GSK3β inhibitor promotes myelination and mitigates muscle atrophy after peripheral nerve injury
Jian Weng, Yan-hua Wang, Ming Li, Dian-ying Zhang, Bao-guo Jiang
Department of Orthopedics and Trauma, Peking University People’s Hospital, Beijing, China
Introduction
Peripheral nerve injury results in developmental atrophy of the target skeletal muscle and also poor functional recovery when surgery is delayed (Gigo-Benato et al., 2010; Gu et al.,2010; Willand et al., 2014; Zhang et al., 2017). The peripheral nervous system is different from the central nervous system as it has the capacity to regenerate after injury (David and Aguayo, 1985; Schmitt et al., 2003; Hall, 2005; Höke and Brushart, 2010). However, regeneration of the peripheral nerve after injury is a slow process (1–3 mm/d). Axons can take more than 3 months to regenerate into the distal target organs or tissues, meanwhile the distal nerve stump and skeletal muscle usually atrophy (Aydin et al., 2004). To improve the functional recovery of target muscle after periph-eral nerve injury, it is essential to reduce the reinnervation time and reduce the atrophy of the denervated muscle (Moimas et al., 2013).
Previous studies have revealed that the Wallerian degeneration occurs at the lesion site after peripheral nerve injury(Wagner et al., 1998; Pesini et al., 1999; Wang et al., 2013;Xin et al., 2013). Promoting the migration and proliferation of the Schwann cells has benefits for the improvement of myelination and nerve regeneration (Le et al., 1988; Wang et al., 2016; Wen et al., 2017). Muscle regeneration also depends on resident satellite cells, which are located between the sarcolemma and basement membrane of muscle fibers(Montarras et al., 2005; Le and Rudnicki, 2007; Lepper et al., 2011). Restoration of myotube and myogenesis differentiation has been linked to a reduction of muscle atrophy(Sorci et al., 2003; Johnson et al., 2013; Lee et al., 2017).We hypothesize a therapy that has a positive effect on both Schwann cells and muscle cells, leading to a short reinnervation interval and good muscular function after peripheral nerve injury. In recent years, studies on the peripheral nerve injury have increasingly focused on the role of various signaling pathways, including the Wnt signaling pathway.
The discovery of the wingless gene found by Sharma in 1973 was the basis of the important Wnt signaling pathway(Sharma and Chopra, 1976). Since then other studies have shown that the Wnt/β-catenin signaling pathway has a direct role in myelin gene expression (Chew et al., 2011; Tawk et al., 2011; Meffre et al., 2015; Hichor et al., 2017). Wnt/β-catenin signals act as positive regulators during remyelination (Fancy et al., 2009; Makoukji et al., 2011). Recently,the Wnt/β-catenin signaling pathway has been considered the main molecular mechanism in age-related skeletal muscle atrophy (Rajasekaran et al., 2017). Wnt/β-catenin was involved in impaired muscle repair, such as loss of satellite cell number, muscle cell dysfunction, decreased myoblast proliferation and attenuated differentiation (Carlson et al.,2009). 3-(2,4-Dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione (SB216763) is a typical inhibitor of glycogen synthase kinase 3 beta (GSK3β), which stimulates canonical Wnt/β-catenin signal (Li et al., 2012). Our previous study demonstrated that SB216763 can promote remyelination and myelin protein production (Chen et al., 2016).SB216763 reduces skeletal muscle atrophy (Litwiniuk et al.,2016). Pretreatment of muscle cell cultures with SB216763 prevented loss of myogenic differentiation and myogenesis induced by TNF-α (Verhees et al., 2013).
Considering the importance of the Wnt signaling pathway in nerve and muscle regeneration, we investigated the effect of the GSK3β inhibitor on both Schwann cells and muscle cells. Ourin vitromodel system mimics the progress of myelination and muscle atrophy, and evaluates the effect of SB216763 on RSC96 cells and C2C12 myotubes.
Materials and Methods
Cells culture and SB216763 treatment
Murine RSC96 Schwann cell and C2C12 myoblast cell lines,passages 1–3, were obtained from American Type Culture Collection (Manassas, VA, USA). Cells were cultured in growth media: RSC96 cells with Dulbecco’s modified Eagle’s medium (DMEM)/F12 containing 10% fetal bovine serum(Gibco, Grand Island, NY, USA), 100 units/mL penicillin and 100 μg/mL streptomycin (Sigma-Aldrich, St. Louis, MO,USA); C2C12 cells with high-glucose DMEM (Sigma-Aldrich)containing 5 mM glucose, 10% fetal bovine serum and antibiotics., When cells reached 80–90% confluence, the medium was replaced by a differentiation medium (high-glucose DMEM containing 2% calf serum (Gibco) and antibiotics)to induce C2C12 cell myotube formation and differentiation.The differentiation medium was changed daily for 6 days.SB216763 (Apexbio, Houston, TX, USA) was prepared as a 20 mM stock in dimethyl sulfoxide. RSC96 and C2C12 cells were exposed to 10 μM of SB216763 for 48 hours as described previously (Chen et al., 2016). Following these treatments, cells were washed with phosphate-buffered saline (PBS; Gibco) to remove any free drug prior to the sequence experiments.
Scratch wound assay
RSC96 cells were plated in 6-well plates 24 hours before the scratch. A scratch length, approximately 1 mm, was made in the middle area of the plate using a 200 μL pipette tip. All wells were washed with PBS to remove cellular debris. Cells were incubated with growth media as mentioned above. The scratch was photographed at 0, 24 and 48 hours using an inverted fluorescence microscope equipped with microscope camera (BM CAM, Shanghai, China) and ToupView software (ToupTek, Hangzhou, China). Five photographs were taken of the scratched region of each group at each time point. Relative area of the gap was calculated as follows:Relative area in the gap of wound = (area in the gap at 24 or 48 hours/area in the gap at 0 hours) × 100%.
