The effect of increased intra-abdominal pressure on orbital subarachnoid space width and intraocular pressure
Su-meng Liu, Ning-li Wang,, Zhen-tao Zuo, Wei-wei Chen, Di-ya Yang, Zhen Li, Yi-wen Cao
1 Beijing Tongren Eye Center, Beijing Tongren Hospital, Capital Medical University, Beijing Ophthalmology and Visual Sciences Key Laboratory,Beijing, China; Beijing Institute of Ophthalmology, Beijing Tongren Hospital, Capital Medical University, Beijing, China
2 State Key Laboratory of Brain and Cognitive Science, Institute of Biophysics, Chinese Academy of Sciences, Beijing, China
3 Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China
Introduction
Glaucoma is characterized by retinal ganglion cell degeneration, alterations in optic nerve head topography and associated visual field loss. As a leading cause of global irreversible blindness, glaucoma is considered to be a multifactorial disease (Tham et al., 2014; Carreras, 2016). However, it has been recognized that the relationship between cerebrospinal fluid pressure and intraocular pressure (IOP) plays an important role in the progression of glaucomatous optic neuropathy (Jonas et al., 2011; Hou et al., 2016; Zhang et al.,2016).
The difference between IOP and cerebrospinal fluid pressure has been defined as the trans-lamina cribrosa pressure difference (Morgan et al., 1995; Berdahl et al., 2008a,b; Ren et al., 2011). The lamina cribrosa of the optic nerve head is the pressure watershed between the intraocular compartment and the retrolaminar compartment together with the cerebrospinal fluid space (Park et al., 2015). Previous studies considered that a high trans-lamina cribrosa pressure difference may be more important for the development of glaucomatous optic neuropathy than IOP (Berdahl et al., 2008a, b; Killer et al.,2012; Wang et al., 2012). The conventional treatment of glaucoma has been to reduce the IOP. If we accept the trans-lamina cribrosa pressure difference theory, another way to relieve glaucomatous neuropathy might be to elevate the cerebrospinal fluid pressure. Elevating intracranial pressure (ICP) with a non-invasive method in a clinic is not feasible. However, it is well known that rapid changes in intra-abdominal pressure(IAP) may cause a corresponding variation in cerebrospinal fluid pressure (Leon et al., 1994; Vear-Brozovic et al., 2008;Zander et al., 2009; Kamine et al., 2014). As the cerebrospinal fluid around the optic nerve is continuous with the central nervous system, a higher cerebrospinal fluid pressure could give rise to a dilation of the optic nerve sheath (Fichtner et al.,2016). Many previous studies have suggested that the orbital subarachnoid space (OSAS) is strongly correlated with cerebrospinal fluid pressure (Cennamo et al., 1987; Gangemi et al.,1987; Tamburelli et al., 1993; Dubourg et al., 2011). Recently,magnetic resonance imaging (MRI) and ultrasound have detected and been used to measure the expansion of the OSAS(Shirodkar et al., 2015; Garcia-Armengol et al., 2016; Xu et al., 2016). Lagreze et al. (2007) pointed out that MRI provides more precise measurements than ultrasound. The noninvasive MRI measurement of the OSAS has been used to provide surrogate markers for early elevated cerebrospinal fluid pressure(Kimberly and Noble, 2008; Rohr et al., 2010; Xie et al., 2013).The cerebrospinal fluid in the OSAS around the optic nerve can be differentiated from the surrounding fat tissue and appears as round high-intensity circles on the coronal images by T2-weighted imaging (Lam et al., 1997; Seitz et al., 2002;Shinoda et al., 2017). The dilation of the OSAS, caused by the increased ICP, can be estimated by measuring the orbital subarachnoid space width (OSASW) (Gass et al., 1996; Imamura et al., 1996; Brodsky and Vaphiades, 1998). The short-term change of the OSAS resulting from the increased cerebrospinal fluid pressure has been proven. However, the relationship between OSAS and cerebrospinal fluid pressure increased by elevated IAP remains to be investigated in terms of absolute changes, reversibility, time course and response linearity.
Therefore, to determine the variation of the OSAS in early elevated cerebrospinal fluid pressure, we measured OSASW with MRI to observe the changes before and after increasing IAP.
Participants and Methods
Participants
A total of 15 healthy volunteers (age 22–30 years, mean:24.25 years; 12 women, 3 men; student volunteers from medical school) were recruited at State Key Laboratory of Brain and Cognitive Science, Institute of Biophysics, Chinese Academy of Sciences, China in July 2015. An informed consent was signed by each subject. This study was registered in the Chinese Clinical Trial Registry (registration number: ChiCTR-ONRC-14004947).
Participants with one or more of the following conditions were excluded from this study:
(1) IOP > 21 mmHg, visual acuity < 1.0
(2) History of ocular or intracranial tumors
(3) History of orbital disease, cranial surgery or traumatic brain injury
(4) Previous lumbar puncture within 1 month
(5) Any other problems that disallowed complete MRI examination.
Attentions
All 15 subjects underwent the MRI examination in a standardized manner in a supine position at 9:00 hours. The right eyes of the subjects were examined. The subjects were required to keep their head and body completely still during the whole experiment. All subjects were instructed to stare at a cursor on the MRI machine during the entire experiment. The sequence of measurements was repeated if any motion artifact appeared.
MRI measurement
High-resolution MRI was performed on a 3.0 T scanner(Trio TIM, Siemens, Erlangen, Germany) using a homemade 30-channel phased array coil. A Fast Spin-Echo (FSE)sequence was scanned at an axial direction to get the visual fiber angle. The oblique sagittal FSE sequence was acquired along the visual fiber. Oblique axial FSE sequence was scanned along the visual fiber in the oblique sagittal image.The oblique coronal direction was perpendicular to both the sagittal and axial directions. Fat-saturated Half Fourier Acquisition Single-Shot Turbo Spin-Echo T2-weighted MR images of the orbit were obtained with repetition time/echo time 3,550/122 ms, flip angle 180°, slice thickness 3 mm,matrix 618 × 1,024, field of view 159 mm × 200 mm (inplane resolution 0.195 mm × 0.195 mm). The high white signal was the cerebrospinal fluid, while the dark signal was the optic nerve parenchyma (Figure 1). We measured four positions at 1, 3, 9 and 15 mm behind the globe.
