Obstructive Sleep Apnea and Cardiovascular Disease in Women
Nimeh Najjar, MD , Peter Staiano, MD and Mariam Louis, MD, MSc, FCCP
1 University of Florida, Department of Medicine, Jacksonville, FL, USA
Abstract Obstructive Sleep Apnea (OSA) is a common chronic disorder that af fects 5 - 10% of the US population with a higher prevalence in men compared to women by 2:1 in population studies. By contrast, in patients with cardiovascular disease,its prevalence can exceed 50% depending on the specif c disorder surveyed. Although sex differences have been well described for cardiovascular risk factors; existing data regarding the impact of sex on the relationship between OSA and cardiovascular outcomes is controversial. Similarly, while there is strong evidence for increased prevalence of cardiovascular conditions, such as systemic hypertension, atrial f brillation, as well as heart failure amongst patients suffering from OSA; conf icting evidence exists regarding the incidence and bidirectional relationship between them as well as the impact of treatment of OSA on cardiovascular outcomes. In this paper, we will review the associations between OSA and cardiovascular diseases in women. The data on sex differences is limited, due to a number of reasons, including, but not limited to late presentation of OSA in women, diff culties in diagnosing both OSA and cardiovascular diseases in women and still suboptimal inclusion of adequate number of women in clinical trials. More studies are needed to better delineate sex differences in the clinical presentation as well as the pathophysiology of the associations between OSA and cardiovascular diseases so that we can provide patients with more personalized care.
Keywords: obstructive sleep apnea (OSA); hypertension; cardiovascular; heart failure; atrial f brillation ; women ; sex
Introduction
Obstructive Sleep Apnea (OSA) is a relatively common chronic disease that is def ned by repetitive collapse of the upper airway during sleep that is associated with intermittent hypoxia and arousals from sleep. OSA has been associated with deleterious cognitive impairments such as increased daytime sleepiness and motor vehicle accidents. It has also been associated with adverse medical outcomes including increased cardiometabolic disease, postoperative complications and overall higher mortality.
The biggest risk factor for OSA is obesity, and the higher the BMI, the more the severity of disease.Other risk factors include age, male sex and craniofacial abnormalities such as short mandibular size,a wide craniofacial base and tonsillar and adenoid hypertrophy, post-menopausal status, nasal congestion and smoking.
The overall prevalence of disease varies according to the manner in which OSA is def ned and the distribution of risk factors in the populations being s studied. Refer to Table 1 for def nitions. In NorthAmerica, the estimated prevalence is 20 - 30% in adult males and 10 - 15% of adults in females when OSA is def ned as an apnea-hypopnea index (AHI)of > 5/hour as measured by a polysomongram [ 1,2]. However, if narrower def nitions are used, i.e.an AHI > 5/hour and at least one symptom of disturbed sleep or an AHI > 15/hour (moderate OSA),then the prevalence estimates are 15% in men and 5% in females [ 1, 2]. Regardless of the def nition of OSA, the prevalence rates in the US are expected to increase due to the rise of obesity. In addition, the prevalence of OSA varies by race and ethnicity. OSA is more common among African-Americans who are younger than 35 years of age when compared to Caucasians in the same age group, independent of BMI [ 3]. Finally, although OSA is 2 - 3 times more common in men compared to females, the gap narrows as women reach menopausal age [ 4].

Table 1 Important Def nitions of Sleep Disordered Breathing.
In this article, we will focus OSA and its associations with cardiovascular outcomes in women.In particular, we will discuss OSA in relation to systemic arterial hypertension (HTN), Atrial Fibrillation (AF), cardiovascular events and heart failure (HF). Figure 1 depicts the proposed mechanisms by which these interactions are mediated.Although robust data exists in the overall population, there is a paucity of data as it pertains to women for several factors. First, prior to 1985, women were excluded from clinical trials, and in 1993, the passage of the NIH Revitalization Act, ensured that women were included and that there would be sexspecif c analysis. Second, women tend to develop OSA at an older age compared to men, and so the cumulative exposure to the adverse effects of OSA are limited. Third, OSA is much more challenging to diagnose in women because the classic symptoms of OSA such as snoring, witnessed apneas, and daytime somnolence are not as common; women tend to have more subtle signs and symptoms of OSA with complaints of fatigue and insomnia. As a result, the ratio of men to women diagnosed with OSA is 8:1 and women are often misdiagnosed.
