Detection of thinned corticospinal tract and corticoreticular pathway in a patient with a calf circumference discrepancy
In clinical practice, it is challenging to elucidate the location of the lesion in a patient’s nervous system that is causing the neurologic symptoms, because lesions are often microscopic and cannot be revealed by conventional evaluation methods.However, recently developed techniques may aid clinicians in detecting these microscopic lesions. In particular, diffusion tensor tractography (DTT), derived from diffusion tensor imaging(DTI), possesses the unique advantages of allowing three-dimensional visualization and estimation of neural tracts (Jang and Kwon, 2015; Kim et al., 2015; Chang et al., 2016). Several previous studies have demonstrated that DTT can detect hidden neural tract injuries in various disorders (Jang and Kwon,2015; Kim et al., 2015; Chang et al., 2016). Therefore, DTT is being widely employed to accurately diagnose patients with neurologic symptoms.
In the current study, we describe a patient who presented with calf circumference discrepancy. Employing DTT, we demonstrate thinning of the right corticospinal tract (CST) and corticoreticular pathway (CRP), which correlated with our patient’s symptoms.
An 8-year-old right-handed girl was referred to the rehabilitation department of our hospital because of a discrepancy in her calf circumference (Figure 1). The Institutional Review Board of Yeungnam University Hospital approved study protocols (IRB No. YUMC-2015-07-064).
One week before visiting our department, the patient’s guardian had noticed that the girl’s left calf was noticeably smaller than her right calf. The patient had no specific history of developmental delay or neurological/musculoskeletal disorders. We measured the maximal-calf, mid-thigh, mid-forearm,and mid-upper arm circumferences at the midpoint of the calf,thigh, forearm, and upper arm lengths, respectively. Circumferences were measured using a standardized measurement technique following the guidelines of the CDC’s Anthropometry Procedures Manual (Centers for Disease Control and Prevention, 2000).We observed a calf circumference of 27.5 cm on the right side and 26.0 cm on the left side. The circumferences of the thigh, forearm, and upper arm were similar on both sides(35.0 cm, 15.2 cm, and 18.0 cm, respectively). Further physical examinations revealed slight motor weakness of the left upper and lower extremities (Medical Research Council muscle grade:4+; Jang and Chang, 2013) (Table 1). However, neither the patient’s guardian nor the patient herself had previously noticed this motor weakness. We did not observe any other significant neurological symptoms, including any disruption of upper limb function, gait disturbance, or sensory and cognitive deficits.
We conducted the Purdue Pegboard Test to evaluate fine motor activity, for which the patient achieved scores within normal range (right hand: 15 [cut off < 13], left hand: 11 [cut off <10]) (Desai et al., 2005). Furthermore, the Nottingham Sensory Assessment suggested that tactile and kinesthetic sensations were intact, with the patient scoring full marks for both tactile(20) and kinaesthetic (24) sensations (Lincoln et al., 1998). We also evaluated any discrepancy in leg length (from the anterior superior iliac spine to the medial malleolus of the ankle) and abnormalities of the foot or tibia using scanography and X-ray.These tests revealed no leg length discrepancy nor any tibia or foot abnormalities. Furthermore, ultrasound examination of calf muscles did not reveal any lesions. To rule out peripheral neuropathy and myopathy, nerve conduction tests and electromyography were performed. The results of these electrodiagnostic tests did not indicate any abnormalities. Finally, conventional brain and whole spine MRI did not reveal abnormal lesions.
Subsequently, we conducted DTT analysis to evaluate motor function-related neural tracts in the brain. We focused on the patient’s CST and CRP, which have been identified as the most important neural tracts underlying motor function (York,1987; Matsuyama et al., 2004). We obtained DTI data from the patient and ten age-matched control volunteers (3 boys and 7 girls) using a 1.5-T MRI system (GyroscanIntera; Philips Medical Systems, Best, The Netherlands) equipped with a synergy-L Sensitivity Encoding (SENSE) head coil using a single-shot,spin-echo planar imaging pulse sequence. For each of the 32 noncollinear and noncoplanar diffusion-sensitizing gradients, we acquired 67 contiguous slices parallel to the anterior commissure-posterior commissure line to avoid sphenoidal susceptibility artifacts. Imaging parameters were set as follows:matrix = 128 × 128, field of view = 221 × 221 mm2, repetition time = 10,726 ms, echo time = 76 ms, SENSE factor = 2, echo planar imaging factor = 67 andb= 1,000 s/mm2, number of excitations = 1, and slice thickness = 2.3 mm. Eddy current-induced image distortions were removed using affine multi-scale two-dimensional registration from the Oxford Centre for Functional Magnetic Resonance Imaging of Brain (FMRIB) Software Library. DTI-Studio software (CMRM, Johns Hopkins Medical Institute, Baltimore, MD, USA) was used to reconstruct the CST and CRP. For CST reconstruction, a seed region of interest(ROI) was drawn over the portion of the CST located in the anterior mid-pons on a 2D fractional anisotropy (FA) color map.The target ROI was drawn on the portion of the CST located in the anterior lower pons (Jang et al., 2013). For CRP reconstruction, a seed ROI was placed over the reticular formation of the medulla. The target ROI was placed on the midbrain tegmentum. Fiber tracts passing through both ROIs were designated as final tracts of interest. Termination criteria used for fiber tracking were an FA value of < 0.15 and an angle change of > 60°.