Index of C2C12 myotube contraction
On day 6 of differentiation, cells were stimulated with 100 μM carbachol (Apexbio). Five high-quality images of cells were taken sequentially at 1-minute intervals during carbachol stimulation (Niu et al., 2011). The difference in integrated optical density (IOD) between the first and subsequent images is mainly due to the myotube contraction.Thus, the average IOD of differential image represents the index of contraction. Calculated as below, index of contraction = {∑(| IOD5–IOD4|+|IOD4–IOD3|+|IOD3–IOD2|+|IOD2–IOD1|)}/4. IOD1to IOD5stand for the mean IOD value of the first to fifth images. IOD was analyzed using Image-Pro Plus software (Media Cybernetics, Rockville,MD, USA).
Cell proliferation assay
RSC96 and C2C12 cells were seeded into 96-well plates at a density of 10,000 cells/well. After incubation for 48 hours with or without SB216763, cell viability was evaluated by Cell Counting Kit-8 (CCK-8) assay. Four hours before detection, 10 μL of CCK-8 solution (Sigma-Aldrich) was added into each well, followed by a further 1-hour incubation. The optical density (OD) values were measured at 450 nm with a microplate reader (Bio-Rad, Hercules, CA, USA).
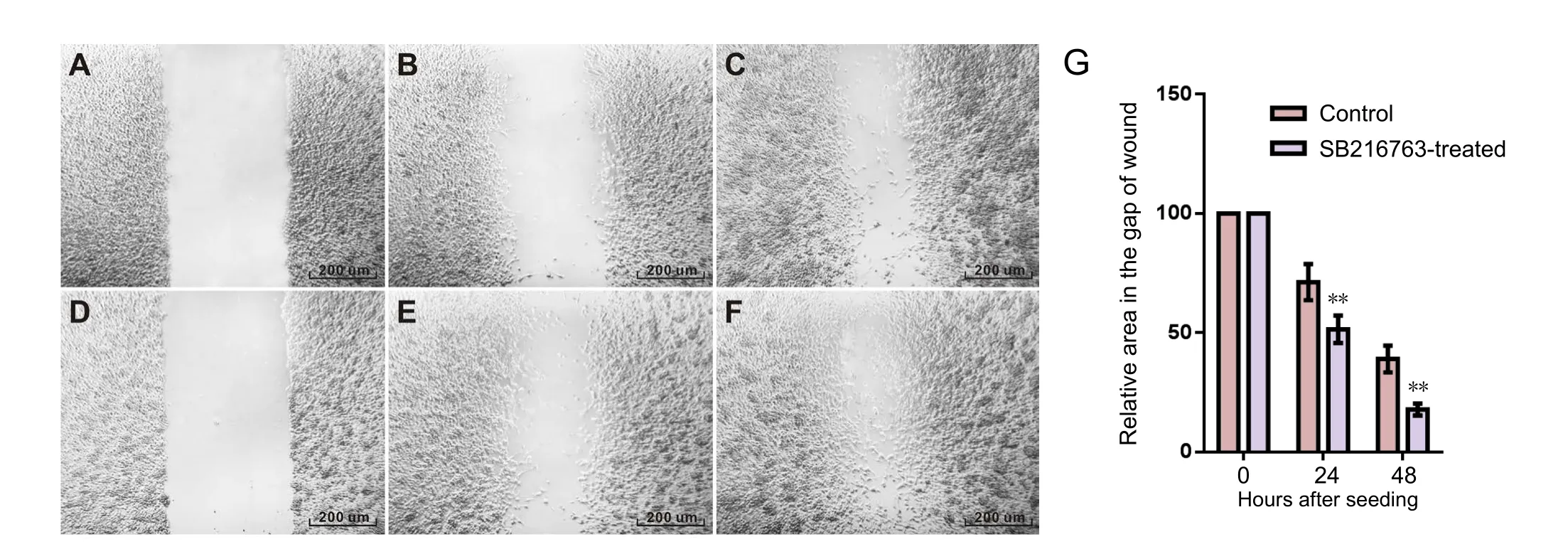
Figure 1 SB216763 promoted gap closure in RSC96 cells.