Experimental procedure
The inflatable abdominal belt was tied to the subjects at the navel level without any pressure to the abdomen before they lay in the MRI machine. A mercury pressure gauge was connected to the abdominal belt by a 5-meter long plastic tube and placed outside the MRI room. Initially, all the subjects lay in the MRI machine in a supine position with their heads stabilized by a fixed pillow for 5 minutes. The baseline images of the OSAS were taken at the four locations mentioned above. To guarantee the stability of the baseline, we repeated each measurement and took the average. The abdominal belt was inflated to exert 40 mmHg pressure (1 mmHg = 0.133 kPa) on the abdomen (which is as high an IAP that a normal person can withstand during an acute test such that they can feel their abdominal aorta beating). After the images were taken at all 4 locations, at 10 minutes intervals for 2 hours,the pressure was released by deflating the air. The MRI measurements of the OSAS were then repeated 10 and 20 minutes after deflation. All the subjects did the IAP elevated trial again outside the MRI machine. Intraocular pressures were measured on the right eyes at the same time points (every 10 minutes during the 2 hours abdominal compression and 10 and 20 minutes after decompression).
Data collection
Three clinicians, blinded to the conditions, measured the images using the image J software (National Institutes of Health,Bethesda, MD, USA). The average between the optic nerve diameter (OND) and the optic nerve sheath diameter (ONSD)equals the mean of the horizontal and vertical diameters. The OSASW (OSASW = ONSD/2 – OND/2) equals half of the optic nerve sheath diameter minus half of the optic nerve diameter (Lam et al., 1997). Observer 1 performed the analysis 3 months later again to assess the intraobserver repeatability.

Figure 1 Oblique coronal image with fat suppression demonstrates the optic nerve/sheath complex taken at 3 mm behind the globe perpendicular to the optic nerve axis in a 23-year-old female.
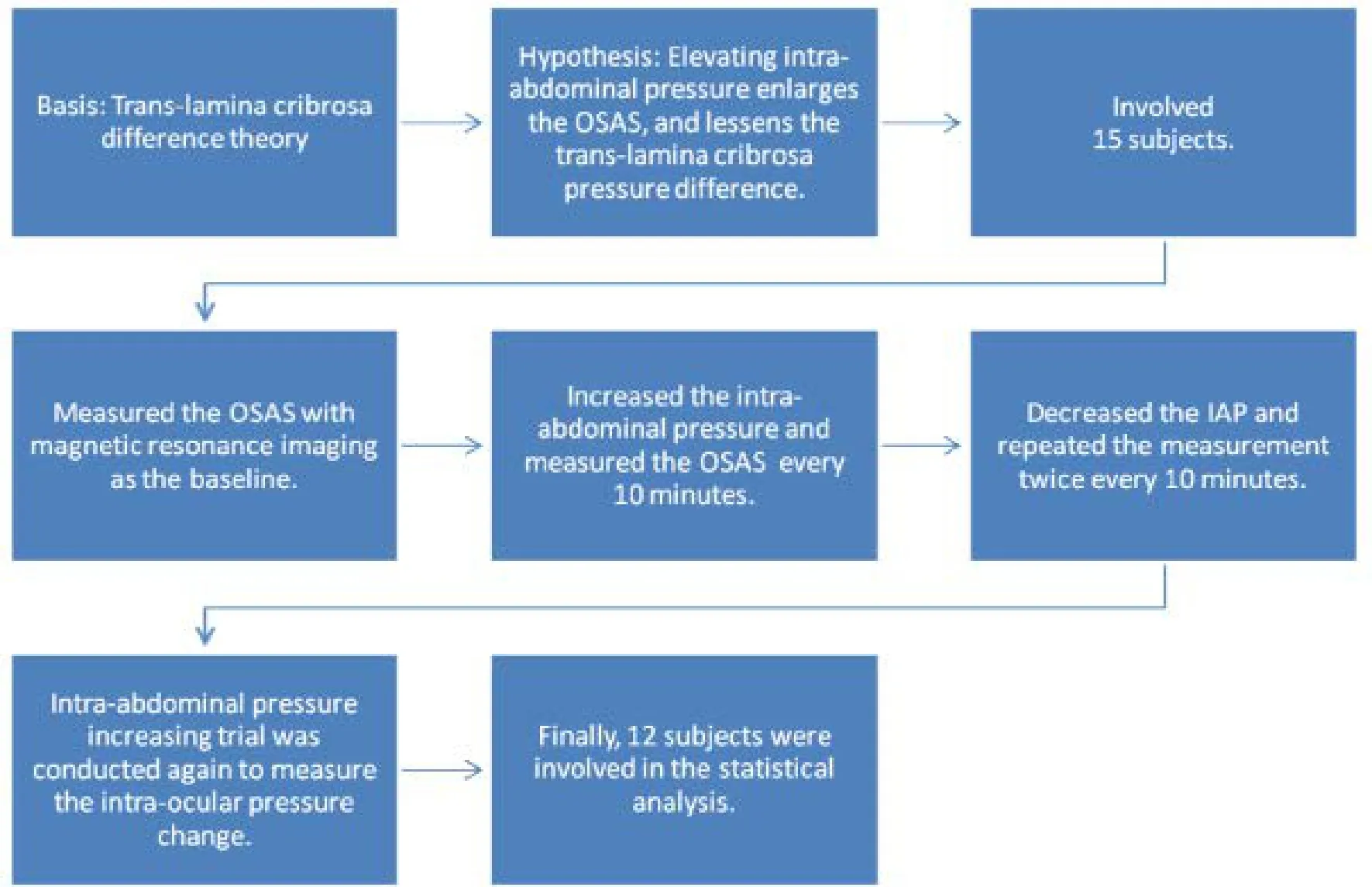
Figure 2 Trial flow chart.
