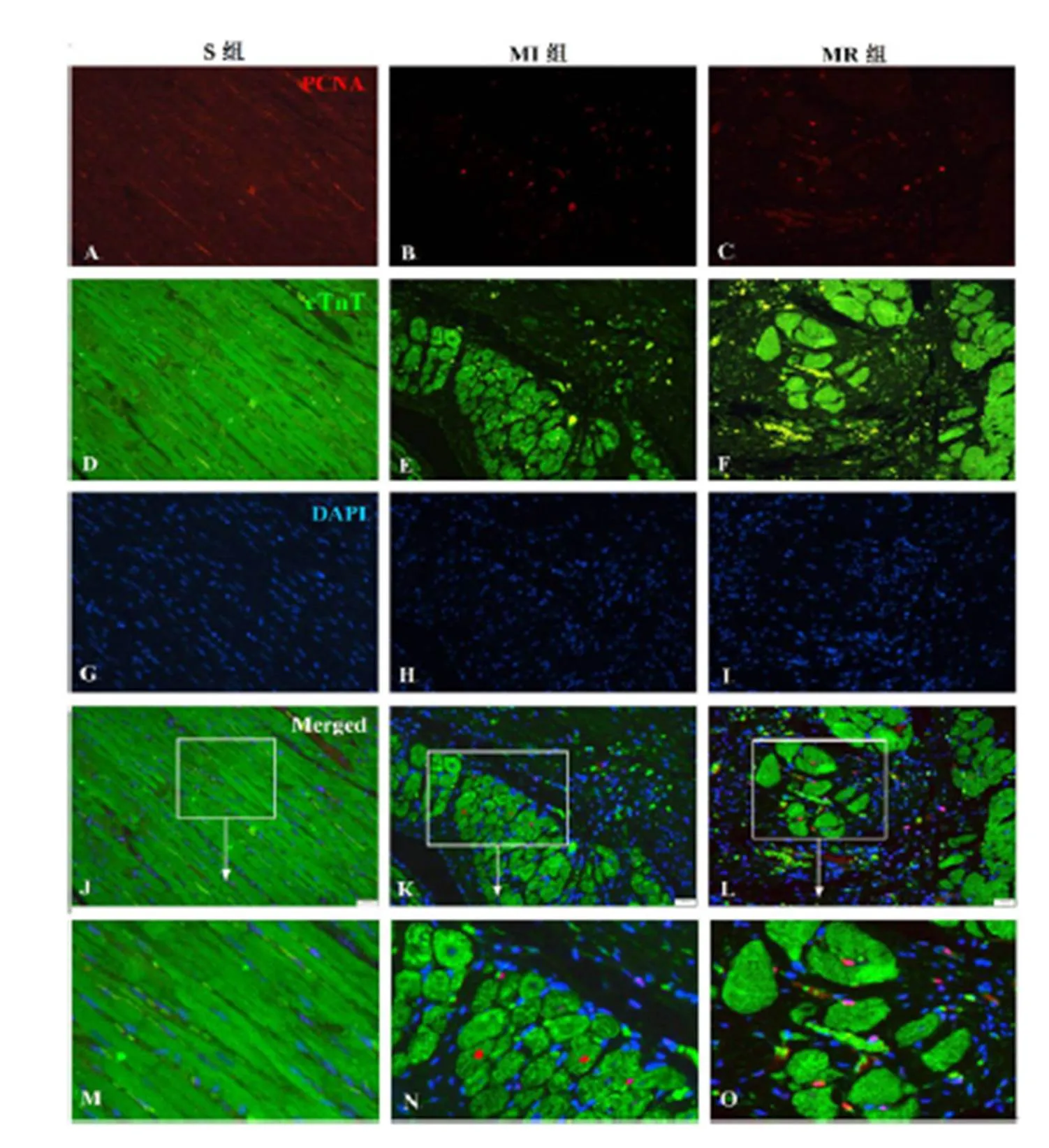
Figure 3. The Immunofluorescence Result of Cardiomyocyte Proliferation in MI Heart
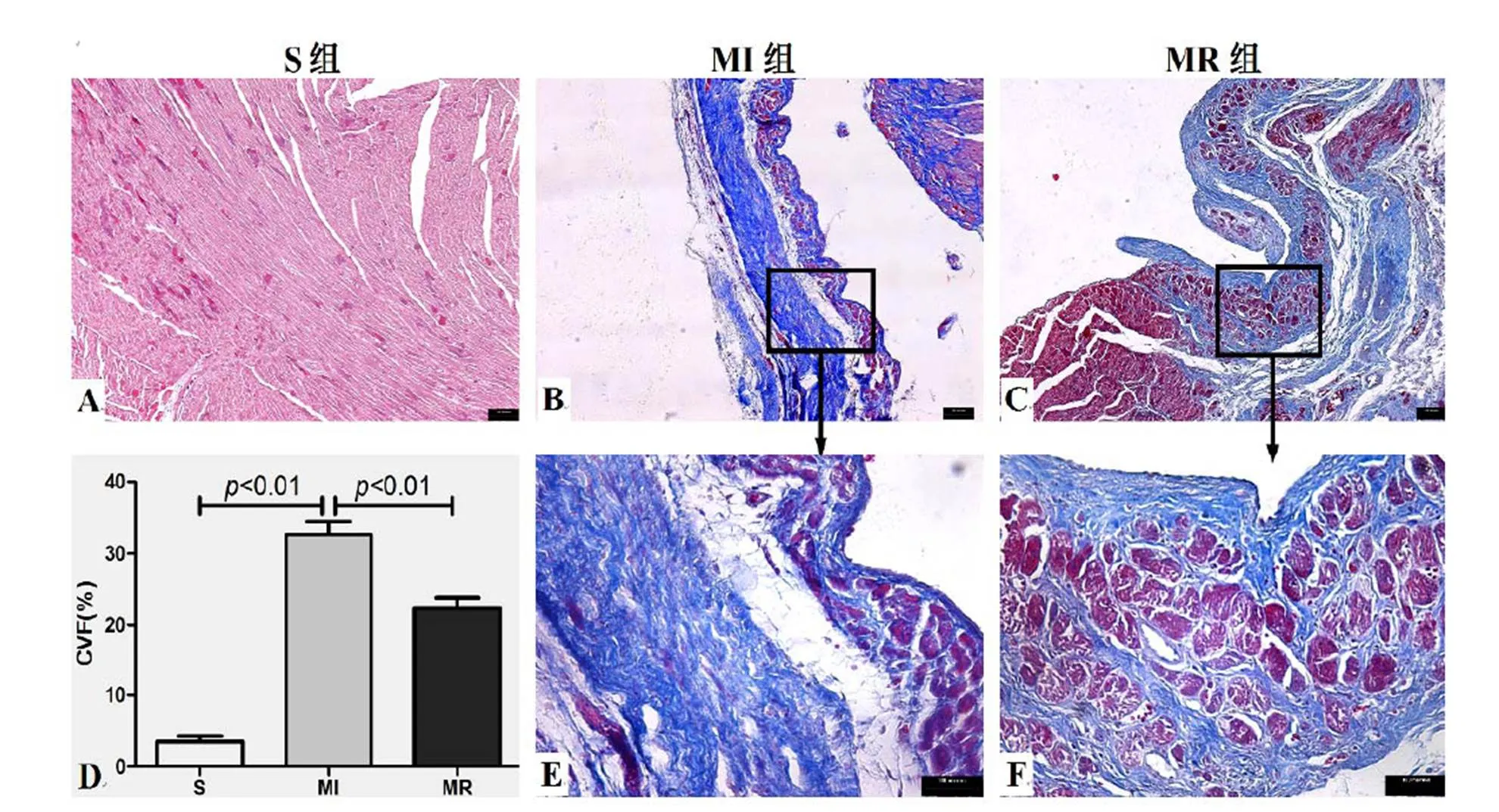
注:Masson染色,心肌细胞为紫红色,胶原纤维为蓝色,细胞核为蓝紫色。A为S组;B为MI组,E为MI组局部放大;C为MR组,F为MR组局部放大;D为胶原容积百分数统计比较。
Figure 4. The Masson Staining Result of Myocardium in MI Heart
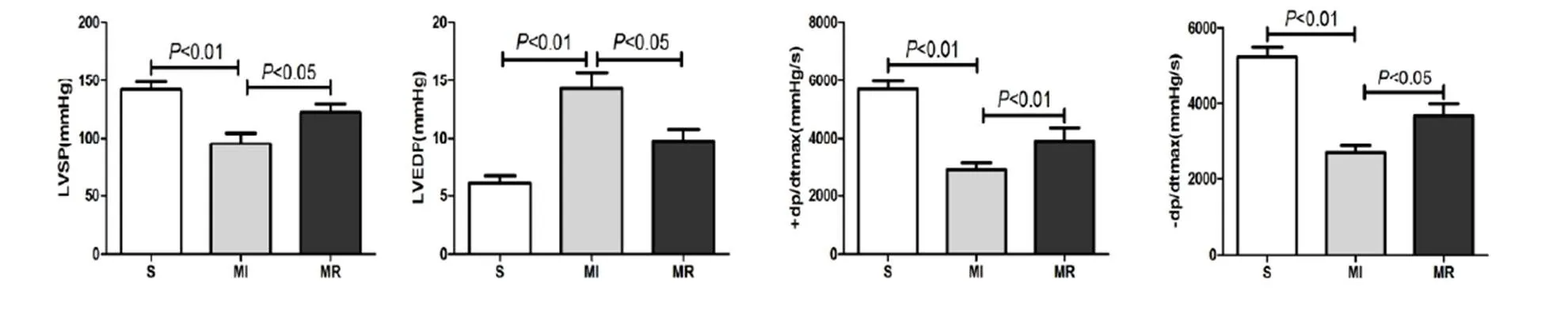
图5 MI心脏血流动力学测试结果
Figure 5. The Result of Hemodynamic Index in MI Heart
光镜观察Masson染色结果显示,细胞核呈蓝紫色,心肌细胞呈红色,胶原纤维呈蓝色。与S组比较,MI组心肌组织发生替代性纤维化,CVF%显著增加(<0.01);与MI组比较,MR组胶原纤维有所减少,CVF%显著降低(<0.01)。表明,MI后胶原纤维过度增生,抗阻运动可抑制胶原纤维的进一步扩大,降低纤维化面积程度(图4,A-F)。
心功能结果显示,与S组比较,MI组LVSP、±dP/dt max均显著降低(<0.01),LVEDP显著升高(<0.01);与MI组比较,MR组LVSP、±dP /dt max均显著上升(<0.05,<0.01,<0.05),LVEDP显著下降(<0.05),表明,MI后心功能显著降低,抗阻运动可显著改善心功能(图5)。