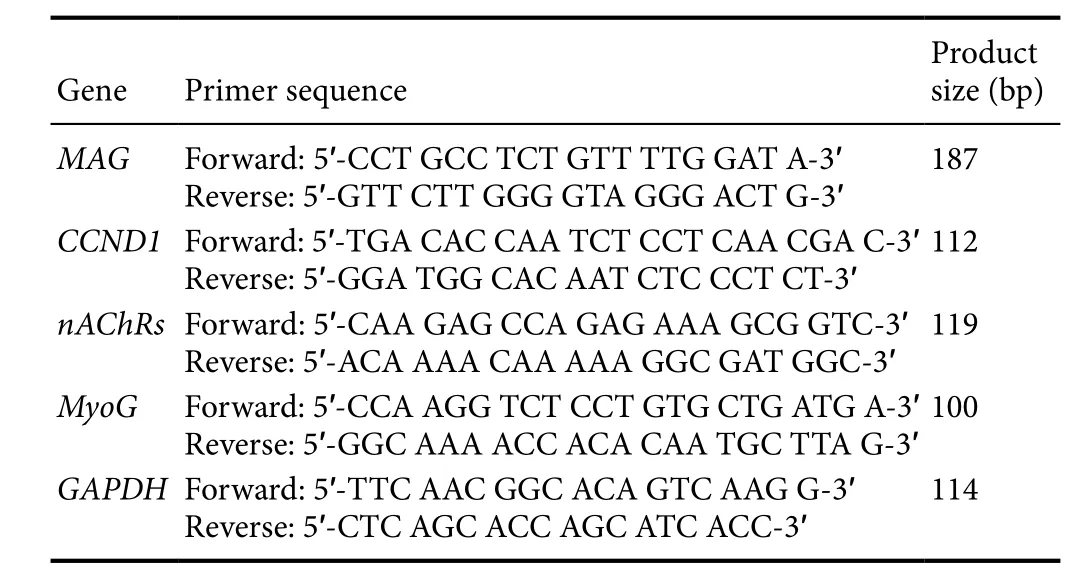
Table 1 Real-time fluorescent quantitative polymerase chain reaction primer sequences
Quantitative real-time polymerase chain reaction (PCR)Total RNA was isolated from RSC96 and C2C12 cells using TRIzol reagent (Thermo Fisher, Waltham, MA, USA).cDNA was synthesized using M-MLV reverse transcriptase(Promega, Madison, WI, USA). The amplification was completed by two steps at each target gene annealing temperature. The specificity of the amplified product was determined according to the fusion curve, and the 2-ΔΔCt method was used to determine the mRNA expression. The primers used for PCR are given inTable 1.
Immunocytochemical staining
RSC96 and C2C12 cells with or without SB216763 treatment were washed three times with PBS and fixed for 15 minutes in 4% paraformaldehyde and 20 minutes in 0.5%Triton X-100 (Amresco, Solon, OH, USA), then deactivated with 3% H2O2for 30 minutes. Subsequently, cell slices were blocked in PBS supplemented with 5% goat serum (Gibco)at room temperature for 20 minutes. Cells were incubated with anti-β-catenin antibody (1:300; Abcam, Cambridge,MA, USA) at 37°C for 1 hour, washed in PBS and incubated with anti-mouse IgG-horseradish peroxidase secondary antibody (1:1,000; GTVision, Shanghai, China) for 30 minutes at room temperature. Following washing with PBS, horseradish peroxidase activity was detected using a diaminobenzidine substrate kit (Vector Labs, Burlingame, CA, USA)according to the manufacturer’s instructions. Five frames per slide were analyzed. Images were collected with a Leica DFC-550 connected to an Olympus IX70 inverted microscope (Olympus, Tokyo, Japan) and analyzed using the soft-ware Leica Application Suite Advanced Fluorescence (Leica,Wetzlar, Germany). The ratio of nuclear localization cells was calculated as follows: The positive ratio was equal to the number of cells with positively stained nuclei/the number of cells with negatively stained nuclei × 100%.
