Voltage adjustment improves rigidity and tremor in Parkinson’s disease patients receiving deep brain stimulation
Shao-hua Xu, Chao Yang, Wen-biao Xian, Jing Gu, Jin-long Liu, Lu-lu Jiang, Jing Ye,, Yan-mei Liu, Qi-yu Guo, Yi-fan Zheng, Lei Wu,Wan-ru Chen, Zhong Pei, Ling Chen,
1 Department of Neurology, National Key Clinical Department and Key Discipline of Neurolory, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong Province, China
2 Department of Neurosurgery, First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong Province, China
3 Department of Medical Statistics and Epidemiology, School of Public Health, Sun Yat-sen University, Guangzhou, Guangdong Province, China
4 Department of Neurology, Tangshan Worker’s Hospital, Tangshan, Hebei Province, China
Introduction
Deep brain stimulation (DBS) in the subthalamic nucleus(STN) is regarded as the most effective therapy for moderate and advanced Parkinson’s disease (PD) (Pahwa et al., 2003;Moldovan et al., 2015; Preda et al., 2016), particularly for those with refractory motor fluctuations and drug-induced complications (Benabid et al., 2009; Rodriguez-Oroz et al., 2012; Odekerken et al., 2013; Jiang et al., 2015). The efficacy of surgery largely depends on the stimulation target (Wodarg et al., 2012;Scarnati et al., 2016), contacts selected for stimulation (Hilliard et al., 2011), programming of stimulation parameters (Kumar,2002; Volkmann et al., 2002, 2006; Bronstein et al., 2011) such as voltage, pulse width, and frequency (Yousif et al., 2012), and medication titration (Kumar, 2002).
China has the largest number of patients with PD worldwide(Dorsey et al., 2007; Jiang et al., 2015), and the number of patients receiving DBS is increasing year by year. But there is still a lack of systematic programming protocols. Moreover, long-term management of stimulation parameters depends on the physician’s experience and repeated testing (Moro et al., 2002; Bronstein et al., 2011). In some patients, satisfactory therapeutic effects can be achieved by adjusting voltage parameters; conversely,in others, there is barely any curative effect even after adjustment of multiple parameters, including double negative, bipolar, or interleaving stimulation (Moro et al., 2002; Vercruysse et al., 2014).Considerable time and resources are often required to achieve the optimal strategy.
The aim of the present retrospective study was to explore which symptom could be best improved by voltage adjustment,and thus identify the most appropriate strategy for STN DBS programming. Moreover, we wish to share our experience to help guide those new programming centers in China.
Subjects and Methods
Subjects
Bilateral STN DBS
In all patients, quadripolar stimulation electrodes (Model 3389S,Medtronic Inc., MN, USA) were implanted bilaterally in the STN and connected to an implantable pulse generator (Kinetra, Medtronic Inc.) in the right subclavicular area. All patients were assessed before surgery, and 1 and 2 years postoperatively,in off-medication and on-medication states (without and with medication, respectively), using the UPDRS III. Tremor, rigidity, bradykinesia, and axial symptoms were assessed.