2.5 心肌FSTL1蛋白表达与细胞增殖百分率、CVF%、心功能变化的相关性
相关性分析显示,MI及MI抗阻运动后,心肌FSTL1蛋白表达与心肌细胞增殖百分率呈显著正相关(=0.761,<0.01),与CVF%呈显著负相关(=-0.822,<0.01)。表明,在MI条件下,随着心肌FSTL1蛋白表达升高,心肌细胞增殖增加,心肌胶原面积减少。心肌细胞增殖百分率与LVSP、+dP /dt max、-dP /dt max呈显著正相关(=0.872,<0.01;=0.708,<0.05;=0.780,<0.01),与LVEDP呈显著负相关(=-0.679,<0.01)。表明,随着心肌细胞增殖的增加,MI大鼠心功能显著改善。
3 分析与讨论
研究证实,FSTL1在促进心肌细胞增殖、降低心肌纤维化[35]、抑制心肌细胞凋亡[21]和诱导心肌血管再生[1]等方面,可作为心脏保护因子发挥重要作用。内源性干预证实,缺血再灌注小鼠内源性高表达,可显著降低心梗面积和细胞凋亡[22],特异性敲除小鼠心肌基因后,压力超负荷心脏表现出心肌病理性肥大和心功能损害[31]。外源性干预表明,注射FSTL1重组蛋白可显著降低心梗面积[21]。表明,FSTL1在抑制病理性心脏的发生发展中发挥重要作用。内皮细胞和心肌细胞膜上有FSTL1受体DIP2A分布,FSTL1与DIP2A结合可激活下游Akt信号,发挥心脏保护作用。FSTL1也可直接激活PI3K-Akt-mTOR和Erk1/2信号[22]。小RNA干扰降低DIP2A表达后,Akt磷酸化水平显著降低[24]。