Statistical analysis
Statistical analysis was performed using SPSS 21.0 for Windows software (IBM, Armonk, NY, USA). All data are presented as the mean ± SD and analyzed with Student’st-test. A value ofPless than 0.05 was considered statistically significant.
Results
GSK3β inhibitor accelerated migration of RSC96 cells
One therapeutic aspect of a GSK-3 inhibitor for the treatment of peripheral nerve injury was the migration ability of Schwann cells as Wallerian degeneration progressed.To compare the motility of RSC96 cells with or without SB216763 treatment, scratch assay was used to detect cell migration in the area of a “wound”, which was scratched into a confluent cell monolayer. The relative area of the gap of wound was measured by the advancement of cells over 24 and 48 hours. The scratch assay demonstrated that the relative area of the gap of wound in control group decreased to 71.2 ± 7.6% at 24 hours and 39.0 ± 5.6% at 48 hours, while
that in the SB216763-treated group decreased to 51.4 ± 5.8%at 24 hours and 17.8 ± 2.5% at 48 hours. The results showed that there were more cells in the gap and the area of the gap was smaller in the SB216763-treated group compared with the control group (P< 0.01;Figure 1).
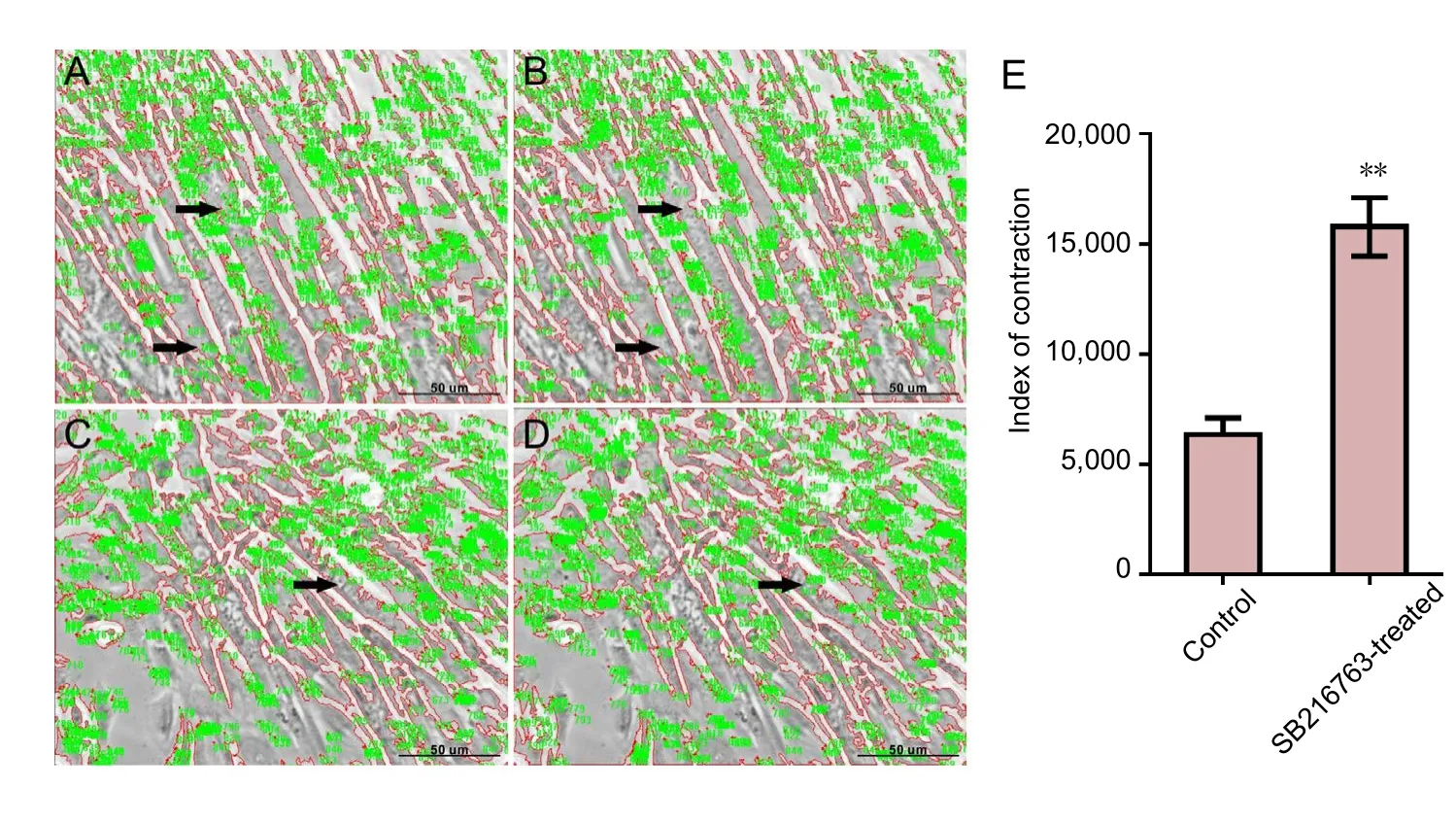
Figure 2 Significant differences in the contraction in C2C12 myotubes after carbachol treatment in each group.

Figure 3 SB216763 promotes the proliferation of RSC96 and C2C12 cells (Cell Counting Kit-8 assay).
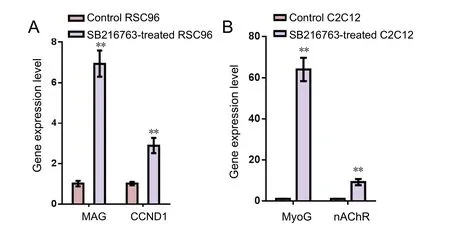
Figure 4 Quantitative polymerase chain reaction of mRNA expression in RSC96 and C2C12 cells in control and SB216763 groups.
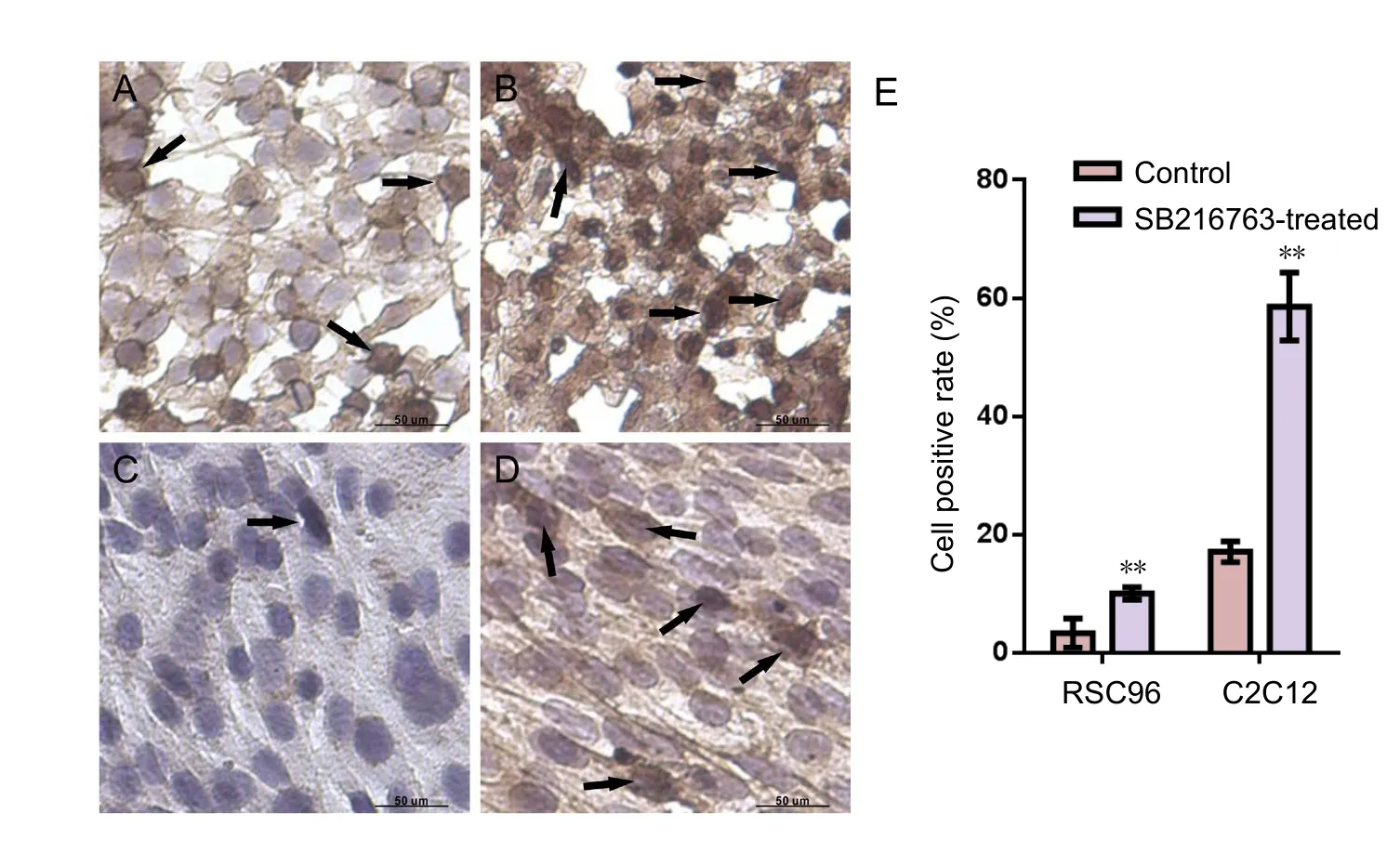
Figure 5 Immunocytochemical staining of β-catenin in RSC96 and C2C12 cells from SB216763-treated group and control group.