OSA and Hypertension
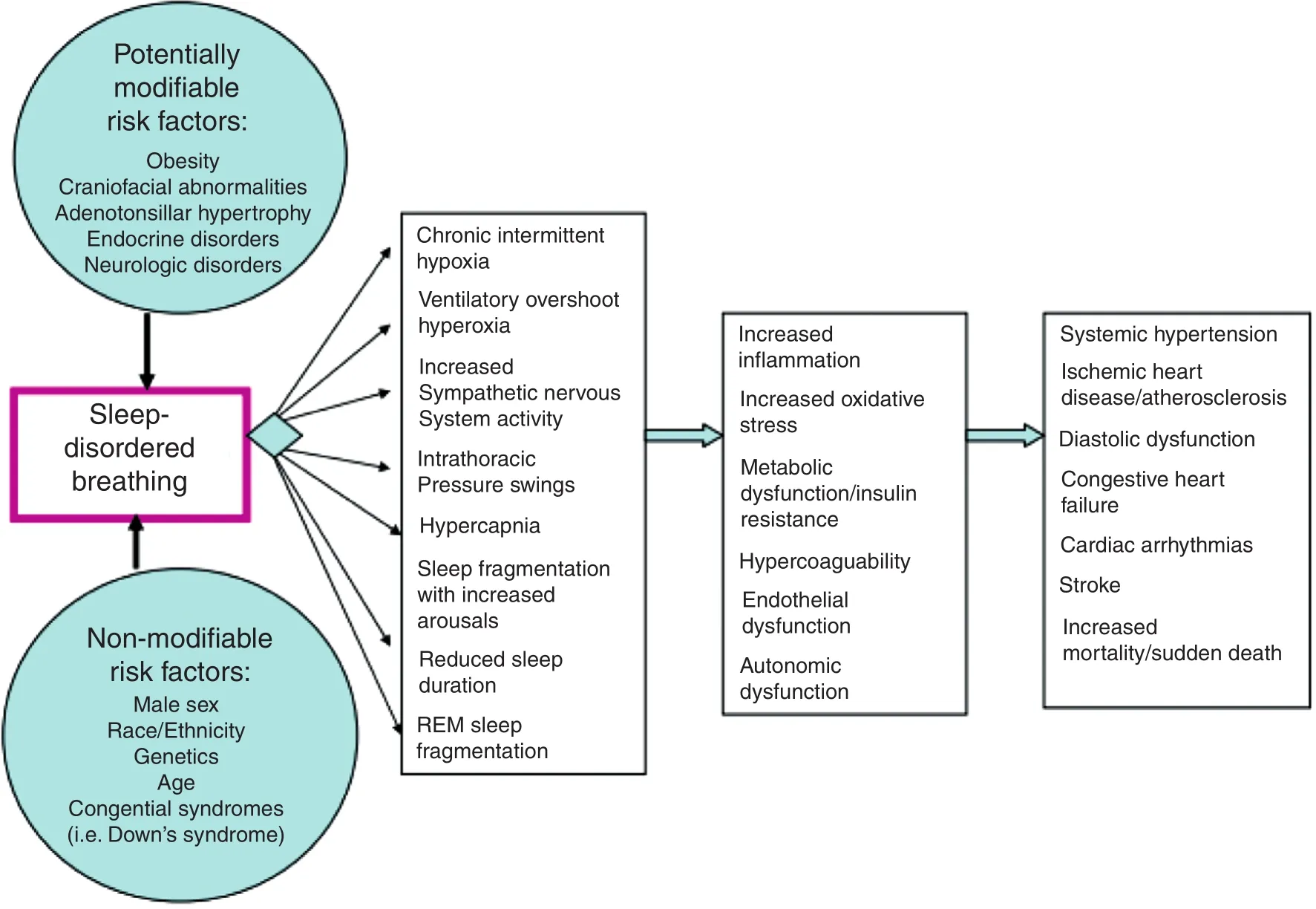
Figure 1 Relationship Between Cardiovascular Disease and Sleep-Disordered Breathing.
There is a strong association between OSA and systemic hypertension (HTN) which has been widely conf rmed by many studies in the past several year s.About 50% of patients with HTN have concomitant OSA. Studies now indicate that OSA may be the most common cause of resistant HTN 5[ ] with a prevalence of OSA in patients with resistant hypertension reaching 83% [ 6]. Indeed, numerous crosssectional population based studies have reported an increased prevalence of HTN in OSA patients[ 7, 8]. The prevalence of HTN increases with OSA disease severity and was not signif cantly different for women and men. Pedrosa et al. [ 9] reported that OSA is common and independently associated with hypertension and increased arterial stif fness in consecutive perimenopausal women. They also found that women with moderate to severe OSA vs those without OSA had a higher prevalence of hypertension, were prescribed more medications for hypertension, and had higher awake blood pressure [ 9]. A recent study showed that the percentage of females with hypertension at dif ferent degrees of OSA severity was stable at about 26%and using an ordinal multivariate logistic regression analysis, hypertension, age, and obesity were associated with OSA severity in males, whereas only age and obesity were associated with OSA severity in females [ 10]. However, unlike other studies, this group reported that although the proportion of subjects with hypertension was higher in females with OSA than in males with OSA, the proportion of subjects with hypertension increased as the severity of OSA increased in males but not in females.
The clinical implication of such a notion is tremendous if we consider the number of patients worldwide suffering from HTN and its deleterious complications on many or gan systems especially the cardiovascular and renal systems. Many studies have proposed def nitions for HTN related to OSA,however none have been accepted thus far. An area of growing research interest is the ef fect OSA has on women ’ s cardiovascular health, especially those in the perimenopausal period. This period is well known as an independent risk factor for cardiovascular disease, although the contribution of OSA is controversial [ 9] .
