幽门螺杆菌感染在非酒精性脂肪性肝病中的作用
蔡 欧,谭诗云
武汉大学人民医院消化内科,湖北 武汉 430060
幽门螺杆菌(Helicobacter pylori,H.pylori)是定植于人体胃内的革兰氏阴性杆菌[1],目前为止,H.pylori的全球感染率约50%,而我国的感染率为23%~63%[2]。H.pylori感染已明确与慢性胃炎、消化性溃疡、胃黏膜相关淋巴组织淋巴瘤和胃癌密切相关[3]。现有证据表明,它可能与消化系统以外的疾病有关,如心脏病、肥胖、Ⅱ型糖尿病[4]等。
非酒精性脂肪性肝病(non-alcoholic fatty liver disease,NAFLD)是一种与胰岛素抵抗(insulin resistance,IR)和遗传易感密切相关的代谢应激性肝脏损伤[5],疾病谱从非酒精性单纯性脂肪肝(non-alcoholic fatty liver,NAFL)到非酒精性脂肪性肝炎(non-alcoholic steatohepatitis,NASH)及其相关肝硬化和肝癌[6]。目前全球发病率约为25%[7],且约25%的NAFL可进展成NASH,严重影响人们的健康和生活质量。早期学者提出NAFLD发病的二重打击学说[8],即脂肪变性基础上发生氧化应激,现“多重打击”学说[9]成为主流,多重因素包括脂毒性、内质网应激、细胞因子、肠道菌群改变、药物、遗传和表观遗传因素、IR等。H.pylori感染与NAFLD的相关性获得更多学者的关注,现对H.pylori感染与NAFLD的相关性研究作一概述。
1 H.pylori感染与NAFLD相关性的临床研究
2008年,CINDORUK等[10]在44岁NASH女性肝穿刺活检标本中发现H.pylori16S rDNA,这一发现引起人们对两者相关性的关注;2009年,PIROUZ等[11]在11例NAFLD患者肝穿刺活检组织中发现,H.pyloriDNA阳性者5例,更证实了两者之间存在某种关联,而H.pylori定植在胃内,是如何进入肝脏的呢?有学者提出两种可能机制:细菌通过十二指肠和胆道从胃到肝脏,或通过肝门静脉的血液循环[12]。
近年来,关于H.pylori感染与NAFLD相关性的临床研究较多。最早希腊的一项研究,共纳入28例肝穿刺活检证实的NAFLD患者(15例NAFL,13例NASH)和25名健康对照者,发现NAFLD组H.pylori抗体阳性率高于对照组(P=0.038),回归分析也显示,H.pylori感染可独立预测NAFLD。但NAFLD组内分析发现,无论H.pylori血清学阳性还是13C呼气试验阳性或两者结合,均不能独立预测NASH,说明H.pylori感染主要在NAFL的发病中起作用,但在NAFL到NASH的进展中可能作用不大[13]。两年后,来自日本的一项纳入130例肝穿刺活检证实的NAFLD患者(43例NAFL,87例NASH)的研究发现,H.pylori抗体阳性患者的NASH发病率高于H.pylori抗体阴性者(P=0.008),且H.pylori抗体阳性者的NAFLD活动度分级和肝细胞气球样变的分级高于H.pylori抗体阴性者(P=0.03),回归分析显示,H.pylori感染是NASH的独立危险因素,证实H.pylori感染可能在NAFL到NASH发展过程中发挥作用[14]。国内刘安楠等[15]纳入14 373名体检人群数据统计发现,H.pylori感染与NAFLD呈正相关。然而,来自韩国和日本的两项分别纳入3 663名和13 737例患者的研究均发现,H.pylori感染与NAFLD不相关[16-17],尚需大样本的前瞻性多中心研究阐明H.pylori感染与NAFLD的相关性。