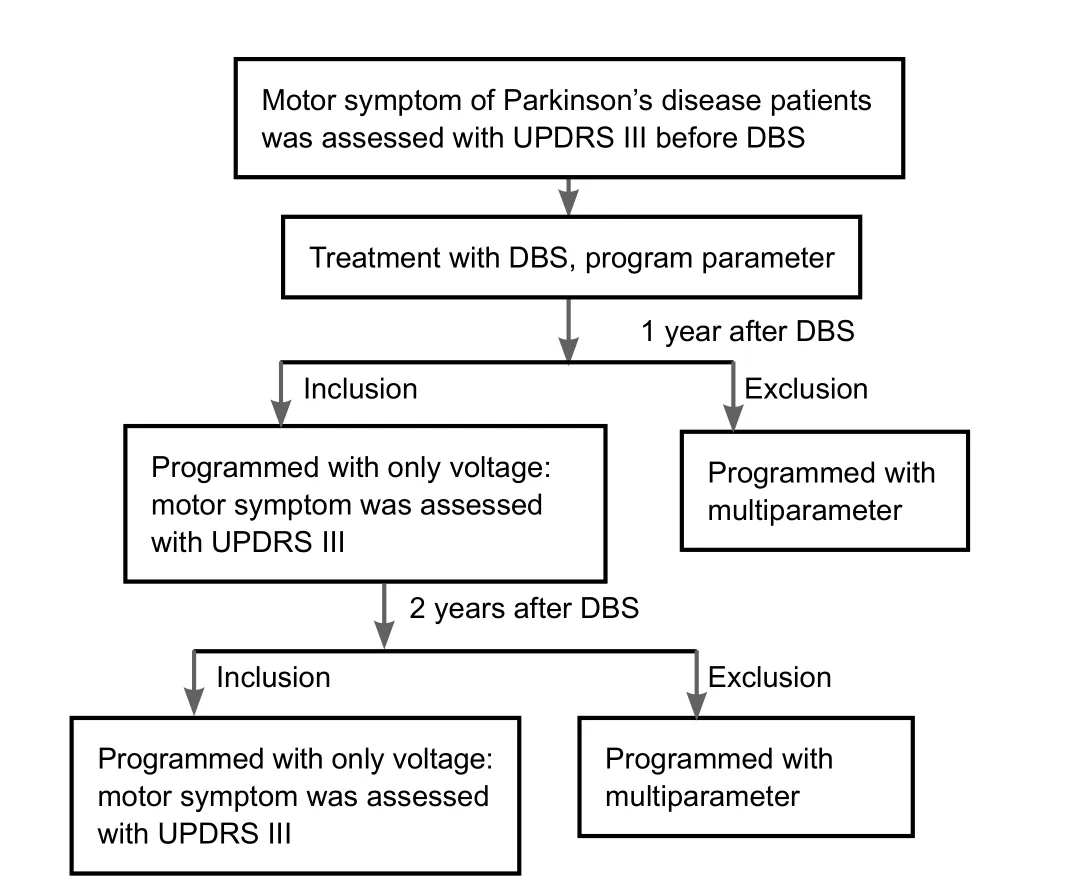
Figure 1 Clinical flow chart.
DBS programming was generally performed at three main stages: (1) patients returned to the clinic for initial programming 1 month after implantation; (2) further programming 2–4 weeks after initial programming; and (3) slight adjustment during the stabilization stage (Volkmann et al., 2006).
Four weeks after surgery, the patients returned for initial programming in the off-medication state. The implantable pulse generator was turned on, and eight contacts were tested in accordance with a standard protocol (Volkmann et al., 2002;Volkmann et al., 2006) to identify the therapeutic window and the side-effect threshold. Usually, the implantable pulse generator was used as the anode and one contact as the cathode. The amplitude was gradually increased from 0 V to 5 V in increments of 0.5–1.0 V, unless unbearable adverse effects occurred,while the pulse width and frequency were maintained at 60 μs and 130 Hz, respectively (Picillo et al., 2016). During the process, adverse effects and improvements in motor symptoms were observed and recorded (Deuschl et al., 2006). Generally,rigidity was the most useful sign for confirming the benefit of stimulation because it occurred several seconds after voltage adjustment. After all contacts were tested, the best contact in each side was chosen as the cathode, and the pulse width and frequency were set at 60 μs and 130 Hz, respectively. The amplitude was set at around 1.5 V, and the final adjustment was made according to the motor symptoms. Two weeks after the implantable pulse generator was turned on, patients came back for reprogramming (small to moderate increase in voltage).A little authority was given to patients, so they could adjust this parameter at home, according to their symptoms. When frequency was set at 130 Hz and pulse width at 60 μs, the therapeutic voltage did not usually exceed 3.5 V.
Assessment
UPDRS III was conducted before surgery, and 1 and 2 years postoperatively. Stimulation settings, including voltage, pulse width, and frequency, were recorded at 1 month, 1 year and 2 years after surgery. During the 2 years of follow-up, the voltage was adjusted at every clinical visit, but the pulse width and fre-quency remained unchanged. Levodopa equivalent daily dose and Hoehn and Yahr stage (Fahn and Elton, 1989) were also noted in detail. Hoehn and Yahr stages were as follows. Stage 1: unilateral involvement only; stage 1.5: unilateral and axial involvement; stage 2: bilateral involvement without impairment of balance; stage 2.5: mild bilateral disease with recovery on pull test; stage 3: mild to moderate bilateral disease, some postural instability, physically independent; stage 4: severe disability, still able to walk or stand unassisted; stage 5: wheelchair bound or bedridden unless aided.