表明,FSTL1直接激活或与DIP2A结合激活PI3K-Akt-mTOR和Erk1/2信号[20]。本研究前期发现,抗阻运动干预显著增加MI心肌的FSTL1蛋白表达[1]。本研究结果显示,MI后进行抗阻运动可显著升高DIP2A蛋白表达,激活Akt和mTOR磷酸化水平。文献报道,运动可激活PI3K-Akt-mTOR信号通路[42],该信号通路在促进心肌细胞增殖与抑制凋亡中发挥重要作用。因此认为,抗阻运动发挥心梗心脏保护效应的机制,可能是通过上调MI大鼠心肌FSTL1及其受体DIP2A蛋白表达,激活Akt-mTOR信号通路而发挥作用。
细胞周期激活是心肌细胞增殖的必备条件,其关键点在G1期和S期之间。Cycin/CDK复合物可调控细胞周期从G1期与S期的转化,启动细胞周期[18]。CyclinD可结合并激活CDK进而诱导心肌细胞增殖,PI3K-Akt信号激活后可上调CyclinD和CDK4的表达,诱导细胞增殖[19,32]。诸多文献表明,跑台或游泳等耐力运动可激活内源性c-kit+心脏干细胞和sca-1+心脏祖细胞分化为心肌细胞[34,38],发挥心脏重塑作用[16]。跑台运动可增加正常大鼠心脏ki67和BrdU标记的心肌细胞数目[34],上调MI大鼠心肌PCNA表达[8];游泳运动可增加缺血再灌注小鼠心肌ki67和EdU表达水平[39],上调压力超负荷致小鼠病理性肥厚心肌的PCNA、磷酸化H3、BrdU和AuroraB表达水平[7]。表明,耐力运动可诱导心脏干/祖细胞的增殖效应。但有关抗阻运动与心脏干/祖细胞研究的报道尚少。本研究结果表明,4周抗阻运动显著增加MI大鼠心脏的CyclinD1、CDK4表达和PCNA+/cTnT+双阳性心肌细胞数目。推测,抗阻运动对MI心脏的保护效应,可能通过刺激FSTL1表达,并与其受体DIP2A结合或直接激活PI3K-Akt-mTOR信号,参与心肌细胞增殖,发挥缺血心脏的保护作用。