GSK3β inhibitor stimulated contraction of C2C12 myotubes
Six days after differentiation, approximately 50% C2C12 cells had fused together to form the typical multinucleated myotubes. After exposure to 100 μM carbachol, vigorous contraction of C2C12 myotubes was readily observed. Examples at the first two times are shown inFigure 2A–D.Measurement of the index of contraction is described above,in the Methods. Analysis of the results revealed that the C2C12 myotubes in SB216763-treated group showed significantly higher contractile activity compared with the control group (Figure 2E).
GSK3β inhibitor induced proliferation of both Schwann cells and muscle cells
CCK-8 assay was used to determine the effects of 48 hours of treatment with SB216763 on the proliferation of RSC96 and C2C12 cells. The results showed that treatment with SB216763 significantly stimulated the growth of Schwann cells and muscle cells (Figure 3).
GSK3β inhibitor treatment resulted in regulation of related genes
The mRNA expression of myelin associated glycoprotein(MAG), cyclin-D1 (CCND1), myogenin (MyoG) and nicotinic acetylcholine receptors (nAChRs) were detected by quantitative PCR. The mRNA expression of both MAG and CCND1 was significantly greater in the SB216763-treated than the control RSC96 group. Similarly, the mRNA expression of MyoG and nAChRs was significantly higher (P< 0.01) in SB216763-treated than in the control C2C12 group (Figure 4).
GSK3β inhibitor promoted β-catenin expression and nuclear localization
Immunocytochemical staining was performed to detect β-catenin expression. Quantification of β-catenin nuclear localization was analyzed by counting the average positive cell ratio from five frames per slide. After SB216763 treatment,the overall β-catenin expression was greatly upregulated and the nuclear concentration of β-catenin was elevated in RSC96 cells. Beta-catenin expression level was higher and greater nuclear localization was shown in C2C12 cells of SB216763-treated group than in the control group (Figure 5). Our results demonstrated that the GSK3β inhibitor enhanced β-catenin expression and nuclear localization and possibly exerted its efficacyviaWnt/β-catenin signaling pathway.
Discussion
Peripheral nerve injury is a common disease and has been a focus in neuroscience research for decades. In a clinical setting of peripheral nerve damage, both nerve and muscles undergo degeneration processes. The identification of the molecular mechanism that fosters nerve regeneration,as well as to protect skeletal muscle from atrophy, is thus required. Enhancing the efficacy of remyelination is one of the main areas of research for the treatment of peripheral nerve injury (Torii and Yamauchi, 2016; Yi et al., 2017). Remyelination failure usually leads to irreversible axonal loss and functional impairment (Brück, 2005; Frohman et al.,2006). For successful axon myelin formation, it is critical to increase the proliferation of Schwann cells to support the injured axon. As reported, any delay of interaction between axon and distal target may lead to axonal degeneration, resulting in neuronal apoptosis (Ohnishi et al., 1985; Scheib and Höke, 2013). In turn, peripheral nerve injury could result in the atrophy of the target skeletal muscle. This denervation process induces some complex changes in the expression of genes involved in muscle atrophy (Durbeej et al., 2003). However, the mechanisms underlying the degeneration and regeneration of Schwann cells and muscle cells remain unclear. In ourin vitromodel, the GSK3β inhibitor,SB216763, plays a role in regulating both myelin formation and myogenesis. The preliminary data have shown that a GSK3β inhibitor may promote myelination and also reverse muscle cell atrophy through regulating the Wnt/β-catenin signaling pathway.
SB216763 directly inhibits GSK3β, leading to β-catenin stabilization and nuclear translocation, thereby activating the Wnt/β-catenin signaling pathway (Lenox and Wang,2003). In the peripheral nervous system, GSK3β inhibitor treatment promotes remyelination of sciatic and facial nerves after crush (Makoukji et al., 2012). Previously we found that up-regulating Wnt/β-catenin signaling by SB216763 induced the clearance of myelin debris and the expression of myelin-related genes (Chen et al., 2016). Others have found that activation of Wnt signaling is important for muscle regeneration (Polesskaya et al., 2003; Brack et al.,2009). Wnt signaling also enhances the proliferation of muscle satellite cells (Otto et al., 2008).
