Novel Imaging Approaches for the Diagnosis of Stable Ischemic Heart Disease in Women
Viviany R. Taqueti, MD, MPH
1 Cardiovascular Imaging Program, Heart and Vascular Center, Division of Cardiology, Department of Medicine, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Brigham and Women’ s Hospital, Harvard Medical School, Boston, MA, USA
Abstract Conventional recommendations for diagnostic testing for the evaluation of stable ischemic heart disease in women have largely paralleled those in men. Although they are designed primarily for the identif cation of obstructive coronary artery disease (CAD), traditional approaches can lead to overtesting in women without differentiating who is truly at risk.Several unique factors related to the presentation, diagnosis, and underlying pathophysiology of stable ischemic heart disease in women necessitate a more specif c approach to the assessment of their risk, complete with separate guidelines when appropriate. This overview highlights how advanced noninvasive imaging tools, including cardiac computed tomography angiography, positron emission tomography, and cardiac magnetic resonance imaging, are enabling very sensitive assessments of anatomic atherosclerotic plaque burden, macrovessel- and microvessel-related ischemia, and myocardial f brosis, respectively. Moving forward, effective diagnostic testing will need to identify women at high risk of adverse cardiovascular events (not anatomically obstructive CAD per se) without overtesting those at low risk. Judicious application of novel imaging approaches will be critical to broadening the def nitions of CAD and ischemia to better ref ect the whole spectrum of pathological phenotypes in women, including nonobstructive CAD and coronary microvascular dysfunction, and aid in the development of needed evidence-based strategies for their management.
Keywords: coronary microvascular dysfunction ; nonobstructive coronary artery disease ; stable ischemic heart disease ;heart disease in women; cardiovascular imaging; positron emission tomography
Introduction
Conventional recommendations for diagnostic testing for the evaluation of heart disease in women have largely paralleled those in men. Based on an assumption that stable ischemic heart disease (SIHD) in women was the same as that in men, except occurring about a decade later, traditional ef forts focused on the identif cation of f ow-limiting, or obstructive,epicardial coronary artery disease (CAD) as the principal cause of symptoms such as chest pain and dyspnea, common in both women and men. There is now growing recognition that several unique factors related to the presentation, diagnosis, and underlying pathophysiology of SIHD in women necessitate a more rigorous and evidence-based approach to the assessment of their risk, complete with guidelines for sex-specif c management strategies when appropriate [ 1, 2]. Still, because most recommendations have derived predominantly from studies performed in men, the diagnostic utility and accuracy of testing in women has been limited at times by discrepancies between predicted and actual probabilities of obstructive CAD in these patients. Currently, few high-quality data exist regarding optimal diagnostic strategies for the assessment of symptomatic women to establish or exclude a diagnosis of SIHD.
Despite this, heart disease remains the leading cause of death in women worldwide [ 3], and important sex differences in the rates of diagnosis, utilization of care, response to therapy, and clinical outcomes have been described [ 4 - 7A ]l.t hough these partly ref ect that women outnumber men in elderly populations at greatest risk of heart disease, women often present with a higher burden of comorbidities and experience worse outcomes than men.Women relative to men have a higher prevalence of persistent angina, nonobstructive CAD, coronary microvascular dysfunction (CMD), spontaneous coronary artery dissection, stress-induced cardiomyopathy, and heart failure with preserved ejection fraction (HFpEF) [ 8 - 16C ]a. rdiovascular risk factors, including diabetes mellitus [ 17] and atrial f brillation [ 18], are associated with higher rates of vascular complications in women versus men, and women account for a disproportionate burden of inf ammatory diseases such as rheumatoid arthritis, which have also been linked to increased cardiovascular morbidity [ 19 , 20 ]. Women presenting with acute coronary syndromes experience higher mortality than men [ 21 - 24w ]i,t h lower use of cardiac device therapy [ 25 - 2d7e ] spite data showing increased benef t [ 28], and are referred for cardiac transplantation at later stages of heart failure [ 29].