动物实验和临床研究表明,MI后早期运动训练可减少心脏胶原纤维含量,降低纤维化,改善心肌收缩力和左心室功能[13,41]。长期有氧运动可抑制大鼠缺血/再灌注心肌凋亡,改善心脏舒/缩功能[42]。适宜强度的抗阻运动在动物和人正常心脏重塑[3]、心血管功能调节及适应过程中[26,25],均发挥积极效应,亦可改善MI致心衰大鼠的心功能,减弱心脏病理性肥大,降低左心室胶原蛋白沉积和炎症反应[4];抑制盐超载诱导的大鼠心室重构和左室舒张压紊乱[5],下调糖尿病大鼠的心脏氧化应激水平[28],改善其心功能[15],因此认为,无论是针对正常心脏或病理性心脏,抗阻运动均可产生积极效应。本研究结果表明, 4周抗阻运动显著降低MI后心肌CVF%和LVEDP,显著提高LVSP和±dP/dt max,且增殖的心肌细胞数目与FSTL1表达水平和心功能改善呈显著正相关,与心肌纤维化面积呈显著负相关。提示,抗阻运动可能通过激活心肌FSTL1-DIP2A-Akt-mTOR信号通路,促进心肌细胞增殖,降低MI心脏纤维化,显著改善心功能。
4 结论
抗阻运动上调FSTL1及其受体DIP2A的表达,激活其下游Akt-mTOR信号通路。表明,FSTL1-DIP2A-Akt-mTOR信号通路在抗阻运动促进心梗大鼠心肌细胞增殖、降低心肌纤维化面积和改善心功能中发挥重要作用。
[1] 席悦, 蔡梦昕, 田振军. 不同运动方式上调FSTL1蛋白表达诱导心梗心脏血管新生[J]. 体育科学, 2016, (36)10: 32-39.