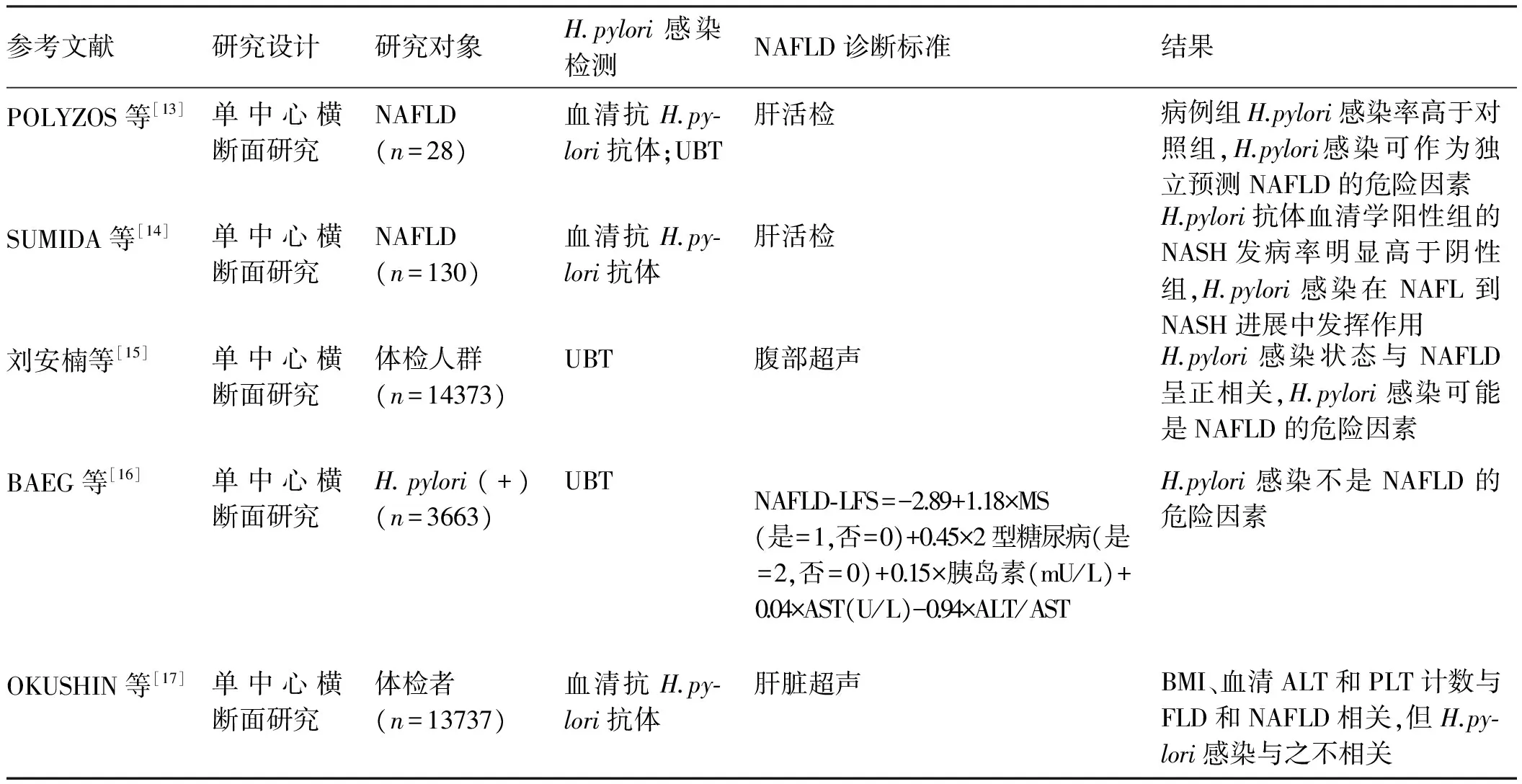
表1 关于H.pylori感染与NAFLD相关性的临床研究Tab 1 Clinical researches about the association between H.pylori infection and NAFLD
注:UBT:尿素呼气试验;HOMA-IR:稳态模型评估IR指数;NAFLD-LFS:NAFLD-肝脂肪分数;MS:代谢综合征;AST:谷草转氨酶;ALT:谷丙转氨酶;BMI:体质量指数;PLT:血小板;FLD:脂肪肝。
2 H.pylori感染在NAFLD中作用的可能机制
2.1IR2005年,AYDEMIR等[18]首先证明慢性H.pylori感染与IR的相关性,H.pylori阳性组的HOMA-IR水平较H.pylori阴性组高(P<0.05)。2009年,日本的GUNJI等[19]发现,IR患者(HOMA-IR≥2.5)的H.pylori血清学阳性率明显高于对照组(HOMA-IR<2.5)(P=0.027),且排除了性别、年龄、BMI、腰围、吸烟、饮酒、活动度等混杂因素后,HOMA-IR评分与H.pylori血清学状态有相关性(P=0.001)。2013年,希腊的POLYZOS等[13]发现,NAFLD组H.pylori感染率高于对照组,而该研究也检测了血糖、胰岛素、HOMA-IR等指标,发现这些指标在NAFLD组高于对照组,在H.pylori感染组高于非感染组,因此推测H.pylori感染可能通过直接或间接地增加IR作用于NAFLD的发病过程。ABENAVOLI等[20]研究发现,根除H.pylori之后的空腹血浆胰岛素水平和HOMA-IR均低于根除治疗之前(P<0.01),提示根除H.pylori可改善IR并可能预防MS和NAFLD的发生。
H.pylori可能是间接通过慢性炎症或直接活化特定的信号通路诱导IR。有研究[21]认为,慢性炎症在IR中起重要的作用。有研究[22-24]报道,H.pylori导致的慢性炎症可增加C-反应蛋白(CRP)、肿瘤坏死因子(TNF)和白介素-6(IL-6)的表达。这些细胞因子可以活化一系列激酶,并最终通过上调丝氨酸磷酸化或抑制胰岛素受体酪氨酸的自体磷酸化而导致IR[25]。另有一项研究[26]针对H.pylori感染对IR作用,建立人和小鼠两种模型,发现H.pylori可通过转录因子c-Jun下调miR-203的表达从而上调胰岛素信号抑制子SOCS3的表达,并最终导致IR,因此认为,H.pylori感染可通过c-Jun/miR-203/SOCS3信号通路诱导IR。
2.2炎性细胞因子很多炎性细胞因子参与H.pylori感染,其中关系最紧密的包括CRP、TNF和IL-6。一项纳入159例H.pylori感染患者的前瞻性研究[22]发现,HOMA-IR和CRP水平高于无H.pylori感染者(P<0.05),而经过H.pylori根除治疗6周后,患者这两项指标水平明显低于根除治疗前(P<0.05),另一项研究[27]发现,hs-CRP水平在NASH组高于非NASH组,且可作为NAFLD非侵入性标记物。POLYZOS等[13]发现,NAFLD组TNF-α水平高于非NAFLD组,而H.pylori感染组的TNF-α水平亦高于H.pylori非感染组,TNF-α可能作为一种介质,直接或间接作用于H.pylori感染导致NAFLD。该研究也在H.pylori感染的NAFLD患者发现更高水平的hs-CRP。