Outcome measures
The primary outcome measure was OSASW. The secondary outcome measure was IOP.
Statistical analysis
All data are expressed as the mean ± SD and were analyzed using SPSS 17.0 software (IBM-SPSS, Chicago, IL, USA). A paired samplet-test was used to assess significant differences of the mean OSASW changes at each time point before and after elevated IAP. The inter- and intra-observer repeatability of the optic nerve/sheath baseline measurements was tested with analysis variance and intraclass correlation coefficient. The changes of IOP before and after the IAP were elevated were assessed using the one-way analysis of variance and least significant difference test. A value ofP< 0.05 was 2-sided and considered statistically significant.
Results
Quantitative analysis of participants
Three of the subjects were excluded as clear MRI images were not obtained from them. The remaining twelve volun-teers were included in the final analysis. The basic information of all the subjects involved is presented inTable 1.
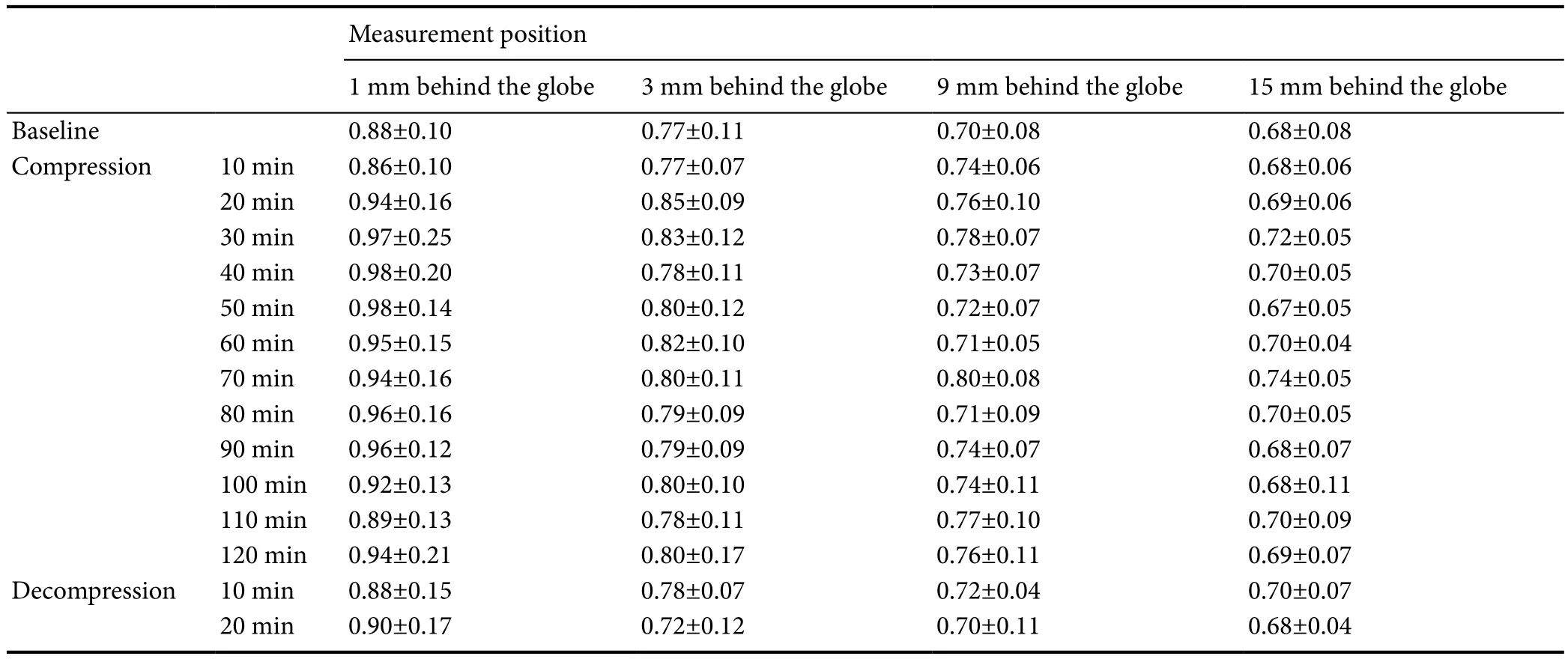
Table 3 Orbital subarachnoid space width before, during and after elevated intra-abdominal pressure

Subject Age (years)Sex Height (m)Weight (kg)Body mass index 1 23 Female 1.60 52 20.31 2 22 Female 1.65 52 19.10 3 29 Male 1.78 75 23.67 4 24 Female 1.60 55 21.48 5 27 Female 1.60 50 19.53 6 23 Female 1.58 62 24.84 7 23 Female 1.65 55 20.20 8 23 Female 1.72 60 20.28 9 22 Female 1.58 50 20.03 10 22 Female 1.55 43 17.9 11 30 Male 1.58 50 20.03 12 23 Female 1.58 45 18.03
Subject baseline analysis
The MRI images of the OSASW taken at 1 and 3 mm behind the globe could be evaluated for all patients. Due to image quality problems and individual differences, the MRI images of the OSASW taken at 9 mm behind the globe could not be evaluated for 1 (8%) patient. Similarly, those taken at 15 mm behind the globe could not be evaluated for 2 (17%) patients. Including all subjects, the mean value of the baseline data (optic nerve diameter, optic nerve sheath diameter, and OSASW) is presented inTable 2.