Using these methods, we estimated the FA value and tract volume (TV; Table 2) of the CST and CRP in the affected hemisphere. We observed that the right CST and CRP were thinner,compared with the left CST and CRP (Figure 2). Moreover,tract volumes estimated from the right CST and CRP were more than two standard deviations smaller than the mean tract volume obtained from control participants (Table 2). FA values for the right CST and CRP were within a two standard deviation margin from the mean FA values obtained from controls.
In the current study, we report a patient with thinning of the right CST and CRP detected using DTT, which was associated with a smaller left calf circumference. Slight motor weakness(neither the patient nor her guardian had noticed it) was diagnosed in the upper and lower left extremities. Furthermore,tract volume estimates for the right CST and CRP were signif icantly decreased in our patient compared with tract volume estimates from control participants.
FA values represent the degree of directionality and integrity of white matter microstructures, such as axons, myelin, and microtubule (Assaf and Pasternak, 2008). Tract volume is estimated by counting the number of voxels contained within a neural tract (Assaf and Pasternak, 2008). Therefore, a decrease in tract volume that is not accompanied by a change of FA value (as observed in our patient) indicates an injury in the respective tract,in this case the right CST and CRP. The CST and CRP are regarded as the most important neural tracts for voluntary movements (York, 1987; Matsuyama et al., 2004). The CST primarily controls movement of distal extremities (York, 1987) and the CRP primarily controls movement of proximal extremities(Matsuyama et al., 2004). Therefore, we think that the observed thinning of the right CST and CRP, and the reduced tract volumes, might be associated with the motor weakness exhibited by our patient in her upper and lower left extremities. Although we did not establish the exact mechanism underlying the calf circumference discrepancy in our patient, we suggest that the smaller left calf circumference can be attributed, at least in part,to her brain lesion and motor weakness.
In line with our observations, several previous studies have reported that central nervous system lesions can affect musculoskeletal maturation, owing to decreased vascularity, hormonal changes, and a lack of mechanical force and nutrients on the affected side (Lin and Henderson, 1996; Demir et al., 2006).Moreover, our patient’s calf circumference discrepancy might represent a decrease in muscle mass due to disuse (Than et al.,2016). It is possible that there is reduced muscle action in her left calf due to her left side motor weakness, which in turn has caused a decrease in the left calf muscle mass.
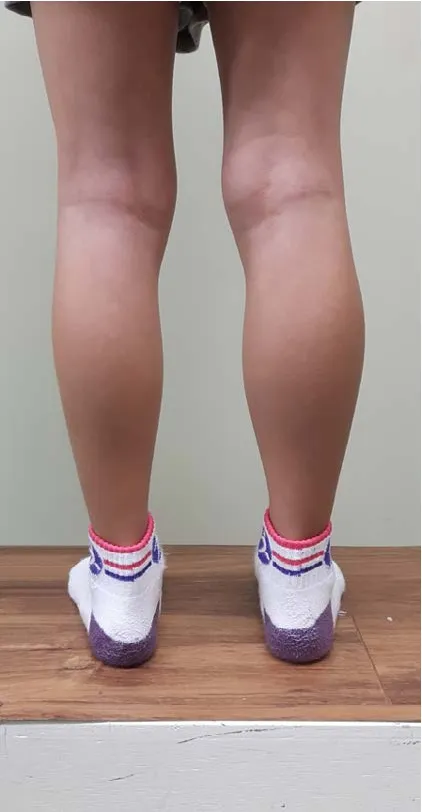
Figure 1 Image showing the patient’s calves.