脂肪组织不仅通过参与能量代谢,而且通过分泌细胞因子,如瘦素和脂联素,导致IR,进而作用于NAFLD。瘦素是一种前炎性细胞因子,且与IL-6有相似的结构基础[28]。研究[29]显示,NAFLD组的血浆瘦素水平升高,且可以作为脂肪变性的独立预测因素。一项纳入133例消化不良患者的研究[30]显示,H.pylori感染与瘦素水平相关(P<0.01),H.pylori可能影响瘦素的产生,瘦素可以通过抑制肝脏相关酶活性减少肝脏极低密度脂蛋白胆固醇和脂肪沉积。因此,我们推测,H.pylori感染可能通过影响脂质代谢或运输相关酶类而导致NAFLD。但也有研究认为,H.pylori感染加速瘦素合成[31]。
2.3血脂NAFLD是脂肪在肝脏的沉积,H.pylori感染也与血脂异常有关。1999年,LAURILA等[32]研究发现,H.pyloriIgG阳性的男性血清甘油三酯(TC)和总胆固醇(TG)含量更高(P<0.001),提示H.pylori感染可能影响脂质代谢。2016年,UPALA等[33]针对27 544名受试者的Meta分析显示,H.pylori感染者有更高的TC,更低的HDL-C(P<0.05)。一项纳入159例NAFLD患者的前瞻性研究[22]显示,H.pylori感染者的HOMA-IR、TC、TG、LDL-C和CRP水平更高,HDL-C水平更低(P<0.05),该研究揭示了H.pylori根除可以改善IR、血脂异常和炎症状态,然而,这一现象发生的具体机制尚不明确。
2.4肠道通透性和肠道菌群近年来,随着基因组测序技术的发展和应用,更多的证据表明,肠道菌群失调与肝脏疾病的相关性,而其在NAFLD中的作用也是热门研究之一。JIANG等[34]研究发现,NAFLD患者肠道通透性明显增加,而且,除外菌群失调,肠道菌群介导的肠道炎症反应及其相关的肠道免疫功能受损也在NAFLD的发病中发挥重要作用。另一项回顾性纳入57例活检证实的NAFLD患者的研究也显示,拟杆菌属丰度在NASH和F≥2的患者中明显增加,而普氏菌属丰度下降,瘤胃球菌属丰度在F≥2的患者中明显增加。多因素回归分析显示,拟杆菌属丰度与NASH独立相关,而瘤胃球菌属丰度与F≥2相关。因此认为,NAFLD严重度与肠道菌群失调相关是NAFLD严重度的预测因子[35]。
2001年,FUKUDA等[36]通过口服蔗糖耐受试验检测肠道通透性发现,存在H.pylori本身即与通透性增加相关。2014年,HEIMESSAT等[37]发现,H.pylori野生株长期感染的蒙古沙鼠可导致远端未感染肠道的菌群结构发生明显改变。2015年,KHOSRAVI等[38]观察到SPF环境的小鼠的H.pylori感染与肠道菌群有关。有学者推测,H.pylori相关的肠道菌群失调所致的NAFLD机制可能是H.pylori侵入肠道黏膜增加肠道通透性、导致肠道菌群失调,为细菌内毒素通过门静脉进入肝内提供便利,进而促进肠道炎症反应[14,36]。
3 总结与展望