OSASW change
Clear changes of the mean OSASW were recorded during the increased IAP (Table 3). In the baseline state, the OSASW narrowed along the optic nerve from just behind the globe to 15 mm away. OSASW at elevated IAP was wider than baseline. Statistically significant OSAS dilation (P<0.05) was found at 1, 3 and 9 mm behind the globe (Table 4), illustrating OSAS sensitivity to the increased IAP. This dilation was especially observed at 9 mm behind the globe where 5 time points showed that the OSASW was statistically significantly enlarged. In one example (Subject No. 9,Female, 23 years), the OSASW at 9 mm behind the globe enlarged by 0.21 mm (from 0.67 to 0.88 mm) after 70 minutes of elevated IAP. The variations of the OSASW induced by elevated IAP demonstrated obvious fluctuations over the time of IAP. After decompression of the abdominal pressure, the OSASW normalized and returned to the baseline value.
Inter- and intra-observer repeatability
The analysis of variance and the intraclass correlation coefficient were performed to reveal the reproducibility on the intra-observer and inter-observer of the optic nerve/sheath baseline measurements (Table 5). The data suggested that there was no significant difference on intra- and inter-observer (P> 0.05).
IOP change
Least significant difference multiple comparison and oneway analysis of variance were constructed to assess the changes of IOP between baseline and IAP compression states (Table 6). Compared to the baseline IOP, there was nosignificant difference in the IOP after the IAP was increased(P> 0.05,F= 0.22).
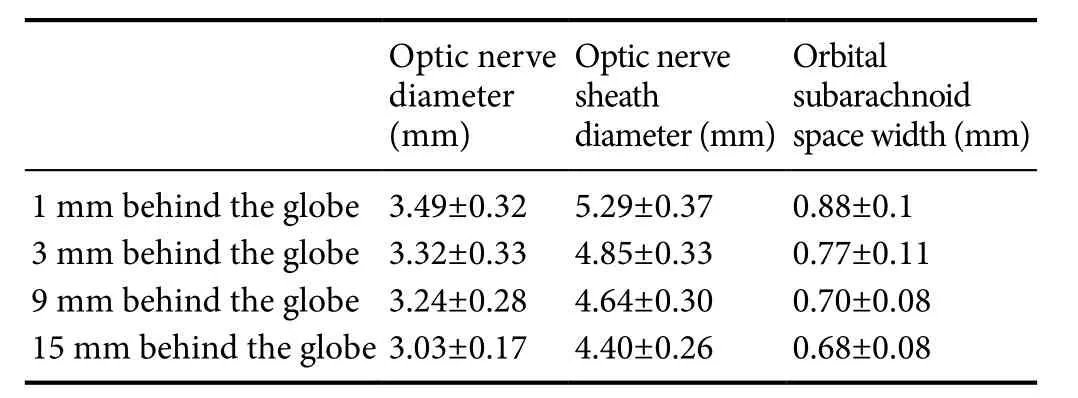
Data are expressed as the mean ± SD from 12 patients.
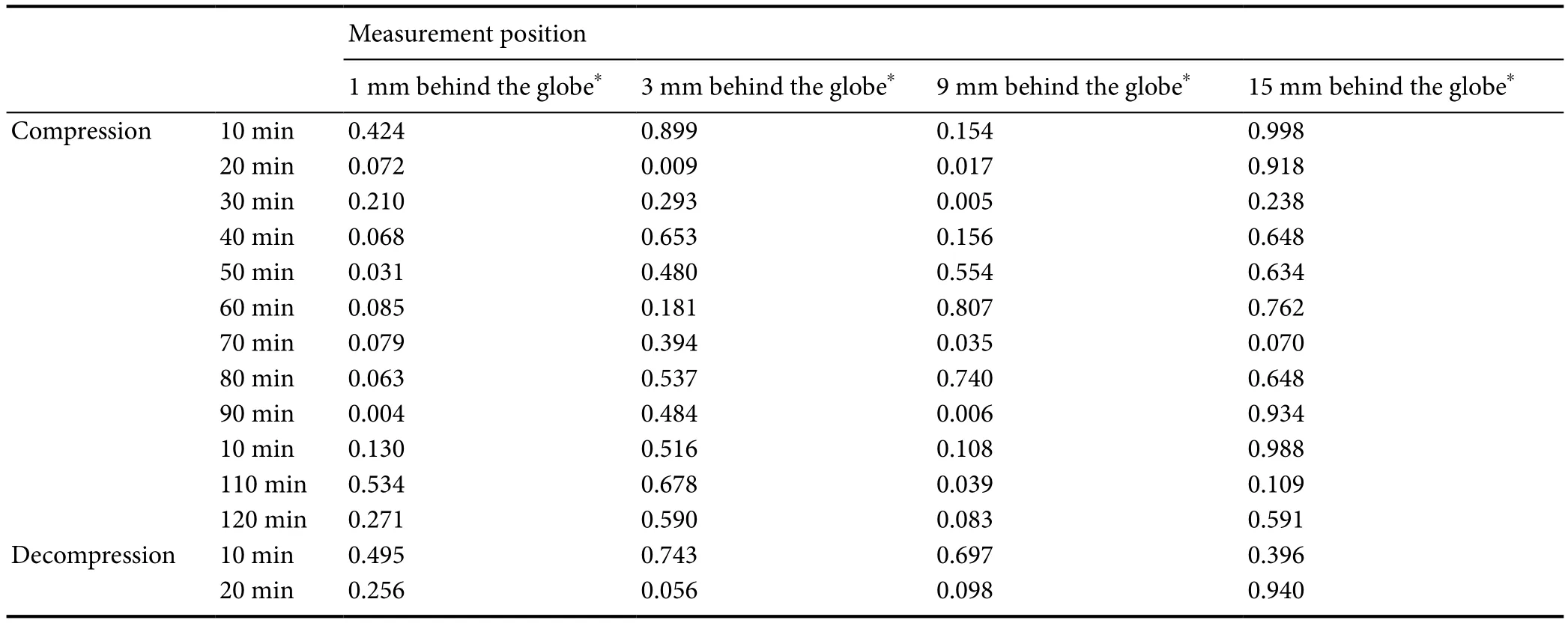
Table 4 P value from paired sample t test of the orbital subarachnoid space width changes before, during and after the elevated intra-abdominal pressure
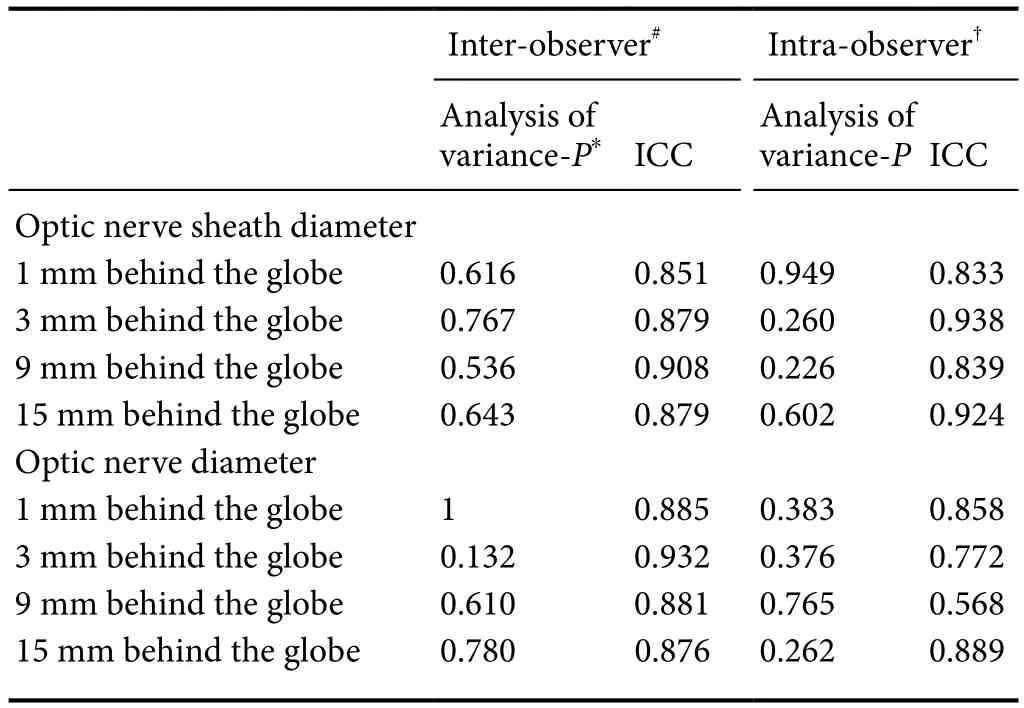
Table 5 Inter-observer and intra-observer repeatability of the optic nerve/sheath baseline measurements
Discussion