The incidence of HTN is OSA patients is also increased, although this has not been a consistent f nding. Early data from the Wisconsin Sleep Cohort showed that over a 4 year period, the odds ratios for the presence of HTN were 1.42 (1.13 - 1.78) with an AHI of 0.1 - 4.9 events per hour at baseline as compared with none, 2.03 (1.29 - 3.17) with an AHI of 5.0- 14.9 events per hour, and 2.89 (1.46 - 5.64) with an AHI of 15.0 or more events per hour [ 11]. This was after adjustment of several factors, including sex. Marin et al. reported similar results, although there was no analysis by sex [ 12]. In contrast, data from the Sleep Health Heart Study [ 13] and Vitoria Sleep Cohort [ 14] did not f nd such an association between OSA and incident HTN. Differences in methodology, selection of population and power may all contribute to the dif ferences in results. To further investigate this, a post-hoc analysis of the Vitoria Sleep Cohort data was performed [ 15]. Over an 8 year timespan, men with moderate OSA had a signif cantly increased OR for stage 2 hypertension(OR, 2.54 [95% CI, 1.09 - 5.95], P = 0.032). This association was not statistically signif cant among women (P = 0.371). Therefore while there is robust data showing an increase in prevalence of HTN amongst OSA patients, more stringent studies are needed to delineate the relationship between OSA and incident HTN.
Several mechanisms have been proposed for the pathogenesis of HTN and OSA [ 16]. Individuals without OSA experience a nocturnal drop in blood pressure; this seems to be altered in patients with OSA. This exact mechanism remains unclear. But it is thought to be due to the activation of the sympathetic system, due to hypoxia and the resultant hypercapnia, leading to elevations in nocturnal systolic and diastolic pressures causing the mean arterial pressure to remain elevated throughout the night [ 17, 18]. This may be evident by the elevation of catecholamine levels detected by urine studies[ 5]. On the molecular level, activation of inf ammatory cascades leads to endothelial dysfunction[ 19] and to elevated inf ammatory markers such as interlukin-1, interleukin-6, interleukin-8 and tumor necrosis factor alpha is thought to portend a worse prognosis but more studies are needed to elucidate these pathways in hypertensive OSA patients[ 5]. The role of the renin-angiotensin-aldosterone system in the development of HTN has been well documented, however its role in hypertensive OSA patient is still unclear [ 20].
Despite the growing evidence recognizing OSA as a risk factor for cardiovascular disease especially HTN in women, it is mainly based on observational studies. Pedrosa et al. [ 9] studied the potential vascular consequences of OSA in perimenopausal women. They concluded that although OSA is common, it remains underdiagnosed in women.Young et al. indicated that greater than 90% of women with moderate to severe OSA were underdiagnosed [ 17]. They attributed this f nding to somnolence being a lesser reason women are evaluated for OSA when compared to men.
Impact of Treatment
The bi-directionality of the relationship between OSA and HTN leads to several questions which are areas of current study interest; does treatment of OSA with positive pressure therapy also treat HTN? Or does tar geting HTN with antihypertensive agents treat OSA ? How much of systemic HTN is attributed to OSA ? The answers are unclear and as a result current treatment modalities are aimed at both [ 21] .
The effect of positive pressure therapy on blood pressure reduction in patients with OSA is very modest, with only about 2 - 3 mmHg decrease in pressure, compared to the signif cant effect of antihypertensive agents [ 22, 23]. However, although the f nding that CPAP reduces systemic blood pressure has been fairly consistent, this has not been a consistent f nding. Differences related to methodology(study size, multi-center or single center, etc.) and patient factors (age, sex, BMI, degree of HTN, adequate antihypertensive treatment, etc.) may explain the disparity. Campos-Rodriguez et al. looked at CPAP treatment on blood pressure in women [ 24].They found that as with the mixed trials, CP AP lowered blood pressure by about 2 mmHg, indicating that there are no sex dif ferences in the response to CPAP on blood pressure.
The evidence on the effect of treatment of HTN with antihypertensive agents on OSA is also lacking. Kraczi et al. studied the effect of some of the most common antihypertensive agents used (atenolol, amlodipine, enalapril, losartan and hydrochlorothiazide) on severity of OSA by assessing AHI [ 25]. Their study did not show any change in AHI based on the antihypertensive agent used.The most promising evidence we have thus far indicates the benef t of using diuretics and antialdosterone agents in hypertensive OSA patients due to lessening the parapharyngeal edema thought to be an important factor in the pathogenesis of OSA [ 26].
Considering the multifactorial pathophysiology of OSA-associated hypertension, the combination of CPAP therapy, antihypertensive drugs and weightloss interventions appears to be the most promising strategy to improve blood pressure control in OSA patients [ 27]. Although antihypertensive drugs such as valsartan have been found to be markedly more effective than CPAP on OSA-associated hypertension the combination of blood pressure-lowering drugs and CPAP has additive effects on blood pressure [ 28, 29].
The relationship between OSA and HTN remains unclear, however, given the great clinical impact both OSA and HTN have larger multi-center studies are necessary to clarify this relationship. Clear def -nitions for HTN related to OSA and treatment algorithms would signif cantly impact many patients.