NAFLD是遗传和环境共同作用导致的一种复杂的临床病理综合征,是隐源性肝硬化的重要原因之一。H.pylori感染可能通过增加IR、介导炎性因子、影响脂质代谢、改变肠道通透性等影响NAFLD的发生及发展,但其具体机制目前尚不明确,尚需高质量的临床和基础研究明确两者相关性,阐明两者内在的关联性,为NAFLD的治疗提供新的思路和线索。
[1] WARREN J R, MARSHALL B. Unidentifid curved bacilli on gastric epithelium in active chronic gastritis [J]. Lancet, 1983, 1(8336): 1273-1275.
[2] 王雪, 李异玲, 吕晓辉. 我国幽门螺杆菌感染的现状分析[J]. 胃肠病学和肝病学杂志, 2017, 26(6): 640-643. DOI: 10.3969/j.issn.1006-5709.2017.06.006.
WANG X, LI Y L, LYU X H. Analysis of Helicobacter pylori infection in China [J]. Chin J Gastroenterol Hepatol, 2017, 26(6): 640-643. DOI: 10.3969/j.issn.1006-5709.2017.06.006.
[3] 钱家鸣. 消化内科学[M]. 北京: 人民卫生出版社, 2014: 54-55.
[4] GONI E, FRANCESCHI F. Helicobacter pylori and extragastric diseases [J]. Helicobacter, 2016, 21 Suppl 1: 45-48. DOI: 10.1111/hel.12340.
[5] 中华医学会肝病学分会脂肪肝和酒精性肝病学组. 非酒精性脂肪性肝病诊疗指南(2010年修订版)[J]. 胃肠病学和肝病学杂志, 2010, 19(6): 483-487. DOI: 10.3969/j.issn.1006-5709.2010.06.001.
The Chinese National Work-shop on Fatty Liver and Alcoholic Liver Disease for the Chinese Liver Disease Association. Guidelines for management of nonalcoholic fatty liver disease: an updated and revised edition [J]. Chin J Gastroenterol Hepatol, 2010, 19(6): 483-487. DOI: 10.3969/j.issn.1006-5709.2010.06.001.
[6] CALDWELL S, ARGO C. The natural history of non-alcoholic fatty liver disease [J]. Dig Dis, 2010, 28(1): 162-168. DOI: 10.1159/000282081.
[7] YOUNOSSI Z M, KOENIG A B, ABDELATIF D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence and outcomes [J]. Hepatology, 2016, 64(1): 73-84. DOI: 10.1002/hep.28431.