[2] Adams D, Larman B, Oxburgh L. Developmental expression of mouse follistatin-like 1 (fstl1): Dynamic regulation during organogenesis of the kidney and lung[J]. Gene Express Patterns, 2007, 7(4): 491-500.
[3] Alves J P, Nunes R B, Ddc F,. High-intensity resistance training alone or combined with aerobic training improves strength, heart function and collagen in rats with heart failure[J]. Am J Transl Res, 2017, 9(12): 5432-5441.
[4] Alves J P, Nunes R B, Stefani G P,. Resistance training improves hemodynamic function, collagen deposition and inflammatory profiles: experimental model of heart failure[J]. Plos One, 2014, 9(10): e110317.
[5] BARRETTI D L M, MELO S F S, OLIVEIRA E M,. Resistance training attenuates salt overload-induced cardiac remodeling and diastolic dysfunction in normotensive rats[J]. Brazilian J Med Bio Res, 2017, 50(9): e6146.
[6] Bei Y, Fu S, Chen X,. Cardiac cell proliferation is not necessary for exercise-induced cardiac growth but required for its protection against ischaemia/reperfusion injury[J]. J Cell Molecular Med, 2017: 1-8.
[7] Bostrom P, Mann N, Wu J,C/EBPβcontrols exercise-induced cardiac growth and protects against pathological cardiac remodeling[J]. Cell, 2010, 143(7): 1072-1083.
[8] Cai M X, Shi X C, Chen T,Exercise training activates neuregulin 1/Erbβ signaling and promotes cardiac repair in a rat myocardial infarction model[J]. Life Sci, 2016, 149: 1-9.
[9] Chaly Y, Blair H C, Smith S M,. Follistatin-like protein 1 regulates chondrocyte proliferation and chondrogenic differentiation of mesenchymal stem cells[J]. Ann Rheumatic Diseases, 2015, 74(7): 1467-1473.
[10] Chow CK, Jolly S, Rao-Melacini P,. Association of diet, exercise, and smoking modifcation with risk of early cardiovascular events after acute coronary syndromes[J]. Circulation, 2010, 121(6): 750–758.
[11] Ellingsen Ø, Halle M, Conraads V M,. High-intensity interval training in patients with heart failure with reduced ejection fraction[J]. Circulation, 2017, 135(9): 839-849.
[12] French J P, Hamilton K L, Quindry J C,Exercise-induced protection against myocardial apoptosis and necrosis: MnSOD, calcium-handling proteins, and calpain[J]. Faseb J , 2008, 22(8): 2862-2871.
[13] Giallauria F, Acampa W, Ricci F,. Exercise training early after acute myocardial infarction reduces stress-induced hypoperfusion and improves left ventricular function[J]. Eur J Nuclear Med Molecular Imaging, 2013, 40(3): 315-324.
[14] Grans C F, Feriani D J, Abssamra M E,Resista-nce training after myocardial infarction in rats: its role on cardiac and autonomic function[J]. Arquivos Brasileiros De Cardiologia, 2014, 103(1): 60-68.
[15] Ko T H, Marquez J C, Kim H K,Resistance exercise improves cardiac function and mitochondrial efficiency in diabetic rat hearts[J]. Pflugers Archiv Eur J Physiol, 2018, 470: 263-275.
[16] Leite C F, Lopes C S, Alves A C,Endogenous resident c-kit cardiac stem cells increase in mice with an exercise-induced, physiologically hypertrophied heart[J]. Stem Cell Res, 2015, 15(1): 151-164.
[17] LIN Q H. The impact of cardiac rehabilitation exercise on cardiopulmonary capacity and life quality of the patients with myocardial infarction[J]. Atlantis Press, 2014: 88-90.