OSA and Atrial Fibrillation
Several studies have clearly documented the association (up to four times higher odds) between OSA and AF independent of obesity and other confounding factors [ 30]. Several cross-sectional and case control studies have found increased prevalence of AF among OSA patients. Guilleminault et al. in 1983 [ 31] found that in 400 patients with moderate-severe OSA, the prevalence of AF was approximately three times higher than the general population. A more recent study [ 32], found a four-fold increase in the prevalence of AF in OSA patients independent of age, sex and BMI (OR 4.0,1.0- 15.7). Furthermore, there seems to be a doseresponse relationship between increasing severity of OSA and prevalence of OSA in older men [ 33].It is not clear based on published reports if sex differences exist.
Interestingly, it appears that a bidirectional relationship between AF and OSA exists. The prevalence of OSA in patients with AF is increased, with estimates ranging from 30 to 80% [ 34, 35]. This association is independent of shared risk factors.The implication of this association remains unclear;is OSA a causative factor of AF or just a common coexisting condition, among others, in patients with AF [ 36] ?
There is robust evidence to suggest that OSA may be a risk factor for incident AF. In a recent retrospective study [ 37], patients with OSA had a 55% risk of incident AF over a median follow-up of 12 years. This is supported by prospective data in older men in which the risk of AF was doubled in patients with sleep disordered breathing over a 6.5 year follow up [ 32]. In another prospective study [ 38] following patients with OSA for just under 5 years, the cumulative probability of incident AF was 14%. Univariate predictors of AF were age, male sex, hypertension, coronary artery disease, heart failure, smoking, body mass index, OSA(hazard ratio 2.18, 95% CI 1.34 - 3.54) and multiple measures of OSA severity. In subjects < 65 years old,independent predictors of incident AF were age,male sex, coronary artery disease, body mass index(per 1 kg/m2, hazard ratio 1.07, 95% CI 1.05 - 1.10).
In addition, there is accumulating evidence that OSA is a risk factor for recurrent AF following cardioversion or ablation. In a meta-analysis, Ng et al. [ 39], looked at 3995 patients and found that overall, patients with OSA have a 25% greater risk of AF recurrence after catheter ablation than those without OSA (risk ratio 1.25, 95% conf dence interval 1.08 - 1.45, P = 0.003). In another meta-analysis[ 40] examining 3743 patients with AF found that patients with OSA had a 31% greater risk of AF recurrence after catheter ablation than did patients without OSA [relative ratio (RR) = 1.31, P = 0.00],and this risk increased by 57% in patients with OSA not undergoing CPAP therapy (RR = 1.57, P = 0.00).However, CPAP users had a risk of AF recurrence similar to that of patients without OSA (RR = 1.25,P = 0.37), and this similarity was maintained even after the removal of study heterogeneity (RR = 0.99,P = 0.39). Furthermore, based on data from another meta-analysis [ 41] it appears that the benef t of CPAP was stronger for younger, obese, and male patients. Despite this data, there is a paucity of examining the association between OSA and AF in women per se.

Figure 2 Proposed Mechanism of Atrial Fibrillation in OSA.
The proposed mechanisms by which OSA and AF are associated are poorly understood [ 42]. Figure 2 depicts the proposed mechanisms by which this interaction is mediated. Cardiac remodeling such as increases in left ventricular mass index and left atrial volume has been linked to the development of AF [ 43]. OSA involves repeated episodes of pharyngeal collapse (due to airway edema, diabetic neuropathy, obesity, etc.) during sleep that require the generation of lar ge intrathoracic pressures to overcome this obstruction meanwhile causing hypoxia and hypercapnia. These events are thought to be linked to cardiac structural changes possibly leading to the development of AF [ 43]. Supportive evidence for this mechanism was shown by the effect of continuous positive airway pressure(CPAP) therapy on signif cantly decreasing left ventricular mass index and left atrial volume [ 44].Another proposed mechanism is the activation of sympathetic and parasympathetic systems caused by hypoxia and hypercapnia leading to surges in systolic, diastolic blood pressures and as a result mean arterial pressure. This activation has been noted to precede AF occurrence in animal models [ 45]. On the molecular side, repeated hypoxic episodes and post-apneic re-oxygenation leads to oxidative stress on the myocardium and results in initiation of inf ammation and possibly cardiac remodeling [ 46] .
Impact of Treatment
While the exact relationship between OSA and development of AF remains unclear, there is enough evidence supporting treatment of OSA with CP AP therapy to prevent recurrence of AF and possibly occurrence. Naruse et al. showed that if OSA is not treated with CPAP in patients with AF, pharmacological and non-pharmacological interventions may be futile [ 47]. This has signif cant clinical and economic impact when considering the number of patients affected with AF and the rising prevalence of OSA.
A potential new therapy for OSA and AF is autonomic neuromodulation. The rationale for this treatment is based on the f nding that repeated apneic episodes cause sympathetic activation which increases the risk for development of AF. Targeting this activation may help lessen this risk especially in high risk patients in whom AF would be even more detrimental. Renal nerve denervation (RND)is one such proposed therapy [ 48]. However, more studies are needed to clarify the benef ts of RND and to specify the patient population that would most benef t from such an intervention.