[8] DAY C P, JAMES O F. Steatohepatitis: a tale of two “hits”? [J]. Gastroenterology, 1998, 114(4): 842-845.
[9] BUZZETTI E, PINZANI M, TSOCHATZIS E A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) [J]. Metabolism, 2016, 65(8): 1038-1048. DOI: 10.1016/j.metabol.2015.12.012.
[10] CINDORUK M, CIRAK M Y, UNAL S, et al. Identification of Helicobacter species by 16S rDNA PCR and sequence analysis in human liver samples from patients with various etiologies of benign liver diseases [J]. Eur J Gastroenterol Hepatol, 2008, 20(1): 33-36. DOI: 10.1097/MEG.0b013e3282efa4f2.
[11] PIROUZ T, ZOUNUBI L, KEIVANI H, et al. Detection of Helicobacter pylori in paraffin-embedded specimens from patients with chronic liver diseases, using the amplification method [J]. Dig Dis Sci, 2009, 54(7): 1456-1459. DOI: 10.1007/s10620-008-0522-5.
[12] PELLICANO R, MéNARD A, RIZZETTO M, et al. Helicobacter species and liver diseases: association or causation? [J]. Lancet Infect Dis, 2008, 8(4): 254-260. DOI: 10.1016/S1473-3099(08)70066-5.
[13] POLYZOS S A, KOUNTOURAS J, PAPATHEODOROU A, et al. Helicobacter pylori infection in patients with nonalcoholic fatty liver disease [J]. Metabolism, 2013, 62(1): 121-126. DOI: 10.1016/j.metabol.2012.06.007.
[14] SUMIDA Y, KANEMASA K, IMAI S, et al. Helicobacter pylori infection might have a potential role in hepatocyte ballooning in nonalcoholic fatty liver disease [J]. J Gastroenterol, 2015, 50(9): 996-1004. DOI: 10.1007/s00535-015-1039-2.
[15] 刘安楠, 王蕾蕾, 张晏, 等. 非酒精性脂肪性肝病与幽门螺杆菌感染的相关性[J]. 胃肠病学和肝病学杂志, 2014, 23(12): 1451-1454. DOI: 10.3969/j.issn.1006-5709.2014.12.023.
LIU A N, WANG L L, ZHANG Y, et al. Association between nonalcoholic fatty liver disease and Helicobacter pylori [J]. Chin J Gastroenterol Hepatol, 2014, 23(12): 1451-1454. DOI: 10.3969/j.issn.1006-5709.2014.12.023.
[16] BAEG M K, YOON S K, KO S H, et al. Helicobacter pylori infection is not associated with nonalcoholic fatty liver disease [J]. World J Gastroenterol, 2016, 22(8): 2592-2600. DOI: 10.3748/wjg.v22.i8.2592.
[17] OKUSHIN K, TAKAHASHI Y, YAMAMICHI N, et al. Helicobacter pylori infection is not associated with fatty liver disease including non-alcoholic fatty liver disease: a large-scale cross-sectional study in Japan [J]. BMC Gastroenterol, 2015, 15: 25. DOI: 10.1186/s12876-015-0247-9.
[18] AYDEMIR S, BAYRAKTAROGLU T, SERT M, et al. The effect of Helicobacter pylori on insulin resistance [J]. Dig Dis Sci, 2005, 50(11): 2090-2093. DOI: 10.1007/s10620-005-3012-z.
[19] GUNJI T, MATSUHASHI N, SATO H, et al. Helicobacter pylori infection significantly increases insulin resistance in the asymptomatic Japanese population [J]. Helicobacter, 2009, 14(5): 144-150. DOI: 10.1111/j.1523-5378.2009.00705.x.
[20] ABENAVOLI L, MILIC N, MASARONE M, et al. Association between non-alcoholic fatty liver disease, insulin resistance and Helicobacter pylori [J]. Med Hypotheses, 2013, 81(5): 913-915. DOI: 10.1016/j.mehy.2013.08.011.
[21] HOSSAIN I A, AKTER S, BHUIYAN F R, et al. Subclinical inflammation in relation to insulin resistance in prediabetic subjects with nonalcoholic fatty liver disease [J]. BMC Res Notes, 2016, 9: 266. DOI: 10.1186/s13104-016-2071-x.