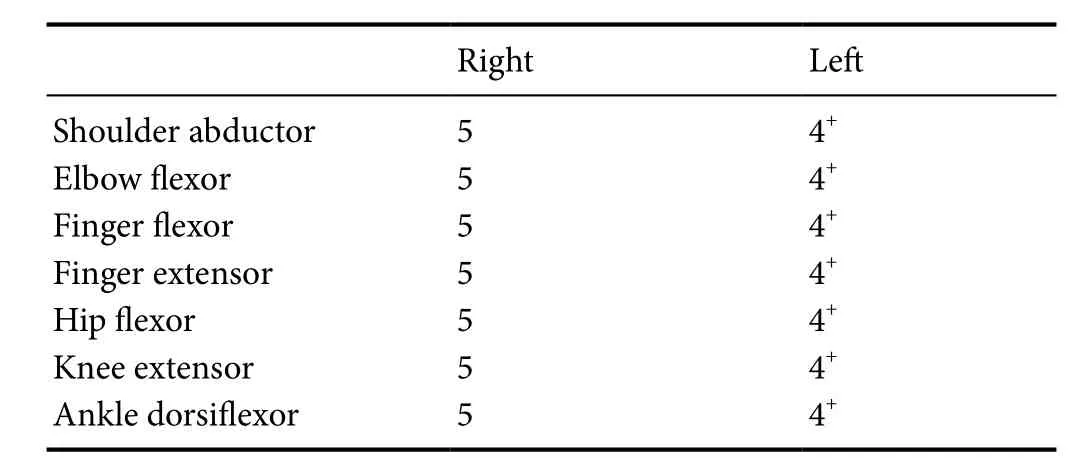
Table 1 Medical Research Council muscle grading in the presented patient
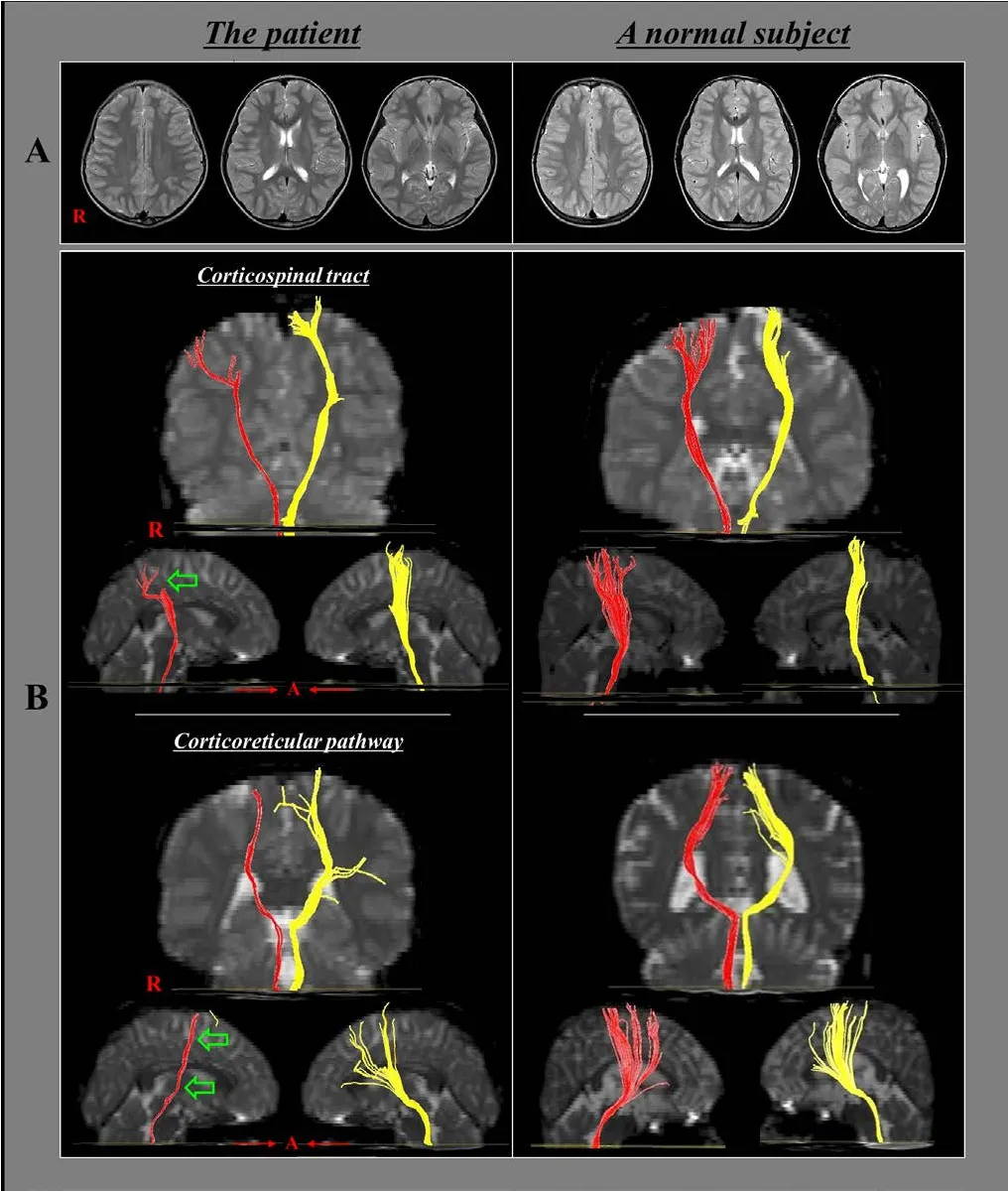
Figure 2 T2-weighted MRI and diffusion tensor tractography images of an 8-year-old right-handed girl with a discrepancy in calfcircumference.