OSA and Cardiovascular Events
Both experimental and population based studies suggest that OSA is a signif cant risk factor for cardiovascular disease. In one of the earliest studies reported, Marin et al. [ 49] found that moderate to severe OSA was associated with higher incidence of fatal and non-fatal cardiac events in male adults when compared to healthy subjects (untreated severe OSA signif cantly increased the risk of fatal(odds ratio 2.87, 95% CI 1.17 - 7.51) and non-fatal(3.17, 1.12 - 7.51) cardiovascular events) over a 10 year follow up. Data from the Sleep Heart Health Study [ 50] found that after adjustment for multiple risk factors, OSA was a signif cant predictor of incident coronary heart disease (myocardial infarction, revascularization procedure, or coronary heart disease death) only in men < or = 70 years of age(adjusted hazard ratio 1.10 [95% conf dence interval 1.00 - 1.21] per 10-unit increase in AHI, but not in older men or in women of any age. Another study published from the Sleep Heart Health cohort [ 51],found that the 10 year incidence of ischemic stroke was higher for men. However, the risk in women increased after AHI of 25/hour with a 10% increase for every 5 unit change in AHI. The major limitation of these data is that the median followup was 8.7 years, which may not be long enough to ascertain the effect of cumulative exposure to OSA.
These data are in contrast to other studies published. Longer term data (24 year follow-up) from the Wisconsin Sleep Cohort that allowed women to have longer exposure to OSA showed a progressive risk of developing incident coronary heart disease with increasing AHI after adjusting for confounders with a HR of 2.6 (1.41 - 6.1) for an AHI of > 30/hour[ 52]. Although not statistically signif cant, the data showed a trend for a higher association between SDB and incident coronary heart disease in women than in men. A robust association between OSA and cardiovascular events was also reported by Campos-Rodriguez et al. [ 53] who followed 1116 women for 6 years. They found that the adjusted hazard ratios for cardiovascular mortality were 3.50 (CI, 1.23 -9.98) for the untreated, severe OSA group and 1.60(CI, 0.52 - 4.90) for the untreated, mild to moderate OSA group.
One of the mechanisms by which some of these effects may be mediated was revealed in a subset analysis of the Sleep Heart Health Study looking at patients over a mean of 13.6 years [ 54]. They found that OSA severity was independently associated with high sensitivity troponin (hs TNT), a marker of subclinical myocardial ischemia. Furthermore,while men did have higher levels of hs TnT, there was no variation across OSA disease severity.However, in women, there was a linear relationship,with increasing levels noted with OSA severity, suggesting that women are more sensitive to the ef fects of OSA. When they did a mediation analyses, they found that the increased troponin level explained 20- 30% of the increased risk of CAD. A second important f nding was that late life left ventricular mass was independently associated with OSA in women, but not men. Overall, the hazard ratio for heart failure or cardiovascular death in this study was statistically higher in women at 1.26 (1.05 -1.50) compared to men at HR 1.12 (0.98 - 1.29) who have moderate-severe OSA.
There are a number of potential reasons for sex differences in OSA and cardiovascular events.First, due to the lower prevalence of OSA in women, more power is needed to detect differences. Second, there may be differences in cumulative exposures to OSA, as men generally develop OSA earlier in life compared to women. However,it should be stressed that OSA remains under-diganosed in women, especially those with moderate to severe OSA. Third, differences in OSA physiological stress (level of hypoxemia) that might play a role. Although women with OSA tend to have generally less hypoxemia than men, they are more likely to have REM-dominant OSA, the sleep state when sympathetic activity is highest and nocturnal ischemia may be greatest. Indeed women demonstrate a greater heart rate response associated with arousals compared to men. Additionally, women with OSA may display greater endothelial dysfunction than men with OSA.
Interestingly, OSA may also exacerbate pre-existing coronary artery disease [ 16]. In a prospective cohort study [ 55], OSA was detected in 51% of patients who had undergone percutaneous coronary intervention for acute coronary syndrome. During a mean follow up of 227 days, the incidence of major adverse cardiac events (cardiac death, reinfarction, target vessel revascularization) was higher among patients with OSA than those without OSA(adjusted hazard ratio [HR] 1 1.6, 95% CI 2.2 - 62.2).The study was not powered to detect sex d fiferences.
Impact of Treatment
Observational data suggest that treatment of OSA with positive airway pressure may reduce the incidence of cardiovascular events, including events related to coronary artery disease [ 49, 56, 57]. A limitation of these studies is the nonrandomized design and the possibility that those who were not adherent with CPAP represent a group at increased cardiovascular risk due to engaging in unhealthy lifestyle behaviors or nonadherence with other aspects of their medical care.