In this study, two kinds ofin vitrocellular models of peripheral nerve injury were used to study the effect of SB216763 on myelination and myotube differentiation. In the two cell lines, RSC96 and C2C12, tested, the beneficial effects of SB216763 on the progression of peripheral nerve repair are not limited to the local injured site. A previous study showed that migration of Schwann cell is essential to create a permissive environment to promote axon sprouting and also inhibit nerve scar formation (Chehrehasa et al., 2010). In our RSC96 model, SB216763 treatment also accelerated the migratory capacities of the cells. We obtained other comparable beneficial results in other assays. SB216763 treatment increased RSC96 cell proliferation, and enhanced myelin-related gene expression (MAG and CCND1). CCND1 is a β-catenin target gene for an important protein in the cell cycle and can promote the proliferation of cells (Zhao et al.,2016). MAG is a myelin-specific marker which plays a key role in the early stage of myelination and the maintenance of stable axonal myelin interaction (Shim and Ming, 2010).These encouraging results revealed that the treatment with a GSK3β inhibitor can increase myelin formation.
Positive effects of SB216763 on C2C12 muscle cell line,in vitro, were also found. SB216763 stimulated C2C12 myotube contraction, which is caused by acetylcholine receptor activation. CCK-8 assay showed that SB216763 induced C2C12 cell proliferation. Quantitative PCR data indicated that SB216763 up-regulated the expression of MyoG and nAChRs of C2C12 cells. MyoG is a muscle-specific transcription factor that is essential for the development of skeletal muscle, and atrophy of denervated muscle could be prevented by high MyoG expression (Teraoka et al., 2012). nAChR acts as an activator of genes encoding endplate-associated proteins (Méjat et al., 2005). These data revealed that GSK3β inhibitor has the potential to restore muscular function as well as accelerate the myelination.
The mechanism by which GSK3β inhibitor exerts its efficacy was explored by immunocytochemical staining. Exposure to SB216763 increased the nuclear localization and expression of β-catenin, the landmark molecular event of the Wnt signaling pathway. All results indicated that SB216763, as a GSK3β inhibitor, may exert its positive effects on Schwann cells and muscle cellsviathe Wnt/β-catenin signaling pathway.
In summary, thisin vitrostudy supports the hypothesis that this GSK3β inhibitor generates a permissive environment for promoting nerve regeneration and decelerating muscle atrophy in the peripheral nervous system. Our results suggest that a GSK3β inhibitor can be considered as an important therapeutic target in myelination and myotube differentiation. This researchin vitroprovides the basis for the hitherto absentin vivoanimal model studies and further investigations into the underlying mechanism.
Author contributions:BGJ and DYZ designed this study. JW performed most of the experiments. YHW and ML contributed to the analysis and acquisition of all data. JW wrote the paper. All authors approved the final version of the paper.
Conflicts of interest:There are no competing interests to declare.
Financial support:This study was supported by the National Basic Research Program of China (973 Program), No. 2014CB542201; the National High Technology Research and Development (863 Program),No. SS2015AA020501; the National Natural Science Foundation of China (General Program), No. 31571235, 31771322, 31671248, 31571236,31271284, 31171150, 81171146, 31471144, 30971526, 31100860,31040043, 31371210, and 81372044. The funding sources had no role in the design, execution, and data collection, analysis, and interpretation or decision to submit results.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Open peer reviewer:Yiren Hu, University of California San Diego,USA; Dirk Montag, Head Neurogenetics Special Laboratory, Leibniz Institute for Neurobiology, Neurogenetics Special Laboratory, Germany.Additional file:Open peer review reports 1 and 2.
Aydin MA, Mackinnon SE, Gu XM, Kobayashi J, Kuzon WM (2004)Force deficits in skeletal muscle after delayed reinnervation. Plast Reconstr Surg 113:1712-1718.
Brack AS, Murphy-Seiler F, HanifiJ, Deka J, Eyckerman S, Keller C,Aguet M, Rando TA (2009) BCL9 is an essential component of canonical Wnt signaling that mediates the differentiation of myogenic progenitors during muscle regeneration. Dev Biol 335:93-105.
Brück W (2005) The pathology of multiple sclerosis is the result of focal inflammatory demyelination with axonal damage. J Neurol 252 Suppl 5:3-9.
Carlson ME, Suetta C, Conboy MJ, Aagaard P, Mackey A, Kjaer M,Conboy I (2009) Molecular aging and rejuvenation of human muscle stem cells. EMBO Mol Med 1:381-391.
Chehrehasa F, Windus LC, Ekberg JA, Scott SE, Amaya D, Mackay-Sim A, St JJA. 2010. Olfactory glia enhance neonatal axon regeneration.Mol Cell Neurosci 45:277-288.
Chen Y, Weng J, Han D, Chen B, Ma M, Yu Y, Li M, Liu Z, Zhang P,Jiang B (2016) GSK3β inhibition accelerates axon debris clearance and new axon remyelination. Am J Transl Res 8:5410-5420.
Chew LJ, Shen W, Ming X, Senatorov VV, Chen HL, Cheng Y, Hong E, Knoblach S, Gallo V (2011) SRY-box containing gene 17 regulates the Wnt/β-catenin signaling pathway in oligodendrocyte progenitor cells. J Neurosci 31:13921-13935.
David S, Aguayo AJ (1985) Axonal regeneration after crush injury of rat central nervous system fibres innervating peripheral nerve grafts.J Neurocytol 14:1-12.
Durbeej M, Sawatzki SM, Barresi R, Schmainda KM, Allamand V, Michele DE, Campbell KP (2003) Gene transfer establishes primacy of striated vs. smooth muscle sarcoglycan complex in limb-girdle muscular dystrophy. Proc Natl Acad Sci U S A 100:8910-8915.