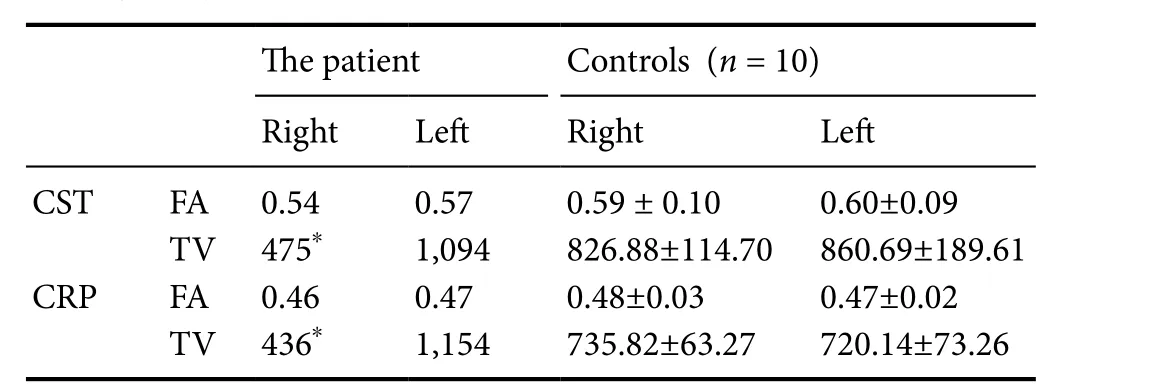
Table 2 Fractional anisotropy and tract volume values for the corticospinal tract and corticoreticular pathway in our patient and 10 age-matched controls measured using diffusion tensor tractography
In our patient, we did not observe any abnormalities using conventional MRI. However, neural tract lesions were revealed by DTT. We believe that our patient belongs to a category of patients with hemiplegic cerebral palsy (CP) because she showed brain pathology in DTT and neurologic symptoms indicative of hemiplegic CP, even though no abnormalities were detected using conventional brain MRI and there was only slight motor weakness of left upper and lower extremities (Bax,1964). Furthermore, the calf circumference discrepancy in our patient is consistent with results from previous studies demonstrating decreased sizes of affected extremities compared with the unaffected side in patients with hemiplegic CP (Stevenson et al., 1995; Lin and Henderson, 1996; Demir et al., 2006).
Along with our study, several DTT studies have revealed microscopic injury of neural tracts present in various disorders,including cerebral palsy, motor neuron disease, encephalitis,and traumatic brain injury (Son et al., 2007; Jang and Kwon,2015; Kim et al., 2015; Chang et al., 2016). Therefore, when no abnormalities are observed in conventional MRI or CT of patients showing neurologic symptoms indicative of brain lesions, we recommend performing DTT to identify microscopic lesions in neural tracts.
In conclusion, using DTT we detected thinning of the CST and CRP in the right hemisphere, which seemed to be correlated with a smaller circumference of the left calf of the patient.To the best of our knowledge, the current study is the first to demonstrate neural tract lesions in a patient with limb discrepancy between the right and left side. However, some limitations should be considered when interpreting these results. First, we were unable to explain why the circumference discrepancy only occurred in the calf muscles and not in other muscles. Second,we could not identify a cause for the lesions found in the CST and CRP. Third, we could not clearly demonstrate the relationship between the discrepancy in calf circumference and the lesions in the CST and CRP. Last, the present study presents only a single case. Therefore, further studies are required to fully establish the connection between limb size discrepancies and lesions of the CST and CRP.