Statistical analysis
Continuous variables are presented as the mean ± SD and were analyzed using pairedt-tests. Statistical analyses were performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics of patients
After we excluded those who received pulse width and frequency adjustments, or double cathode or bipolar stimulation,six of 33 patients remained. Changes in voltage alone were programmed in these patients for 2 years postoperatively. Their baseline characteristics are shown inTable 1.
Motor symptoms of patients with PD treated by DBS
Total UPDRS III score, tremor, rigidity, bradykinesia and axial symptoms in the off-medication and on-medication states are shown inTable 2. Rigidity, tremor and bradykinesia improved well. In the off-medication (on-stimulation) state, total UPDRS III scores and rigidity sub-scores improved from baseline by 57.1% at 1 year and 65.4% at 2 years (P< 0.01). Bradykinesia score improved from baseline by 48.5% at 1 year (P< 0.01)and 34.7% at 2 years (P< 0.05). Moreover, tremor scores improved by 76.4% between baseline and 2 years (P< 0.05), and axial scores improved by 31.7% between baseline and 1 year(P< 0.01). In the on-medication state, rigidity was further improved at 2 years compared with baseline (P< 0.01).
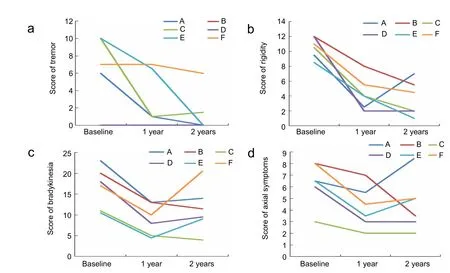
Figure 2 UPDRS III of six patients at 1 and 2 years after surgery in the off-medication state.
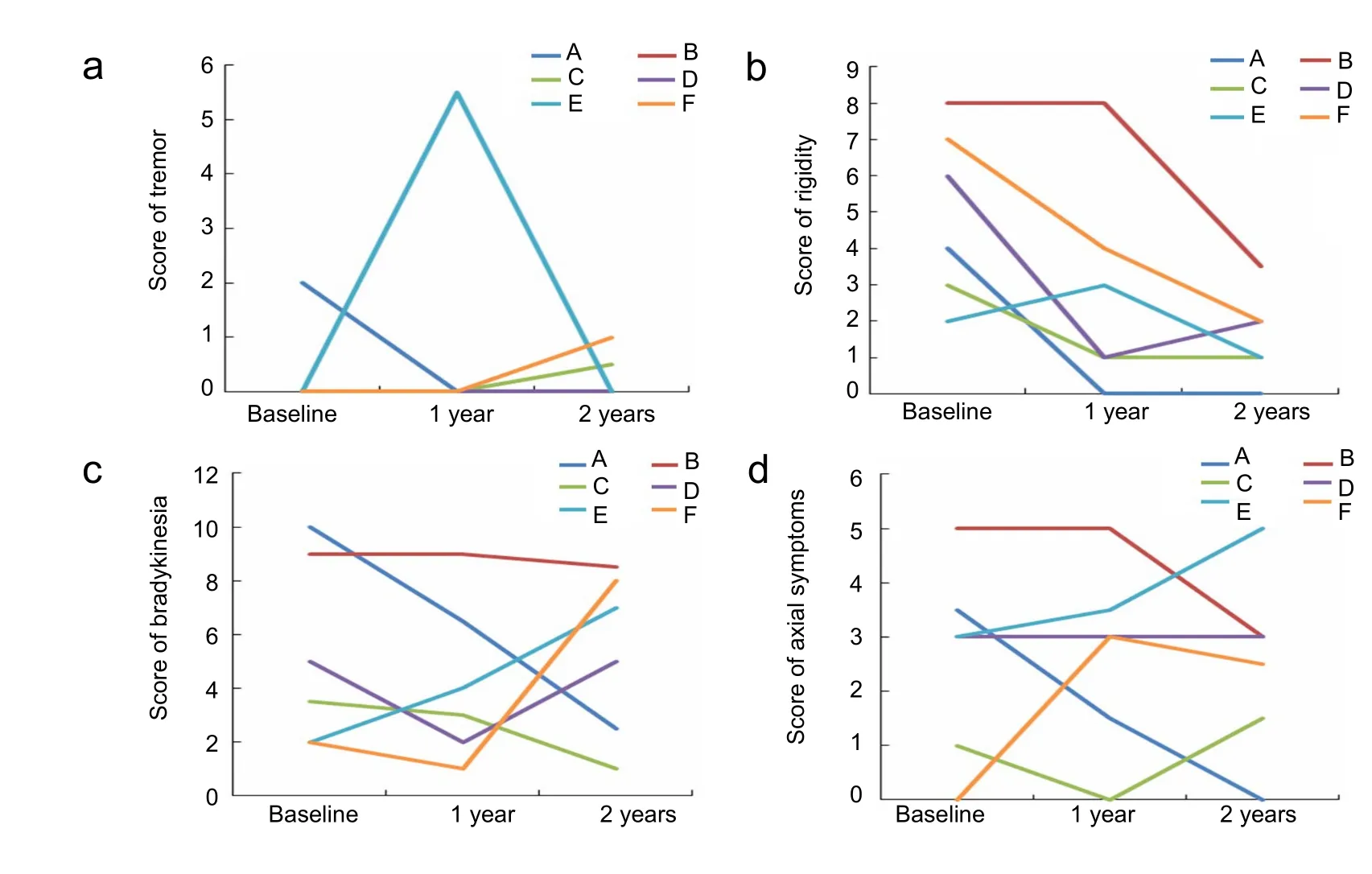
Figure 3 UPDRS III of six patients at 1 and 2 years after surgery in the on-medication state
In the off-medication state, four patients (A, C, E, and F)showed significant improvement in tremor at 1 and 2 years postoperatively. The remaining two (B and D) did not develop tremor, even by the 2-year follow-up.
Rigidity was greatly ameliorated at 1 year; four patients (B, C,E, and F) showed further improvement at 2 years, but patient A showed slightly worse rigidity at 2 years than at 1 year, and patient D showed no change between 1 and 2 years.
All patients showed marked improvement in bradykinesia at 1 year; but at 2 years, four (A, D, E, and F) showed worse symptoms than at 1 year.