[18] MATTHEW P S, ANDREW W J, HELEN R F,. CDK substrate phosphorylation and ordering the cell cycle[J]. Cell, 2016, 167(7): 1750-1761.
[19] Muisehelmericks R C, Grimes H L, Bellacosa A,Cyclin D expression is controlled post- transcriptionally via a phosphatidylinositol 3-kinase/akt-dependent pathway[J]. J Bio Chem, 1998, 273(45): 29864-29872.
[20] NOVOYATLEVA T, SAJJAD A, POGORYELOV,FGF1-mediated cardiomyocyte cell cycle reentry depends on the interaction of FGFR-1 and Fn14[J]. FASEB J, 2014, 28(6): 2492-2503.
[21] Ogura Y, Ouchi N, Ohashi K,. Therapeutic impact of follistatin-like 1 on myocardial ischemic injury in preclinical models[J]. Circulation, 2012, 126(14): 1728-1738.
[22] Oshima Y, Ouchi N, Sato K,. Follistatin-like 1 is an akt-regulated cardioprotective factor that is secreted by the heart[J]. Circulation, 2008, 117(24): 3099-3108.
[23] Ouchi N, Oshima Y, Ohashi K,. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism[J]. J Bio Chem, 2008, 283(47): 32802-32811.
[24] Ouchi N, Yohashi A, Ohashi K,. DIP2A functions as a FSTL1 receptor[J]. J Bio Chem, 2010, 285 (10): 7127-7134.
[25] Piras A, Persiani M, Damiani N,. Peripheral heart action (PHA) training as a valid substitute to high intensity interval training to improve resting cardiovascular changes and autonomic adaptation[J]. Eur J Appl Physiol, 2015, 115(4): 763-773.
[26] Queiroz A C, Kanegusuku H, Forjaz C L. Effects of resistance training on blood pressure in the elderly[J]. Arquivos Brasileiros De Cardiol, 2010, 95(1): 135-140.
[27] Rochette L, Vergely C.“Pro-youthful” factors in the “labyrinth” of cardiac rejuvenation[J]. Experiment Gerontol, 2016, 83: 1-5.
[28] SAMADI A, GAEINI A, RAVASI A,The effect of resistance exercise on oxidative stress in cardiac and skeletal muscle tissues of streptozotocin-induced diabetic rats[J]. J Physics Condensed Matter, 2013, 18(39): 9055-9070.
[29] Shen M, Yu M, Li J,Effects of exercise training on kinin receptors expression in rats with myocardial infarction[J]. Archives Physiol Biochem, 2017: 1-6.
[30] Shibanuma M, Mashimo J, Mita A,. Cloning from a mouse osteoblastic cell line of a set of transforming-growth-factor-β1-regulated genes, one of which seems to encode a follistatin-related polypeptide[J]. Eur J Biochem, 1993, 217(1): 13-19.
[31] Shimano M, Ouchi N, Nakamura K,. Cardiac myocyte follistatin-like 1 functions to attenuate hypertrophy following pressure overload[J]. Proc Natl Acad Sci U S A, 2011, 108(43): E899-906.
[32] Tamamori-Adachi M, Ito H, Sumrejkanchanakij P,Critical role of cyclin D1 nuclear import in cardiomyoc-yte proliferation[J]. Cir Res, 2003, 92(1): 12-19.
[33] Tanaka K, Valeromuñoz M, Wilson R M,Follistatin like 1 regulates hypertrophy in heart failure with preserved ejection fraction[J]. Jacc Basic Translation Sci, 2016, 1(4): 207-221.
[34] Waring C D, Carla V, Angela P,. The adult heart responds to increased workload with physiologic hypertrophy, cardiac stem cell activation, and new myocyte formation[J]. Eur Heart J, 2014, 35(39): 2722-2731.
[35] Wei K, Serpooshan V, Hurtado C,. Epicardial fstl1 reconstitution regenerates the adult mammalian heart[J]. Nature, 2015, 525(7570): 479-485.
[36] WIDERA C, GIANNITIST E, KEMPF T,Identification of follistatin-like 1 by expression cloning as an activator of the growth differentiation factor 15 gene and a prognostic biomarker in acute coronary syndrome[J]. Clin Chem, 2012, 58(8): 1233-1241.