Han Do Lee, Min Cheol Chang*
Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, Namku, Daegu, Republic of Korea*Correspondence to:Min Cheol Chang, wheel633@hanmail.net.
orcid:0000-0002-7629-7213 (Min Cheol Chang)
Author contributions:MCC designed the study. HDL analyzed the data.Both authors co-wrote the paper and approved the final version of this paper.
Conflicts of interest:None declared.
Financial support:None.
Research ethics: The study was approved by the Institutional Review Board of Yeungnam University Hospital (IRB No. YUMC- 2015-07-064).
Declaration of participant consent:The authors certify that they have obtained all appropriate participant consent forms. In the form, participants have given their consent for their images and other clinical information to be reported in the journal. The participants understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Data sharing statement:Datasets analyzed during the current study areavailable from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Assaf Y, Pasternak O (2008) Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci 34:51-61.
Bax MC (1964) Terminology and classification of cerebral palsy. Dev Med Child Neurol 6:295-297.
Centers for Disease Control and Prevention (CDC). Anthropometry Procedures Manual. National Health and Nutrition Examination Survey.Revised.CDC; 2000. Available at: www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf. Accessed August 1,2014.
Chang MC, Yeo SS, Do Lee H, Jang SH (2016) Diffusion tensor tractography in a patient with memory impairment following encephalitis. Acta Neurol Belg 116:629-631.
Demir SO, Oktay F, Uysal H, Seluk B (2006) Upper extremity shortness in children with hemiplegic cerebral palsy. J Pediatr Orthop 26:764-768.
Desai K, Kene K, Dochi M, More S (2005) Normative data of purdue pegboard on Indian population. Indian J Occup Ther 37:69-72.
Jang SH, Chang MC (2013) Motor outcomes of patients with a complete middle cerebral artery territory infarct. Neural Regen Res 8:1892-1897.
Jang SH, Choi BY, Chang CH, Kim SH, Chang MC (2013) Prediction of motor outcome based on diffusion tensor tractography findings in thalamic hemorrhage. Int J Neurosci 123:233-239.
Jang SH, Kwon HG (2015) Injury of the dentato-rubro-thalamic tract in a patient with mild traumatic brain injury. Brain Inj 29:1725-1728
Kim JW, Lee HD, Jang SH (2015) Severe bilateral anterior cingulum injury in patients with mild traumatic brain injury. Neural Regen Res 10:1876-1878.
Lin PP, Henderson RC (1996) Bone mineralization in the affected extremities of children children. Dev Med Child Neurol 38:782-786.
Lincoln NB, Jackson JM, Adams SA (1998) Reliability and revision of the Nottingham sensory assessment for stroke patients. Physiotherapy 84:358-365.
Matsuyama K, Mori F, Nakajima K, Drew T, Aoki M, Mori S (2004) Locomotor role of the corticoreticular-reticulospinal-spinalinterneuronal system. Prog Brain Res 143:239-249.
Son SM, Ahn YH, Sakong J, Moon HK, Ahn SH, Lee H, Yu IK, Shin YJ, Jang SH (2007) Diffusion tensor imaging demonstrates focal lesions of the corticospinal tract inhemiparetic patients with cerebral palsy. Neurosci Lett 420:34-38.
Stevenson RD, Roberts CD, Vogtle L (1995) The effects of non-nutritional factors on growth in cerebral palsy. Dev Med Child Neurol 37:124-130.
Than C, Tosovic D, Seidl L, Mark Brown J (2016) The effect of exercise hypertrophy and disuse atrophy on muscle contractile properties: a mechanomyographic analysis. Eur J Appl Physiol 116:2155-2165.
York DH (1987) Review of descending motor pathways involved with transcranial stimulation. Neurosurgery 20:70-73.
- 中国神经再生研究(英文版)的其它文章
- Relationship of distraction rate with inferior alveolar nerve degeneration-regeneration shift
- The effect of increased intra-abdominal pressure on orbital subarachnoid space width and intraocular pressure
- Voltage adjustment improves rigidity and tremor in Parkinson’s disease patients receiving deep brain stimulation
- Effect of electrical stimulation on neural regeneration via the p38-RhoA and ERK1/2-Bcl-2 pathways in spinal cord-injured rats
- Proteomic analysis of trans-hemispheric motor cortex reorganization following contralateral C7 nerve transfer
- GSK3β inhibitor promotes myelination and mitigates muscle atrophy after peripheral nerve injury