Axial symptoms were slightly alleviated by long-term DBS at year 1, but reverted to the previous level at year 2 (Figure 2).
In the on-medication state, tremor was absent in five patients(B, C, D, E, and F) before surgery. At 1 year postoperatively, patient E started off with tremor, but it was controlled with longterm DBS and medication at 2 years. Tremor worsened in two of the five patients (C and F) 2 years after operation. Four patients (A, C, D, and F) showed improvement in rigidity at 1 and 2 years. Patient B showed no improvement in rigidity until 2 years. Rigidity in patient E was slightly aggravated at 1 year, but was alleviated at 2 years. In terms of bradykinesia, three patients(A, B, and C) showed improvement at 1 and 2 years. Patients D and F had less symptomatic improvement at 2 years. Manifestations in patient E were exacerbated at the 2-year follow-up. Axial symptoms showed no notable improvement. The condition of patients A and B was slightly better under chronic stimulation.The condition of patient D remained stable. The condition of the other three patients (C, E, and F) worsened slightly (Figure 3).
Generally, tremor and rigidity showed the greatest improvement bilaterally at 1 year, with further improvement at 2 years.Bradykinesia was less severe at 1 year than at 2 years. Improvement in axial symptoms was inferior to that in other symptoms.
Stimulation parameters
Voltage parameters for all patients at 1 month, 1 year and 2 years postoperatively are listed inTable 3. The voltage increased with time. In the limb of onset, voltage increased in four patients, remained unchanged in one, and decreased slightly in another. The mean voltage at 1 month was 1.76 ± 0.29 V in the limb of onset and 1.47 ± 0.24 V in the contralateral limb. At 1 year, the voltage was 2.27 ± 0.20 V in the limb of onset and 1.88 ± 0.34 V in the contralateral limb. The mean value increased to 2.33 ± 0.22 V in the limb of onset and 2.03 ± 0.43V in the contralateral limb at 2 years. There were statistically significant differences in voltage between 1 month and 1 year,and between 1 month and 2 years, in the limb of onset (P<0.05). The increases in voltage over time may be due to disease progression and reductions in medication.