Fancy SP, Baranzini SE, Zhao C, Yuk DI, Irvine KA, Kaing S, Sanai N,Franklin RJ, Rowitch DH (2009) Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev 23:1571-1585.
Frohman E, Costello F, Zivadinov R, Stuve O, Conger A, Winslow H,Trip A, Frohman T, Balcer L (2006) Optical coherence tomography in multiple sclerosis. Lancet Neurol 5:853-863.
Gigo-Benato D, Russo TL, Geuna S, Domingues NR, Salvini TF, Parizotto NA (2010) Electrical stimulation impairs early functional recovery and accentuates skeletal muscle atrophy after sciatic nerve crush injury in rats. Muscle Nerve 41:685-693.
Gu S, Shen Y, Xu W, Xu L, Li X, Zhou G, Gu Y, Xu J (2010) Application of fetal neural stem cells transplantation in delaying denervated muscle atrophy in rats with peripheral nerve injury. Microsurgery 30:266-274.
Hall S. 2005. The response to injury in the peripheral nervous system. J Bone Joint Surg Br 87:1309-1319.
Hichor M, Sampathkumar NK, Montanaro J, Borderie D, Petit PX,Gorgievski V, Tzavara ET, Eid AA, Charbonnier F, Grenier J, Massaad C (2017) Paraquat induces peripheral myelin disruption and locomotor defects: crosstalk with LXR and Wnt pathways. Antioxid Redox Signal 27:168-183.
Höke A, Brushart T. 2010. Introduction to special issue: challenges and opportunities for regeneration in the peripheral nervous system. Exp Neurol 223:1-4.
Johnson AN, Mokalled MH, Valera JM, Poss KD, Olson EN (2013)Post-transcriptional regulation of myotube elongation and myogenesis by Hoi Polloi. Development 140:3645-3656.
Le BJ, LaCorbiere M, Powell HC, Ellisman MH, Schubert D (1988)Extracellular fluid conditioned during peripheral nerve regeneration stimulates Schwann cell adhesion, migration and proliferation. Brain Res 459:93-104.
Le GF, Rudnicki M (2007) Satellite and stem cells in muscle growth and repair. Development 134:3953-3957.
Lee H, Lee SJ, Bae GU, Baek NI, Ryu JH (2017) Canadine from Corydalis turtschaninovii stimulates myoblast differentiation and protects against myotube atrophy. Int J Mol Sci 18:E2748.
Lenox RH, Wang L (2003) Molecular basis of lithium action: integration of lithium-responsive signaling and gene expression networks.Mol Psychiatry 8:135-144.
Lepper C, Partridge TA, Fan CM (2011) An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138:3639-3646.
Li Y, Gao Q, Yin G, Ding X, Hao J (2012) WNT/β-catenin-signaling pathway stimulates the proliferation of cultured adult human Sertoli cells via upregulation of C-myc expression. Reprod Sci 19:1232-1240.
Litwiniuk A, Pijet B, Pijet-Kucicka M, Gajewska M, Pająk B,Orzechowski A (2016) FOXO1 and GSK-3β are main targets of insulin-mediated myogenesis in c2c12 muscle cells. PLoS One 11:e0146726.
Makoukji J, Belle M, Meffre D, Stassart R, Grenier J, Shackleford G,Fledrich R, Fonte C, Branchu J, Goulard M, de Waele C, Charbonnier F, Sereda MW, Baulieu EE, Schumacher M, Bernard S, Massaad C (2012) Lithium enhances remyelination of peripheral nerves. Proc Natl Acad Sci U S A 109:3973-3978.
Makoukji J, Shackleford G, Meffre D, Grenier J, Liere P, Lobaccaro JM,Schumacher M, Massaad C (2011) Interplay between LXR and Wnt/β-catenin signaling in the negative regulation of peripheral myelin genes by oxysterols. J Neurosci 31:9620-9629.
Meffre D, Massaad C, Grenier J (2015) Lithium chloride stimulates PLP and MBP expression in oligodendrocytes via Wnt/β-catenin and Akt/CREB pathways. Neuroscience 284:962-971.
Moimas S, Novati F, Ronchi G, Zacchigna S, Fregnan F, Zentilin L,Papa G, Giacca M, Geuna S, Perroteau I, Arnež ZM, Raimondo S(2013) Effect of vascular endothelial growth factor gene therapy on post-traumatic peripheral nerve regeneration and denervation-related muscle atrophy. Gene Ther 20:1014-1021.
Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M (2005) Direct isolation of satellite cells for skeletal muscle regeneration. Science 309:2064-2067.
Méjat A, Ramond F, Bassel-Duby R, Khochbin S, Olson EN, Schaeffer L (2005) Histone deacetylase 9 couples neuronal activity to muscle chromatin acetylation and gene expression. Nat Neurosci 8:313-321.
Niu W, Bilan PJ, Yu J, Gao J, Boguslavsky S, Schertzer JD, Chu G, Yao Z,Klip A (2011) PKCε regulates contraction-stimulated GLUT4 traffic in skeletal muscle cells. J Cell Physiol 226:173-180.
Ohnishi A, Chua CL, Kuroiwa Y (1985) Axonal degeneration distal to the site of accumulation of vesicular profiles in the myelinated fiber axon in experimental isoniazid neuropathy. Acta Neuropathol 67:195-200.
Otto A, Schmidt C, Luke G, Allen S, Valasek P, Muntoni F, Lawrence-Watt D, Patel K. (2008) Canonical Wnt signalling induces satellite-cell proliferation during adult skeletal muscle regeneration.J Cell Sci 121:2939-2950.