A multicenter randomized trial that followed patients without cardiovascular disease who had moderate to severe OSA and no daytime sleepiness were assigned to receive CP AP therapy or no active intervention for 4 years [ 58]. There was no signif cant difference in the rate of systemic hypertension or cardiovascular events (including nonfatal myocardial infarction, nonfatal stroke, transient ischemic attack, hospitalization for unstable angina or arrhythmia, or cardiovascular death) in patients treated with CP AP, compared with controls (incidence density ratio 0.83, 95% CI 0.63 - 1.1). A post-hoc analysis suggested that CPAP therapy did reduce hypertension and cardiovascular events in those patients who adhered to CPAP for at least four hours per night. In the SA VE trial [ 59] which followed over 2700 patients with moderate-severe OSA and established cardiovascular disease for a total of 3.7 years, CP AP was not associated with signif cant reduction in cardiovascular events.Although the rate of cardiovascular events was slightly improved in those adherent to CP AP (i.e.,≥ 4 hours per night), the benef t was not statistically signif cant. However, there are several limitations to this study, including the exclusion of patients with “ sleepy” OSA, patients at high risk of an accident and patients with severe hypoxemia, as well as use of automated oximetry data rather than polysomnography for diagnosis. Based on the data, it is not clear if sex differences exist with regards to the cardio protective effects of CPAP in OSA patients.
It is clear that further well designed randomized trials that follow patients for longer times are required to fully elucidate whether or not clinically meaningful cardiovascular benef t can be derived from CPAP in patients with OSA.
OSA and Congestive Heart Failure
Although this review article is predominately focused on OSA, central sleep apnea (CSA), frequently accompanies OSA in patients with heart failure. The type of central sleep apnea commonly encountered is Cheyne-Stokes breathing (CSR), a central apnea that is characterized by a crescendodecrescendo pattern. Most studies will combine the two types of apneas and it is coined as Sleep disordered breathing (SDB). It is commonly observed in patients with CHF, with a prevalence of about 50- 75% in those with a reduced ejection fraction and portends a worse prognosis in those who have both [ 60]. It is less common in patients preserved ejection fraction (HFpEF), although the data is minimal in comparison [ 61]. Cheyne-Stokes breathing is pathophysiologically distinct from OSA,and involves a complex interaction of increased afterload and a hypersensitive respiratory control center that leads to cyclical crescendo-decrescendo hypercapnia and hypocapnia. During sleep, CO2rises until an “ apnea threshold ” is reached, which stimulates spontaneous breathing. In heart failure,patients often hyperventilate due to pulmonary edema and congestion, resulting in hypocapnia[ 62 , 63] Consequently, CO2falls below the apnea threshold which results in periodic apneic events.Upper airway obstruction is also seen due to loss of airway tone at the end of an apneic event [ 64], thus creating overlap between CSA and OSA. Multiple observational studies have found that heart failure accompanied by SDB is associated with a worse prognosis than heart failure in the absence of SDB.Risk factors for CSA-CSB in patients with heart failure include male sex, advanced age, atrial f brillation, and hypocapnia [ 65]. Therefore, while SDB plays a key factor in CHF, for the purposes of this paper however, we will focus on the association of OSA and heart failure.
There is evidence that OSA may itself be a risk factor for incident heart [ 50, 52]. Patients from the Sleep Heart Health Study were followed prospectively for 8.7 years [ 50]. They found that OSA predicted incident heart failure in men but not in women (adjusted hazard ratio 1.13 [95% conf -dence interval 1.02 - 1.26] per 10-unit increase in AHI) and that men with AHI > or = 30 were 58%more likely to develop heart failure than those with AHI < 5/hour. However, there may have been an insuff cient sample size to detect a signif cant association as well as a short cumulative risk in women who tend to present later in life with OSA. As such,data from the Wisconsin Sleep Cohort studies that followed participants for 24 years are slightly different. As with the Sleep Heart Health Data, they showed that after adjusting for age, sex, body mass index, and smoking, estimated hazard ratios (95%conf dence interval) of incident CHD or HF were 1.5 (0.9 - 2.6) for AHI > 0 - 5, 1.9 (1.05 - 3.5) for AHI 5 ≤ 15, 1.8 (0.85 - 4.0) for AHI 15 ≤ 30, and 2.6 (1.1 - 6.1) for AHI > 30 compared to AHI = 0 (P trend = 0.02). However, their data showed a trend for a higher association between SDB and incident CHD in women than in men [ 52].
In addition to increased incident CHF, OSA may also worsen the outcomes of patients with pre-existing CHF. Wang et al. [ 66] found that over a mean followup of 2.9 years in patients with a reduced ejection fraction, the death rate was signif cantly greater in the patients who had untreated moder-ate-severe OSA after controlling for confounders.A more recent trial, called the OSA-CARE, found that among patients with acute cardiogenic pulmonary edema, 61% of patients had OSA and that there was signif cantly higher rates of recurrent pulmonary edema, myocardial infarctions and deaths in those with OSA compared to patients without OSA over a 1 year followup [ 67]. These studies were not powered to detect sex differences. According to the American College of Cardiology, white women have the highest proportion of heart failure with preserved ejection fraction (HFpEF), making up about 60% of hospitalizations for this heart condition. Cardiovascular disease remains the leading cause of morbidity and mortality in postmenopausal women. This is disproportionately higher in female minorities, especially African American and Hispanic women and clearly more studies are needed to further assess this association.