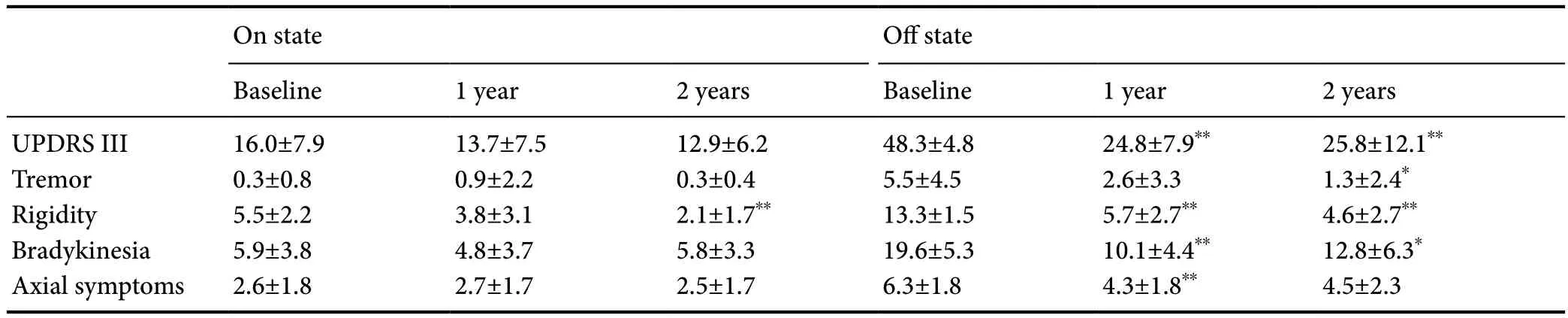
Table 2 UPDRS III and sub-scores at baseline, 1 year and 2 years in off-medication and on-medication states

Table 3 Voltage parameters (V) in each patient at 1 month, 1 year and 2 years after STN DBS surgery
Medication and Hoehn & Yahr stage
The mean levodopa equivalent daily dose at baseline, 1 year,and 2 years was 671.58 ± 203.85 mg, 402.38 ± 188.89 mg, and 391.27 ± 120.78 mg, respectively. The levodopa equivalent daily dose reduced markedly at 1 and 2 years. The Hoehn and Yahr stage in the off-medication state was 2.83 ± 0.26 at baseline, 2.42± 0.38 at 1 year and 2.58 ± 0.74 at 2 years. In the on-medication state, the Hoehn & Yahr stage was 2.33 ± 0.41 at baseline,2.00 ± 0.00 at 1 year and 2.00 ± 0.55 at 2 years.
Discussion
Improvement in motor symptoms after parameter programming
In the present study, we evaluated 1-year and 2-year follow-up data from patients who received STN DBS and subsequent voltage adjustments only, and analyzed the relationship between voltage and motor symptom improvement. Our study showed that voltage adjustment could improve parkinsonism, specifically rigidity, tremor and bradykinesia. The findings were consistent with those of a previous study (Moro et al., 2002).
In the off-medication state, tremor was less severe 1 year after DBS surgery than before surgery, and was further improved at 2 years after surgery. Similarly, rigidity in limbs was alleviated bilaterally, with a greater improvement at 2 years. Tremor and rigidity showed greater improvement in the limb of onset than in the contralateral limb. Improvement in bradykinesia was better at 1 year than at 2 years postoperatively, and in the limb of onset than in the contralateral limb at 2 years, although the reverse was true at 1 year. Although axial symptoms were improved at 1 and 2 years, improvement was not as good as in other symptoms. Together, the results indicate that voltage adjustments best improved tremor, rigidity, and bradykinesia.Motor symptoms were alleviated better in the limb of onset than in the contralateral limb. The improvement in axial symptoms revealed the benefit of voltage programming, suggesting that adjusting amplitude in DBS does not markedly influence axial symptoms. This finding was identical to that reported previously(Rodriguez-Oroz et al., 2005; Fasano et al., 2010).