The recognition that the trans-lamina cribrosa pressure difference plays an important role in the progression of the glaucomatous optic neuropathy has been known for many years (Wang et al., 2013). Scientists are making great efforts to search for a new clinical treatment centered on this theory.Simultaneously, it has been shown that a significant positive relationship has been found between optic nerve sheath diameter (ONSD), measured by MRI, and ICP (r =0.71) (Geeraerts et al., 2008). Our study demonstrates thein vivoresponse of the OSASW to alterations in IAP pressure. Enlargements of the OSAS, especially at 3 mm and 9 mm behind the globe,were clearly detected by 3.0 T MRI. The maximum dilation of the ONSD was 31.4% compared with the baseline. The obvious fluctuation of the dilations was also observed over the time of IAP. The OSASW returned to the baseline value after releasing the IAP. The IOP of the same eye did not show any corresponding change while the IAP changed.
As a part of the central nervous system, the optic nerve is encircled by cerebrospinal fluid that flows in a meningeal sheath (Hayreh, 1964; Lemmens et al., 2016). This optic nerve sheath is anatomically continuous with the dura mater(Geeraerts et al., 2008). The connection between the optic nerve sheath and subarachnoid space allows the increased intracranial pressure (ICP) to pass through the optic nervesheath and alter its diameter (Hayreh, 1964). Le et al. (2009)found that increased ICP impels the inflow of cerebrospinal fluid into the OSAS to increase the ONSD, whereas cerebrospinal fluid hypotension reduced the ONSD by reducing the inflow. The relationship between ONSD and ICP has been confirmed by several studies (Hansen and Helmke, 1997;Kimberly and Noble, 2008; Xie et al., 2013). Others (Watanabe et al., 2008a,b; Rohr et al., 2010) clarified a positive linear relation between optic nerve sheath diameter and ICP.Watanabe et al. (2008) confirmed the widening of the OSAS measured by MRI after successful treatment of spontaneous intracranial hypotension. Our trial, for the first time, showed obvious changes of OSASW measured by MRI while the IAP elevated.