[37] Xiao F, Kimura W, Sadek H A. A hippo "akt" regulates cardiomyocyte proliferation[J]. Cir Res, 2015, 116(1): 3-5.
[38] XIAO J J, XU T Z, LI J,. Exercise-induced physiological hypertrophy initiates activation of cardiac progenitor cells[J]. Int J Clin Experiment Pathol, 2014, 7(2): 663-669.
[39] Xiao J, Shi J, Bei Y,. TCTAP A-038 miR-17-3p contributes to rxercise-induced cardiac growth and protects against myocardial ischemia-reperfusion injury[J]. J Am College Cardiol, 2017, 7(3): 664-676.
[40] Xiao L, He H, Ma L,Effects of miR-29a and miR-101a expression on myocardial interstitial collagen generation after aerobic exercise in myocardial-infarcted rats[J]. Archives Med Res, 2017, 48(1): 27-34.
[41] Xu X, Wan W, Powers A S,Effects of exercise training on cardiac function and myocardial remodeling in post myocardial infarction rats[J]. J Molecular Cellular Cardiol, 2008, 44(1): 114-122.
[42] Zhang K R, Liu H T, Zhang H F,Long-term aerobic exercise protects the heart against ischemia/reperfusion injury via PI3 kinase-dependent and Akt-mediated mechanism[J]. Apoptosis, 2007, 12(9): 1579-1588.
Resistance Training Activates the Signaling Pathway of FSTL1-Akt-mTOR and Induces Cardiomyocyte Proliferation in Rats with Myocardial Infarction
TIAN Zhen-jun,HAO Mei-li,XI Yue
Shaanxi Normal University Xi' an 710062, China.
Objectives: This study aimed at discussing the effect of resistance training on expression of Follistatin-like protein 1(FSTL1) and its receptor Disco-interacting protein 2 homolog A( DIP2A) , downstream signaling pathway of Akt-mTOR and cardiomyocyte proliferation in rats with MyocardialInfarction(MI). Methods: 30 male Sprague-Dawley rats, weight about 180-220g were randomly divided into three groups: Sham-operated group(S), sedentary MI group(MI) and MI with resistance training group(MR) after the MI model was established by ligation of left anterior descending coronary artery. After surgery 1 week, rats in MR took adaptability climbing up ladder unload training for 1 week. Then subjected progressive loading training for 4 weeks. The 24 hours after training, rats were anesthetized, the LVSP, LVEDP, ±dp/dt max were tested in order to evaluate cardiac function. The protein expression of FSTL1, DIP2A, pAkt, Akt, p-mTOR, mTOR, CyclinD1 and CDK4 in Myocardium were measured by Western blot, cardiomyocyte proliferation was observed and analyzed by immunofluorescence. Collagen volume fraction(%) of Myocardium were calculated by Masson Staining. Results: Compared with S, the protein expression of FSTL1, DIP2A, pAkt/Akt, p-mTOR/mTOR, CyclinD1 and CDK4, cardiomyocyte proliferation, the CVF% and LVEDP of MI increased, the LVSP and ±dp/dt max significantly decreased; Compared with MI, the protein expression of FSTL1, DIP2A, pAkt/Akt, p-mTOR/mTOR, CyclinD1 and CDK4, cardiomyocyte proliferation, the CVF% and LVEDP of MR significantly upregulated, the LVSP, ±dp/dt max significantly downregulated. Conclusions: Resistance training may via upregulate the expression of FSTL1 and its receptor DIP2A in Myocardium, activates the signaling pathway of Akt-mTOR, induces cardiomyocyte proliferation, and improves cardiac function.
1000-677X(2018)03-0040-08
10.16469/j.css.201803005
G804.5
A
2017-07-07;
2018-02-27
国家自然科学基金(31371199)资助项目。
田振军,男,教授,博士生导师,主要研究方向为运动与心血管健康,Email:tianzj611@hotmail.com; 郝美丽,女,在读硕士生,主要研究方向为运动与心血管健康; 席悦,男,在读博士生,主要研究方向为运动与心血管健康。