In the on-medication state, we observed a slight loss of stimulation efficacy from voltage programming. This suggested that the combination of medication and stimulation produced an effect similar to that of an overdose of levodopa and allowed stable treatment of parkinsonism. We predict that as levodopa-resistant symptoms develop and reduce the initial benefit, there will be a gradual worsening of on-medication state motor function.
Programming parameters
In our study, six out of 33 patients with PD who underwent STN DBS showed sustained benefit from voltage-adjusted programming alone. This might be because the symptoms in these patients were relatively mild compared with those of patients needing multiparameter programming. Thus, adjusting only the stimulation amplitude can achieve satisfactory clinical benefits. Moreover, some patients cannot tolerate the side effects caused by increasing pulse width or frequency, which include dizziness, blurred vision, worsening of speech and gait, and stimulation-induced dyskinesia (Fasano et al., 2015; Nonnekes et al., 2015; Ramdhani et al., 2015; Vallabhajosula et al., 2015;Baizabal-Carvallo and Alonso-Juarez, 2016). Voltage adjustment might be the best choice in such cases.
Therefore, for patients with mild to moderate tremor- or rigidity-dominant parkinsonism, we suggest voltage adjustment with fixed frequency and pulse width as the first strategy.This can relieve symptoms as well as extending battery life.Frequency or pulse width adjustment, or other changes in configuration, can then be considered in those who fail to obtain bene fit from changing the voltage alone.
Anti-parkinsonism drugs and Hoehn & Yahr stage
Voltage programming can enable drug doses to be reduced while retaining satisfactory clinical effects. We observed that as the stimulation amplitude gradually increased, the dose of anti-parkinsonism drugs reduced. This decrease in medication dose will also reduce drug-induced side effects. This finding was consistent with the reports from other centers (Rodriguez-Oroz et al., 2005; Gan et al., 2007; Lilleeng et al., 2015).
The Hoehn and Yahr stage showed a decreasing trend at 1 and 2 years after surgery, compared with the baseline. This indicated that voltage parameter programming might not only improve motor symptom severity, but also slow the progression of disease (Rodriguez-Oroz et al., 2005; Gan et al., 2007;Lilleeng et al., 2015).
Together, the present results and existing knowledge of programming indicate that selecting appropriate patients is vital to ensuring the effects of parameter programming postoperatively. For those with rigidity, bradykinesia, or tremor as the main symptom, priority selection with voltage adjustment is possible. Further rigorous studies should be conducted to validate the present findings.
In conclusion, voltage adjustments can improve movement in patients with PD, as demonstrated in UPDRS III assessments at baseline and at 1 and 2 years after surgery. Rigidity, tremor and bradykinesia were the symptoms that showed the best alleviation after voltage adjustment, and effects were more pronounced in the limb of onset than in the contralateral limb.
The study had some limitations. First, the sample size was small. Second, for ethical reasons, this was a retrospective study without randomization. A prospective study with a larger sample size and longer follow-up time, together with a detailed comparison of the effect of each parameter, might shed more light on the best parameter setting in bilateral STN stimulation for PD.
Author contributions:LC conceived and designed the study. SHX, YML,YC, WBX, LLJ, QYG, JLL, JY, YFZ, LW, and WRC performed the experiments. GJ analyzed data. PZ reviewed the paper. LC and SHX reviewed and edited the paper. All authors approved the final version of the paper.
Conflicts of interest:The abstract as written communication was presented at the 18th Zhongshan International Neurology Summit Conference at November 28, 2015.
Research ethics:The study protocol was approved by the Medical Ethical Committee of the First Affiliated Hospital, Sun Yat-sen University, China(approval No. [2008]20). The study was conducted in accordance with the Declaration of Helsinki. The trial was registered at ClinicalTrials.gov(identifier: NCT01934881).
Financial support:None.
Declaration of patient consent:The authors certify that they have obtained all appropriate patient consent forms. In the form, patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Open peer reviewers:L Cocito, Università Degli Studi Di Genova, Italy;Lilla Bonanno, IRCCS Centro Neurolesi “Bonino-Pulejo”, Italy.
Additional file:Meaning of UPDRS III and subscores.
Baizabal-Carvallo JF, Alonso-Juarez M (2016) Low-frequency deep brain stimulation for movement disorders. Parkinsonism Relat Disord 31:14-22.
Benabid AL, Chabardes S, Mitrofanis J, Pollak P (2009) Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol 8:67-81.
Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, Horak FB, Okun MS, Foote KD, Krack P, Pahwa R, Henderson JM,Hariz MI, Bakay RA, Rezai A, Marks WJ, Jr., Moro E, Vitek JL, Weaver FM, Gross RE, et al. (2011) Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol 68:165.
Defer GL, Widner H, Marié RM, Rémy P, Levivier M (1999) Core assessment program for surgical interventional therapies in Parkinson’s disease (CAPSIT-PD). Mov Disord 14:572-584.
Deuschl G, Herzog J, Kleiner-Fisman G, Kubu C, Lozano AM, Lyons KE, Rodriguez-Oroz MC, Tamma F, Troster AI, Vitek JL, Volkmann J, Voon V (2006) Deep brain stimulation: postoperative issues. Mov Disord 21 Suppl 14:S219-237.
Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG,Kieburtz K, Marshall FJ, Ravina BM, Schifitto G, Siderowf A, Tanner CM (2007) Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 68:384-386.
Fahn S, Elton R (1989) Unified Parkinson’s disease rating scale. In: UPDRS Development Committee Recent Developments in Parkinson’s Disease (Fahn S, Calne DB, Goldstein M, eds), pp 153-163. Florham Park: Macmillan.
Fasano A, Aquino CC, Krauss JK, Honey CR, Bloem BR (2015) Axial disability and deep brain stimulation in patients with Parkinson disease.Nat Rev Neurol 11:98-110.
Fasano A, Romito LM, Daniele A, Piano C, Zinno M, Bentivoglio AR, Albanese A (2010) Motor and cognitive outcome in patients with Parkinson’s disease 8 years after subthalamic implants. Brain 133:2664-2676.
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189-198.
Gan J, Xie-Brustolin J, Mertens P, Polo G, Klinger H, Mollion H, Benatru I, Henry E, Broussolle E, Thobois S (2007) Bilateral subthalamic nucleus stimulation in advanced Parkinson’s disease: three years follow-up.J Neurol 254:99-106.
Hilliard JD, Frysinger RC, Elias WJ (2011) Effective subthalamic nucleus deep brain stimulation sites may differ for tremor, bradykinesia and gait disturbances in Parkinson’s disease. Stereotact Funct Neurosurg 89:357-364.
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181-184.
Jiang LL, Liu JL, Fu XL, Xian WB, Gu J, Liu YM, Ye J, Chen J, Qian H, Xu SH, Pei Z, Chen L (2015) Long-term efficacy of subthalamic nucleus deep brain stimulation in Parkinson’s disease: a 5-year follow-up study in China. Chin Med J (Engl) 128:2433-2438.
Jiang MF, Shi F, Niu GM, Xie SH, Yu SY (2015) A novel method for evaluating brain function and microstructural changes in Parkinson’s disease. Neural Regen Res 10:2025-2032.
Kumar R (2002) Methods for programming and patient management with deep brain stimulation of the globus pallidus for the treatment of advanced Parkinson’s disease and dystonia. Mov Disord 17 Suppl 3:S198-207.
Lilleeng B, Gjerstad M, Baardsen R, Dalen I, Larsen JP (2015) Motor symptoms after deep brain stimulation of the subthalamic nucleus.Acta Neurol Scand 131:298-304.
Moldovan AS, Groiss SJ, Elben S, Südmeyer M, Schnitzler A, Wojtecki L(2015) The treatment of Parkinson’s disease with deep brain stimulation: current issues. Neural Regen Res 10:1018-1022.
Moro E, Esselink RJ, Xie J, Hommel M, Benabid AL, Pollak P (2002) The impact on Parkinson’s disease of electrical parameter settings in STN stimulation. Neurology 59:706-713.
Nonnekes J, Snijders AH, Nutt JG, Deuschl G, Giladi N, Bloem BR (2015)Freezing of gait: a practical approach to management. Lancet Neurol 14:768-778.
Odekerken VJ, van Laar T, Staal MJ, Mosch A, Hoffmann CF, Nijssen PC,Beute GN, van Vugt JP, Lenders MW, Contarino MF, Mink MS, Bour LJ, van den Munckhof P, Schmand BA, de Haan RJ, Schuurman PR,de Bie RM (2013) Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. Lancet Neurol 12:37-44.