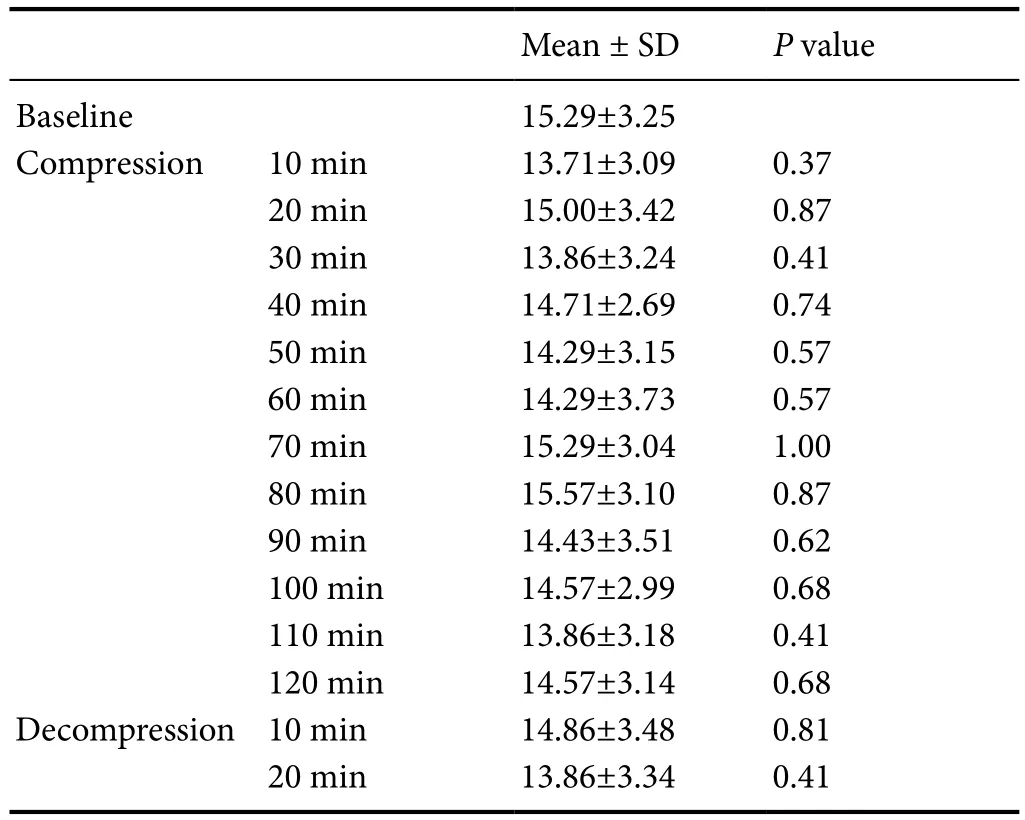
Table 6 Intra-ocular pressure change before and after elevated IAP
The phenomenon that elevated IAP leads to corresponding changes in ICP has been demonstrated for many years. However, earlier reports did not reach a definite conclusion on the mechanism. Josephs et al. (1994) indicated that pneumoperitoneum increased ICP, which can be explained by the modified Monroe-Kellie doctrine. He declared that the intracranial contents can be divided into four compartments: vascular,cerebrospinal fluid, osseous, and parenchymal. A natural pressure buffering system used in this study can maintain a stable ICP in most situations. However, rapid changes in one or more compartments lead to the inadequate compensation of the remaining parts and the ICP rises. Beigel et al. (2013)pointed out that elevated intracranial pressure was due to restriction of outflow from the lumbar venous plexus in an abdominal compression test. Porth et al. (1984) considered that the increase in intrathoracic pressure caused the decreased venous return and elevation in the peripheral venous pressure,thereby affecting the ICP. Although the conclusions have not been unified, the relationship between IAP and ICP has been determined. Some studies (Hansen et al., 1996; Helmke et al.,1996a, b) demonstrated that the ONSD increased by up to 60% at a distance of 3 mm behind the globe compared with only 35% at 10 mm. Newman et al. (2002) chose the position 3 mm behind the globe for ultrasound measurement, because they considered that was where the ultrasound contrast was greatest, the results were more reproducible, and the anterior nerve was anatomically most distensible. Liu and Kahn (1993)noted that the bulbous part of the optic nerve protruded and inflated along with the ICP increase in cadaversin vivobut the remaining nerve showed no obvious change. An study(Hansen and Helmke, 1997) confirmed their findings with intrathecal infusion tests. Our results also verified that the dilatation of the optic nerve sheath diameter induced by elevated ICP was significant at positions 3 mm and 9 mm behind the globe.
The results of the present study are encouraging. Cases in which dilatation of the optic nerve sheath was caused by increased IAP were identified. The elevated IAP possibly increased the ICP and thus led to expansion of the optic nerve sheath, however, the mechanism requires further investigation. In the light of the trans-lamina cribrosa pressure difference theory, reducing the trans-lamina cribrosa pressure difference to a healthy range may be a new way to relieve the glaucomatous optic neuropathy. From our experimental results, increasing the IAP may become a noninvasive way to expand the OSAS and to moderate the abnormal trans-lamina cribrosa pressure difference. Nevertheless, our study could not clarify whether a long-term recovery of the optic nerve sheath diameter is possible or whether any permanent surplus expansion remains. Further investigations are required to monitor the course of the optic nerve sheath diameter increase or decrease over a long time (maybe 24 hours or 1 month). In this study, the changes of OSASW were observed before and after the intra-abdominal pressure increase in each subject. Due to the limited space, this section of data is not shown.
OSASW is positively correlated with an increased IAP in an acute trial based on MRI measurements. The variations of the OSASW induced by elevated IAP demonstrate obvious fluctuations over the period of IAP. The IOP did not show correspondingly increase on the same eye. These findings could open the possibility of a potential treatment that elevates IAP, to enlarge the OSAS, and lessens the trans-lamina cribrosa pressure difference.
Author contributions:NLW, SML and WWC designed the study. DYY and ZL provided critical revision. SML, WWC and ZTZ performed experiments.SML and YWC analyzed data. SML wrote the paper. All authors approved the final version of the paper.
Conflicts of interest:The authors declare that they have no competing interests.Financial support:This study received no specific funding agency in the public, commercial, or not-for-profit sectors.
Research ethics:The study protocol was approved by the Ethics Committee of Beijing Tongren Hospital, Capital Medical University (approval number:P-20140220-1). The trial was registered in the Chinese Clinical Trial Registry(registration number: ChiCTR-ONRC-14004947).
Declaration of participant consent:The authors certify that they have obtained all appropriate participant consent forms. In the form, the participants have given their consent for their images and other clinical information to be reported in the journal. The participants understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Open peer review:Whi-Young Kim, Pierce College, Mechanical Engineering, USA.
Beigel R, Goland S, Siegel RJ (2013) Comparison of the effect on right atrial pressure of abdominal compression versus the valsalva maneuver. Am J Cardiol 113:183-186.
Berdahl JP, Allingham RR, Johnson DH (2008) Cerebrospinal fluid pressure is decreased in primary open-angle glaucoma. Ophthalmology 115:763-768.
Berdahl JP, Fautsch MP, Stinnett SS, Allingham RR (2008) Intracranial pressure in primary open angle glaucoma, normal tension glaucoma, and ocular hypertension: a case-control study. Invest Ophthalmol Vis Sci 49:5412-5418.