[22] GEN R, DEMIR M, ATASEVEN H. Effect of Helicobacter pylori eradication on insulin resistance, serum lipids and low-grade inflammation [J]. South Med J, 2010, 103(3): 190-196. DOI: 10.1097/SMJ.0b013e3181cf373f.
[23] SIREGAR G A, HALIM S, SITEPU V R. Serum TNF-α, IL-8, VEGF levels in Helicobacter pylori infection and their association with degree of gastritis [J]. Acta Med Indones, 2015, 47(2): 120-126.
[24] TSAI C C, KUO T Y, HONG Z W, et al. Helicobacter pylori neutrophil-activating protein induces release of histamine and interlukin-6 through G protein-mediated MAPKs and P13K/Akt pathways in HMC-1 cells [J]. Virulence, 2015, 6(8): 755-765. DOI: 10.1080/21505594.2015.1043505.
[25] DANDONA P, AIJADA A, BANDYOPADHYAY A. Inflammation: the link between insulin resistance, obesity and diabetes [J]. Trends Immunol, 2004, 25(1): 4-7.
[26] ZHOU X, LIU W, GU M, et al. Helicobacter pylori infection causes hepatic insulin resistance by the c-Jun/miR-203/SOCS3 signaling pathway [J]. J Gastroenterol, 2015, 50(10): 1027-1040. DOI: 10.1007/s00535-015-1051-6.
[27] MALEKI I, RASTGAR A, HOSSEINI V, et al. High sensitive CRP and pentraxine 3 as noninvasive biomarkers of nonalcoholic fatty liver disease [J]. Eur Rev Med Pharmacol Sci, 2014, 18(11): 1583-1590.
[28] LA CAVA A, MATARESE G. The weight of leptin in immunity [J]. Nat Rev Immunol, 2004, 4(5): 371-379. DOI: 10.1038/nri1350.
[29] POLYZOS S A, ARONIS K N, KOUNTOURAS J, et al. Circulating leptin in non-alcoholic fatty liver disease: a systematic review and meta-analysis [J]. Diabetologia, 2016, 59(1): 30-43. DOI: 10.1007/s00125-015-3769-3.
[30] HEMMASI G, ZAMANI F, KHONSARI M, et al. Association between Helicobacter pylori and serum leptin in Iranian dyspeptic patients [J]. Middle East J Dig Dis, 2013, 5(3): 158-162.
[31] NISHI Y, ISOMOTO H, UOTANI S, et al. Enhanced production of leptin in gastric fundic mucosa with Helicobacter pylori infection [J]. World J Gastroenterol, 2005, 11(5): 695-699.
[32] LAURILA A, BLOIGU A, NYHS, et al. Association of Helicobacter pylori infection with elevated serum lipids [J]. Atherosclerosis, 1999, 142(1): 207-210.
[33] UPALA S, JARUVONGVANICH V, RIANGWIWAT T, et al. Association between Helicobacter pylori infection and metabolic syndrome: a systematic review and meta-analysis [J]. J Dig Dis, 2016, 17(7): 433-440. DOI: 10.1111/1751-2980.12367.
[34] JIANG W, WU N, WANG X, et al. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease [J]. Sci Rep, 2015, 5: 8096. DOI: 10.1038/srep08096.
[35] BOURSIER J, MUELLER O, BARRET M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota [J]. Hepatology, 2016, 63(3): 764-775. DOI: 10.1002/hep.28356.doi:10.3969/j.issn.1006-5709.2018.02.004
[36] FUKUDA Y, BAMBA H, OKUI M, et al. Helicobacter pylori infection increases mucosal permeability of the stomach and intestine [J]. Digestion, 2001, 63 Suppl 1: 93-96. DOI: 10.1159/000051918.
[37] HEIMESAAT M M, FISCHER A, PLICKERT R, et al. Helicobacter pylori induced gastric immunopathology is associated with distinct microbiota changes in the large intestines of long-term infected Mongolian gerbils [J]. PLoS One, 2014, 9(6): e100362. DOI: 10.1371/journal.pone.0100362.
[38] KHOSRAVI Y, SEOW S W, AMOVO A A, et al. Helicobacter pylori infection can affect energy modulating hormones and body weight in germ free mice [J]. Sci Rep, 2015, 5: 8731. DOI: 10.1038/srep08731.