Pesini P, Kopp J, Wong H, Walsh JH, Grant G, Hökfelt T (1999) An immunohistochemical marker for Wallerian degeneration of fibers in the central and peripheral nervous system. Brain Res 828:41-59.
Polesskaya A, Seale P, Rudnicki MA (2003) Wnt signaling induces the myogenic specification of resident CD45+adult stem cells during muscle regeneration. Cell 113:841-852.
Rajasekaran MR, Kanoo S, Fu J, Nguyen ML, Bhargava V, Mittal RK(2017) Age-related external anal sphincter muscle dysfunction and fibrosis: possible role of Wnt/β-catenin signaling pathways. Am J Physiol Gastrointest Liver Physiol 313:G581-588.
Scheib J, Höke A (2013) Advances in peripheral nerve regeneration.Nat Rev Neurol 9:668-676.
Schmitt AB, Breuer S, Liman J, Buss A, Schlangen C, Pech K, Hol EM,Brook GA, Noth J, Schwaiger FW (2003) Identification of regeneration-associated genes after central and peripheral nerve injury in the adult rat. BMC Neurosci 4:8.
Sharma RP, Chopra VL (1976) Effect of the Wingless (wg1) mutation on wing and haltere development in Drosophila melanogaster. Dev Biol 48:461-465.
Shim S, Ming GL (2010) Roles of channels and receptors in the growth cone during PNS axonal regeneration. Exp Neurol 223:38-44.
Sorci G, Riuzzi F, Agneletti AL, Marchetti C, Donato R (2003) S100B inhibits myogenic differentiation and myotube formation in a RAGE-independent manner. Mol Cell Biol 23:4870-4881.
Tawk M, Makoukji J, Belle M, Fonte C, Trousson A, Hawkins T, Li H,Ghandour S, Schumacher M, Massaad C (2011) Wnt/beta-catenin signaling is an essential and direct driver of myelin gene expression and myelinogenesis. J Neurosci 31:3729-3742.
Teraoka M, Hato N, Takahashi H, Komobuchi H, Sawai N, Okada M,Hakuba N (2012) Myogenin expression in facial muscle following damage to the facial nerve. Acta Otolaryngol 132:783-787.
Torii T, Yamauchi J (2016) Gas6-Tyro3 signaling is required for Schwann cell myelination and possible remyelination. Neural Regen Res 11:215-216.
Verhees KJ, Pansters NA, Baarsma HA, Remels AH, Haegens A, deTheije CC, Schols AM, Gosens R, Langen RC (2013) Pharmacological inhibition of GSK-3 in a guinea pig model of LPS-induced pulmonary inflammation: II. Effects on skeletal muscle atrophy. Respir Res 14:117.
Wagner R, Heckman HM, Myers RR (1998) Wallerian degeneration and hyperalgesia after peripheral nerve injury are glutathione-dependent. Pain 77:173-179.
Wang Y, Zhao Y, Sun C, Hu W, Zhao J, Li G, Zhang L, Liu M, Liu Y,Ding F, Yang Y, Gu X (2016) Chitosan degradation products promote nerve regeneration by stimulating schwann cell proliferation via miR-27a/FOXO1 axis. Mol Neurobiol 53:28-39.
Wang Z, Zhang P, Kou Y, Yin X, Han N, Jiang B (2013) Hedysari extract improves regeneration after peripheral nerve injury by enhancing the amplification effect. PLoS One 8:e67921.
Wen J, Qian C, Pan M, Wang X, Li Y, Lu Y, Zhou Z, Yan Q, Li L, Liu Z,Wu W, Guo J (2017) Lentivirus-mediated rna interference targeting RhoA slacks the migration, proliferation, and myelin formation of schwann cells. Mol Neurobiol 54:1229-1239.
Willand MP, Holmes M, Bain JR, de Bruin H, Fahnestock M (2014)Sensory nerve cross-anastomosis and electrical muscle stimulation synergistically enhance functional recovery of chronically denervated muscle. Plast Reconstr Surg 134:736e-745e.
Xin ZT, Liu XZ, Zhang MJ, Li W, Chen BH, Ma XX (2013) Exogenous cardiotrophin-1:The possibility to protect PC12 cells and Schwann cells. Zhongguo Zuzhi Gongcheng Yanjiu 17:7265-7271.
Yi S, Wang QH, Zhao LL, Qin J, Wang YX, Yu B, Zhou SL (2017) miR-30c promotes Schwann cell remyelination following peripheral nerve injury. Neural Regen Res 12:1708-1715.
Zhang W, Fang X, Zhang C, Li W, Wong WM, Xu Y, Wu W, Lin J(2017) Transplantation of embryonic spinal cord neurons to the injured distal nerve promotes axonal regeneration after delayed nerve repair. Eur J Neurosci 45:750-762.
Zhao A, Yang L, Ma K, Sun M, Li L, Huang J, Li Y, Zhang C, Li H, Fu X (2016) Overexpression of cyclin D1 induces the reprogramming of differentiated epidermal cells into stem cell-like cells. Cell Cycle 15:644-653.
- 中国神经再生研究(英文版)的其它文章
- Detection of thinned corticospinal tract and corticoreticular pathway in a patient with a calf circumference discrepancy
- Relationship of distraction rate with inferior alveolar nerve degeneration-regeneration shift
- The effect of increased intra-abdominal pressure on orbital subarachnoid space width and intraocular pressure
- Voltage adjustment improves rigidity and tremor in Parkinson’s disease patients receiving deep brain stimulation
- Effect of electrical stimulation on neural regeneration via the p38-RhoA and ERK1/2-Bcl-2 pathways in spinal cord-injured rats
- Proteomic analysis of trans-hemispheric motor cortex reorganization following contralateral C7 nerve transfer