Brodsky MC, Vaphiades M (1998) Magnetic resonance imaging in pseudotumor cerebri. Ophthalmology 105:1686-1693.
Carreras FJ (2016) Glaucoma and amyotrophic lateral sclerosis, two kindred diseases? Neural Regen Res 11:1415-1417.
Cennamo G, Gangemi M, Stella L (1987) The correlation between endocranialpressure and optic nerve diameter: an ultrasonographic study. Ophthalmic Echography 7:603-606.
Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B (2011) Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: a systematic review and meta-analysis. Intensive Care Med 37:1059-1068.
Fichtner J, Ulrich Christian T, Christian F, Jan G, Andreas R, Jürgen B (2016)Ultrasound of the optic nerve sheath diameter before and after microsurgical closure of a dural CSF-fistula as discriminator in spontaneous intracranial hypotension. German Med Sci 6:12-15.
Gangemi M, Cennamo G, Maiuri F, D’Andrea F (1987) Echographic measurement of the optic nerve in patients with intracranial hypertenion. Neurochirugica 30:53-55.
Garcia-Armengol R, Domenech S, Botella-Campos C, Goncalves FJ, Menéndez B, Teixidor P, Muñoz-Narbona L, Rimbau J (2016) Comparison of elevated intracranial pressure pulse amplitude and disproportionately enlarged subara chnoid space (DESH) for prediction of surgical results in suspected idiopathic normal pressure hydrocephalus. Acta Neurochirurgica 158:1-7.
Gass A, Barker GJ, Riordan-Eva P, MacManus D, Sanders M, Tofts PS, Mc-Donald WI, Moseley IF, Miller DH (1996) MRI of the optic nerve in benign intracranial hypertension. Neuroradiology 38:769-773.
Geeraerts T, Newcombe VFJ, Coles JP, Abate MG, Perkes IE, Hutchinson PJA, Outtrim JG, Chatfield DA, Menon DK (2008) Use of T2-weighted magnetic resonance imaging of the optic nerve sheath to detect raised intracranial pressure. Crit Care 12:R114.
Geeraerts T, Duranteau J, Benhamou D (2008) Ocular sonography in patients with raised intracranial pressure: the papilloedema revisited. Crit Care 12:150.
Hansen HC, Helmke K (1996) The subarachnoid space surrounding the optic nerves. An ultrasound study of the optic nerve sheath. Surg Radiol Anat 18:323-328.
Hansen HC, Helmke K (1997) Validation of the optic nerve sheathc response to changing cerebrospinal fluid pressure: ultrasound findings during intrathecal infusion tests. J Neurosurg 7:34-40.
Hayreh SS (1964) Pathogenesis of oedema of the optic disc (papilloedema). A preliminary report. Br J Ophthalmol 48:522-543.
Helmke H, Hansen HC (1996a) Fundamentals of transorbital sonographic evaluation of optic nerve sheath expansion under intracranial hypertension. I Experimental study. Paediatr Radiol 26:701-705.
Helmke H, Hansen HC (1996b) Fundamentals of transorbital sonographic evaluation of optic nerve sheath expansion under intracranial hypertension. II Patient study. Paedaitr Radiol 26:706-710.
Imamura Y, Mashima Y, Oshitari K, Oguchi Y, Momoshima S, Shiga H (1996)Detection of dilated subarachnoid space around the optic nerve in patients with papilloedema using T2 weighted fast spin echo imaging. J Neurol Neurosurg Psychiatry 60:108-109.
Jonas JB, Berenshtein E, Holbach L (2003) Anatomic relationship between lamina cribrosa, intraocular space, and cerebrospinal fluid space. Invest Ophthalmol Vis Sci 44:5189-5195.
Josephs LG, Este-McDonald JR, Birkett DH, Hirsch EF (1994) Diagnostic laparoscopy increases intracranial pressure. J Trauma 36:815-819.
Kamine TH, Papavassiliou E, Schneider BE (2014) Effect of abdominal insufflation for laparoscopy on intracranial pressure. JAMA Surg 149:380-382.
Killer HE, Miller NR, Flammer J, Meyer P, Weinreb RN, Remonda L, Jaggi GP (2012) Cerebrospinal fluid exchange in the optic nerve in normal-tension glaucoma. Br J Ophthalmol 96:544-548.
Kimberly HH, Noble VE (2008) Commentary: using MRI of the optic nerve sheath to detect elevated intracranial pressure. Crit Care 12:181.
Lagreze WA, Lazzaro A, Weigel M, Hansen HC, Hennig J, BleyTA (2007)Morphometry of the retrobulbar human optic nerve: comparison between conventional sonography and ultrafast magnetic resonance sequences. Invest Ophthalmol Vis Sci 48:1913-1917.
Lam BL, Glasier CM, Feuer WJ (1997) Subarachnoid fluid of the optic nerve in normal adults. Ophthalmology 104:1629-1633.
Le A, Hoehn ME, Smith ME, Spentzas T, Schlappy D, Pershad J (2009) Bedside sonographic measurement of optic nerve sheath diameter as a predictor of increased intracranial pressure in children. Ann Emerg Med 53:785-791.
Lemmens K, Hove IV, Moons L (2016) Complementary research in mammals and fish indicates MMP-2 as a pleiotropic contributor to optic nerve regeneration. Neural Regen Res 11:740-742.
Liu D, Kahn M (1993) Measurement and relationship of subarachnoid pressure of the optic nerve to intracranial pressures in fresh cadavers. Am J Ophthalmol 116: 548-556.
Morgan WH, Yu DY, Cooper RL, Alder VA, Cringle SJ, Constable IJ (1995)The influence of cerebrospinal fluid pressure on the lamina cribrosa tissue pressure gradient. Invest Ophthalmol Vis Sci 36:1163-1172.