Pahwa R, Wilkinson SB, Overman J, Lyons KE (2003) Bilateral subthalamic stimulation in patients with Parkinson disease: long-term follow up. J Neurosurg 99:71-77.
Picillo M, Lozano AM, Kou N, Puppi Munhoz R, Fasano A (2016) Programming deep brain stimulation for Parkinson’s disease: the Toronto western hospital algorithms. Brain Stimul 9:425-437.
Preda F, Cavandoli C, Lettieri C, Pilleri M, Antonini A, Eleopra R, Mondani M, Martinuzzi A, Sarubbo S, Ghisellini G, Trezza A, Cavallo MA,Landi A, Sensi M (2016) Switching from constant voltage to constant current in deep brain stimulation: a multicenter experience of mixed implants for movement disorders. Eur J Neurol 23:190-195.
Ramdhani RA, Patel A, Swope D, Kopell BH (2015) Early use of 60 Hz drequency subthalamic stimulation in Parkinson’s disease: a case series and review. Neuromodulation 18:664-669.
Rodriguez-Oroz MC, Moro E, Krack P (2012) Long-term outcomes of surgical therapies for Parkinson’s disease. Mov Disord 27:1718-1728.
Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto JL, Pollak P, Rehncrona S, Kulisevsky J, Albanese A, Volkmann J, Hariz MI, Quinn NP,Speelman JD, Guridi J, Zamarbide I, Gironell A, Molet J, Pascual-Sedano B, Pidoux B, Bonnet AM, Agid Y, et al. (2005) Bilateral deep brain stimulation in Parkinson’s disease: a multicentre study with 4 years follow-up. Brain 128:2240-2249.
Scarnati E, Vitale F, Capozzo A, Mazzone P (2016) Cholinergic input from the pedunculopontine nucleus to the cerebellum: implications for deep brain stimulation in Parkinson’s disease. Neural Regen Res 11:729-730.
Vallabhajosula S, Haq IU, Hwynn N, Oyama G, Okun M, Tillman MD,Hass CJ (2015) Low-frequency versus high-frequency subthalamic nucleus deep brain stimulation on postural control and gait in Parkinson’s disease: a quantitative study. Brain Stimul 8:64-75.
Vercruysse S, Vandenberghe W, Munks L, Nuttin B, Devos H, Nieuwboer A (2014) Effects of deep brain stimulation of the subthalamic nucleus on freezing of gait in Parkinson’s disease: a prospective controlled study. J Neurol Neurosurg Psychiatry 85:871-877.
Volkmann J, Moro E, Pahwa R (2006) Basic algorithms for the programming of deep brain stimulation in Parkinson’s disease. Mov Disord 21 Suppl 14:S284-289.
Volkmann J, Herzog J, Kopper F, Deuschl G (2002) Introduction to the programming of deep brain stimulators. Mov Disord 17 Suppl 3:S181-187.
Wodarg F, Herzog J, Reese R, Falk D, Pinsker MO, Steigerwald F, Jansen O, Deuschl G, Mehdorn HM, Volkmann J (2012) Stimulation site within the MRI-defined STN predicts postoperative motor outcome.Mov Disord 27:874-879.
Yousif N, Borisyuk R, Pavese N, Nandi D, Bain P (2012) Spatiotemporal visualization of deep brain stimulation-induced effects in the subthalamic nucleus. Eur J Neurosci 36:2252-2259.(Copyedited by Slone-Murphy J, Hindle A, Yu J, Li CH, Qiu Y, Song LP,Zhao M)
- 中国神经再生研究(英文版)的其它文章
- Detection of thinned corticospinal tract and corticoreticular pathway in a patient with a calf circumference discrepancy
- Relationship of distraction rate with inferior alveolar nerve degeneration-regeneration shift
- The effect of increased intra-abdominal pressure on orbital subarachnoid space width and intraocular pressure
- Effect of electrical stimulation on neural regeneration via the p38-RhoA and ERK1/2-Bcl-2 pathways in spinal cord-injured rats
- Proteomic analysis of trans-hemispheric motor cortex reorganization following contralateral C7 nerve transfer
- GSK3β inhibitor promotes myelination and mitigates muscle atrophy after peripheral nerve injury