The mechanism by which OSA may lead to incident CHF and worsen pre-existing disease is not completely understood. However, there are several possible pathways that likely interact (see Figure 3 ).First, normally during non-REM sleep, sympathetic activity decreases and parasympathetic activity increases. This leads to decreases in blood pressure, heart rate, cardiac workload, and adrenergic activity [ 68]. However, during REM sleep, the brain has emotional responses to dreams which cause an increase in the indices just previously mentioned.The frequent jarring arousals interrupting sleep,hypercapnia, and hypoxemia from OSA leads to increased sympathetic and decreased parasympathetic responses, which cause increase in heart rate,blood pressure, afterload, and release of catecholamines [ 68]. Second, OSA is also strongly associated with hypertension, which if left untreated can lead to cardiac remodeling and subsequent progression to CHF. Finally, the large negative intrathoracic pressures generated due to obstruction during sleep further increases afterload, decreases cardiac output,and causes endothelial damage due to hypoxemia and oxidative free radicals [ 69]. This is theorized to promote development of coronary artery disease,ischemic cardiac remodeling, pulmonary congestion, and cardiac arrhythmias [ 70].
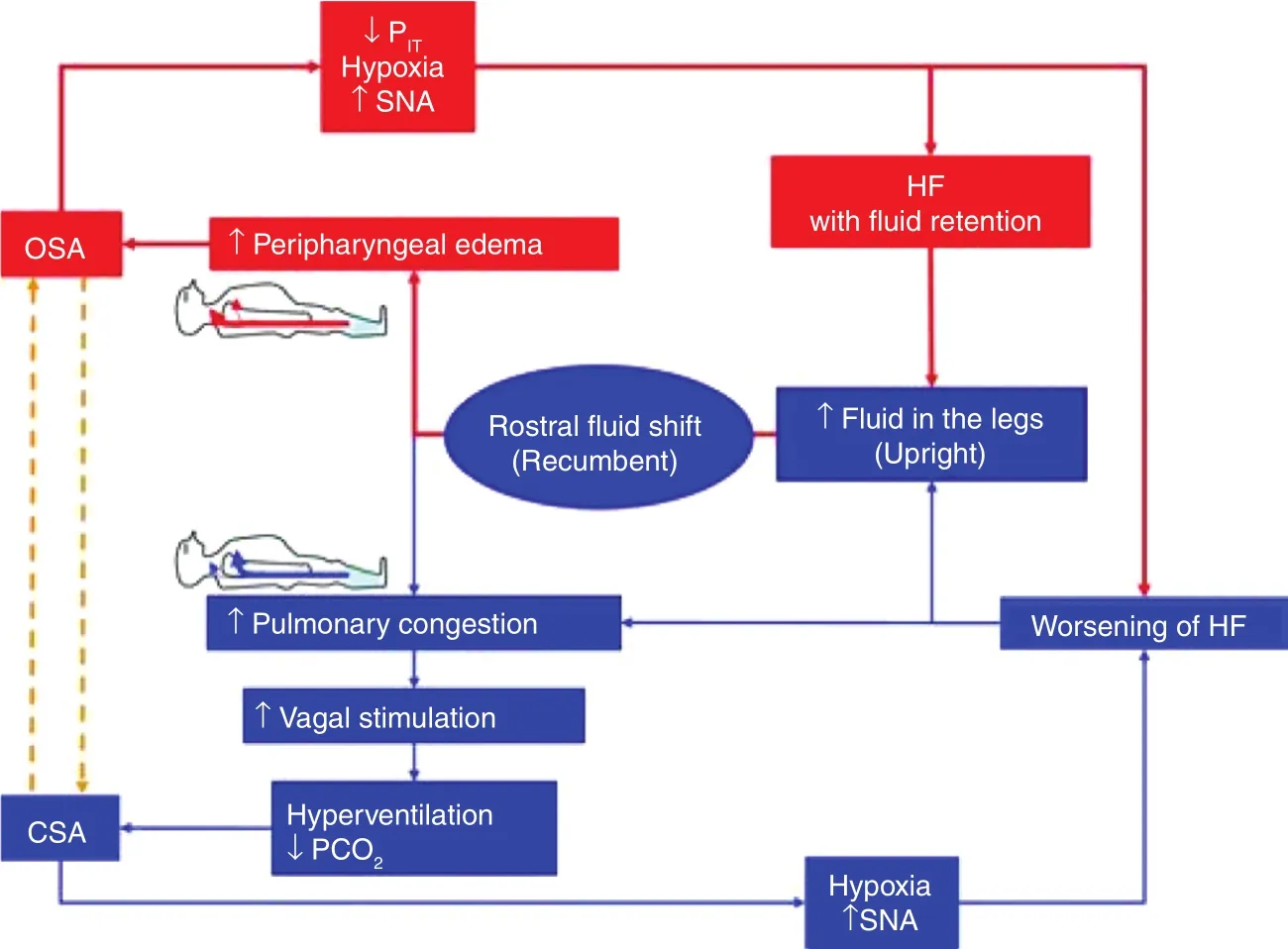
Figure 3 A Visual Example of the Proposed Bidirectional Relationship between Sleep Disordered Breathing and Heart Failure.