Newman WD, Hollman AS, Dutton GN, Carachi R (2005) Measurement of optic nerve sheath diameter by ultrasound: a means of detecting acute raised intracranial pressure in hydrocephalus. Br J Ophthalmol 86:1109-1113.
Park SC, Brumm J, Furlanetto RL, Netto C, Liu Y, Tello C, Liebmann JM,Ritch R (2015) Lamina cribrosa depth in different stages of glaucoma. Invest Ophthalmol Vis Sci 56:2059-2064.
Porth CJ, Bamrah VS, Tristani FE, Smith JJ (1984) The valsalva maneuver:mechanisms and clinical implications. Heart Lung 13:507-518.
Ren R, Jonas JB, Tian G, Zhen Y, Ma K (2010) Cerebrospinal fluid pressure in glaucoma.A prospective study. Ophthalmology 117:259-266.
Ren R, Wang N, Zhang X, Cui T, Jonas JB (2011) Trans-lamina cribrosa pressure difference correlated with neuroretinal rim area in glaucoma. Graefes Arch Clin Exp Ophthalmol 249:1057-1063.
Rohr A, Jensen U, Riedel C, Fruehauf MC, Bartsch T, Hedderich J, Doerner L, Jansen O (2010) MR imaging of the optic nerve sheath in patient with craniospinal hypotension. Am J Neuroradiol 10:1752-1757.
Hou R, Zhang Z, Yang D, Wang H, Chen W, Li Z, Sang J, Liu S, Cao Y, Xie X,Ren R, Zhang Y, Sabel BA, Wang N (2016) Intracranial pressure (ICP) and optic nerve subarachnoid space pressure (ONSP) correlation in the optic nerve chamber: the Beijing Intracranial and Intraocular Pressure (iCOP)study. Brain Res 1635:201.
Shinoda N, Hirai O, Hori S, Mikami K, Bando T, Shimo D, Kuroyama T, Kuramoto Y, Matsumoto M, Ueno Y (2017) Utility of MRI-based disproportionately enlarged subarachnoid space hydrocephalus scoring for predicting prognosis after surgery for idiopathic normal pressure hydrocephalus:clinical research. J Neurosurg 2:1-7.
Shirodkar CG, Munta K, Rao SM, Mahesh MU (2015) Correlation of measurement of optic nerve sheath diameter using ultrasound with magnetic resonance imaging. Indian J Crit Care Med 19:466-470.
Seitz J, Held P, Strotzer M, Müller M, Völk M, Lenhart M, Djavidani B,Feuerbach S (2002) Magnetic resonance imaging in patients diagnosed with papilledema: a comparison of 6 different high-resolution T1- and T2(⋆)-weighted 3-dimensional and 2-dimensional sequences. J Neuroimaging 12:164-171.
Tamburelli C, Aricle C, Mangiola A, Falsini B, Palma P (1993) CSF dynamic parameters and changes of optic nerve diameters measured by standardized echography. Ophthalmic Echography13:101-109.
Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY (2014) Global prevalence of glaucoma and projections of glaucoma burden through 2040:a systematic review and meta-analysis. Ophthalmology 121:2081-2090.
Vear-Brozovic V, Brezak J, Brozovic I (2008) Intra-abdominal hypertension:pulmonary and cerebral complications. Transplant Proc 40:1190-1192.
Wang NL, Xie X,Yang D , Xian J, Li Y, Ren R, Peng X, Jonas JB, Weinreb RN (2012) Orbital cerebrospinal fluid space in glaucoma. Ophthalmology 119:2065-2073.
Wang NL, Yang D, Jonas JB (2013) Low Cerebrospinal fluid pressure in the pathogenesis of primary open-angle glaucoma: epiphenomenon or causal relationship? The Beijing intracranial and intraocular pressure (iCOP)study. J Glaucoma 22 Suppl 5:S11.
Watanabe A, Kinouchi H, Horikoshi T, Uchida M, Ishigame K (2008a) Effect of intracranial pressure on the diameter of the optic nerve sheath. J Neurosurg 8:255-258.
Watanabe A, Horikoshi T, Uchida M, Ishigame K, Kinouchi H (2008b) Decreased diameter of the optic nerve sheath associated with CSF hypovolemia. AJNR Am J Neuroradiol 5:863-64.
Xie X, Zhang X, Fu J, Wang H, Jonas JB, Peng X, Tian G, Xian J, Ritch R, Li L, Kang Z, Zhang S, Yang D, Wang N; Beijing iCOP Study Group (2013)Noninvasive intracranial pressure estimation by orbital subarachnoid space measurement: the Beijing Intracranial and Intraocular Pressure (iCOP)study. Crit Care 17:R162.
Xu W, Gerety P, Aleman T, Swanson J, Taylor J (2016) Noninvasive methods of detecting increased intracranial pressure. Childs Nervous System 32:1-16.
Zander R, Engelhard K, Werner C (2009) Volume causes pressure. Cranial,thoracic, vascular and abdominal. Anaesthesist 58:341-342.
Zhang Z, Wu S, Jonas JB, Zhang J, Liu K, Lu Q, Wang N (2016) Dynein, kinesin and morphological changes in optic nerve axons in a rat model with cerebrospinal fluid pressure reduction: the Beijing Intracranial and Intraocular Pressure (iCOP) study. Acta Ophthamologica 94:266.
- 中国神经再生研究(英文版)的其它文章
- Detection of thinned corticospinal tract and corticoreticular pathway in a patient with a calf circumference discrepancy
- Relationship of distraction rate with inferior alveolar nerve degeneration-regeneration shift
- Voltage adjustment improves rigidity and tremor in Parkinson’s disease patients receiving deep brain stimulation
- Effect of electrical stimulation on neural regeneration via the p38-RhoA and ERK1/2-Bcl-2 pathways in spinal cord-injured rats
- Proteomic analysis of trans-hemispheric motor cortex reorganization following contralateral C7 nerve transfer
- GSK3β inhibitor promotes myelination and mitigates muscle atrophy after peripheral nerve injury