Interestingly, the relationship between OSA and heart failure appears to be bidirectional. Multiple observational studies have evaluated this phenomenon. Fluid accumulated in the lower extremities while upright can disperse to the neck vasculature causing engorgement and soft tissue edema when supine, which increases neck circumference, and leads to further obstruction of the airway during sleep [ 71]. The amount of f uid accumulated is directly proportional to the amount of time spent sitting and a sedentary lifestyle [ 72].
Impact of Treatment
The management of patients with combined OSA and HF is complex. Patients need cardiac optimization to treat their HF, in addition to treatment aimed at their OSA.
Since CSA is a direct consequence of CHF, treatment involves medically optimizing the underlying HF. However, most patients are on appropriate goal directed medical therapy by the time CSA is diagnosed. Studies have shown improvement in CSA in those who underwent cardiac resynchronization[ 64] and atrial overdrive pacing procedures [ 73, 74]which likely is a consequence of improved cardiac output [ 75]. Various medications have been studied, including benzodiazepines, theophylline, and acetazolamide; thus far none have had a statistically signif cant impact. No extensive studies have found any specif c treatment modalities that signif cantly improve patient outcomes.
Not surprisingly, morbidity and mortality is higher in these patients than in either diagnosis alone. This makes adequate treatment for both OSA and CHF of utmost importance, but management is complex.In OSA, positional therapy and positive pressure ventilation with CPAP or BiPAP help decrease the physical obstruction and improve outcomes. CPAP has been shown to improve ventricular function and help alleviate cardiac workload via reduction in sympathetic response, negative intrathoracic pressure swings, and improved ejection fraction [ 72, 76 ,77]. These results were more signif cant in patients with lower ejection fraction and higher apnea hypopnea index (AHI) prior to treatment with CPAP.Additionally, several observational studies have demonstrated that adequate treatment of sleep apnea leads to improved survival in heart failure patients.
Management is further complicated in women because their symptoms are usually more subtle,and are not reported as often in those with both OSA and CHF. One study evaluated this phenomenon, evaluating daytime muscle sympathetic nervous system activity and Epworth sleepiness scale.They showed that patients with lower Epworth sleepiness scales had higher levels of sympathetic activity during the daytime, likely explaining why this patient population was less likely to complain of hypersomnolence [ 77]. This may give the patient and practitioner a false sense of security that the OSA is being adequately treated. Additional studies are needed to further investigate this complex patient population. Data is exceptionally lacking for women.
There is some limited data that goal-directed medical therapy may not have much benef t for OSA in patients with stable heart failure [ 69]. Many medication classes used in HF like beta blockers, ACE inhibitors, ARBs, hydrochlorothiazide, furosemide,and spironolactone have been studied without signif cant benef t in OSA [ 78]. Additionally, multiple other medications for OSA management have been studied, however none were shown to be of statistically signif cant value. A study published in 2013 performed a meta-analysis of 30 trials investigating 25 medications, however it was concluded that none of the medications studied showed suff cient evidence to recommend their use [ 79]. CPAP remains the gold standard in the treatment of OSA,however other modalities like positional therapy devices, dental appliances, or surgical referral to an otolaryngologist or oral maxilla facial surgeon specialist should be pursued as salvage therapy in those who are unable to tolerate CPAP.
The public and practitioners have become increasingly aware of OSA and the detrimental ef fects of this disease. However, there are still a signif cant proportion of OSA cases that either go undiagnosed,or there are long delays in referral for polysomnography. This may be due to the increased diff culty identifying OSA in women. It is imperative that all practitioners, regardless of specialty, be hypervigilant in recognizing the classic and subtler signs of OSA in women, and be prompt in their referral for sleep study. Early referral for polysomnography should be considered in heart failure patients due to the higher prevalence of comorbid OSA in that population. Additionally, frequent counseling to adhere to CPAP treatment in OSA patients with comorbid heart failure is advisable to maximize compliance. Much of the data we have currently on OSA and CHF is in the male population. However, as both these diagnoses are vastly underdiagnosed in women, dedicated studies in the female population are needed to further understand these disease processes.
Conclusion
In conclusion, OSA is an important risk factor for various cardiovascular conditions and the risk appears to be bi-directional via complex mechanisms not yet well-understood but which implicate the sympathetic nervous system and inf ammatory cascade among others. In addition, important sex differences exist in the association of OSA and CV disease with some data suggesting women may have different susceptibility to the effects of OSA. While great strides have been made in trying to understand the pathophysiology and optimal treatment of OSA,more stringent longitudinal and randomized trials as well as more sex specif c research is needed to help close this knowledge gap.
Conflict of Interest
The authors declare no conf ict of interest.
 Cardiovascular Innovations and Applications2018年4期
Cardiovascular Innovations and Applications2018年4期
- Cardiovascular Innovations and Applications的其它文章
- Challenges in Cardiovascular Risk Prediction and Stratification in Women
- Nonobstructive Coronary Artery Disease in Women: Risk Factors and Noninvasive Diagnostic Assessment
- Antiplatelet Therapy Considerations in Women
- Novel Imaging Approaches for the Diagnosis of Stable Ischemic Heart Disease in Women
- Psychosocial Stress, the Unpredictability Schema, and Cardiovascular Disease in Women
- Heart Disease in Pregnancy: A Special Look at Peripartum Cardiomyopathy