As a result of a greater symptom burden and rate of functional disability in women, together with a lower prevalence of obstructive CAD as determined by coronary angiography, the evaluation of SIHD in women as compared with men can present unique challenges to clinicians. The accuracy of standard noninvasive diagnostic testing for ischemia, such as stress testing with exercise electrocardiography, echocardiography, or semiquantitative nuclear myocardial perfusion imaging, can differ signif cantly when evaluated against a gold standard of identifying anatomically obstructive CAD. This is an especially important consideration for women, whose symptoms are less likely to be explained by f ndings on coronary angiography and whose abnormal stress test results in the absence of obstructive CAD are more likely to be interpreted as “ false positives ” [ 1 , 2 ]. Despite this, women with angina and conf rmed ischemia have increased mortality associated with heart disease [ 30]. This overview highlights how the recent application of novel noninvasive clinical diagnostic tools, including cardiac computed tomography angiography (CCTA),positron emission tomography (PET), and cardiac magnetic resonance (CMR) imaging, is leading to a paradigm change in how heart disease is diagnosed[ 31, 32] by broadening the def nitions of CAD and ischemia, respectively, to better ref ect pathological phenotypes more prevalent in women.
Moving Beyond Diagnosis of Obstructive Coronary Artery Disease
Obstructive CAD, def ned anatomically on coronary angiography as luminal narrowing of 70% or greater in the major epicardial arteries (or 50% or greater in the left main artery), is neither necessary nor suff -cient to explain symptoms of SIHD [ 33, 34]. Indeed,women present more frequently than men with symptoms of angina [ 9], but are less likely to manifest obstructive CAD. In a contemporary cohort of 11,223 symptomatic patients (42% women) referred for nonurgent coronary angiography, one-third of men but two-thirds of women had no obstructive CAD, and these patients still experienced an elevated risk of major adverse cardiovascular events(MACEs) [ 10] ( Figure 1 ). Among patients with stable angina who are found to have obstructive CAD,sex differences also exist in the extent and sever-ity of disease, with women less likely than men to have obstructive multivessel disease [ 35, 36]. Sex differences in angiographic f ndings have been demonstrated not only in SIHD patients but also in patients presenting with acute coronary syndromes,including unstable angina and myocardial infarction [ 37 - 39]. In autopsy evaluations of patients who died of ischemic heart disease, women demonstrated less extensive and less obstructive CAD than men,despite pathological evidence of myocardial infarction [ 40], with more evidence of plaque erosion than plaque rupture [ 41]. Despite consistently documented lower angiographic disease burden and more often preserved left ventricular function in women with ischemic heart disease, women and men have similar adverse outcomes [ 42, 43].
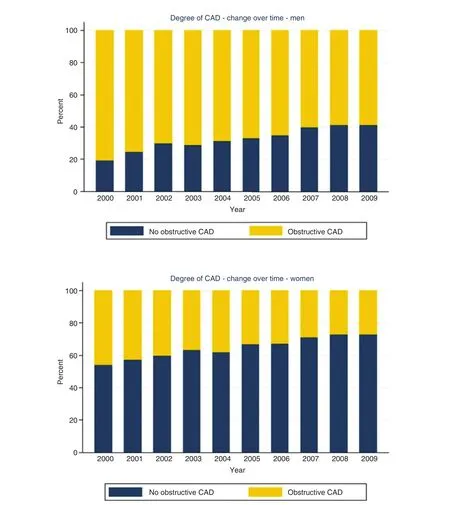
Figure 1 Degree of coronary artery disease (CAD) by sex and year of invasive coronary angiography examination.Reproduced with permission from [ 10 ].
This paradox may be at least partly explained by the f nding that a visually normal coronary angiogram does not necessarily indicate a normal coronary circulation. Beyond obstructive CAD, vascular dysfunction in the form of abnormal coronary reactivity often coexists with dif fuse, nonobstructive atherosclerotic plaques and CMD [ 12, 36,44 , 45 ]. Although invasive coronary angiography using visual assessments of epicardial coronary luminal patency remains a cornerstone of cardiovascular disease diagnosis, it has limited ability to identify dif fuse atherosclerosis and small-vessel dysfunction ( Figure 2 ). The addition of the use of invasive fractional f ow reserve, an assessment of the pressure drop across a focal epicardial stenosis,to coronary angiography has proven benef cial to identify lesion-specif c ischemia and guide revascularization [ 46], but may still underestimate the integrated contribution of diffuse atherosclerosis and small-vessel disease to myocardial ischemia[ 47, 48]. Nonobstructive CAD and CMD have been implicated in adverse cardiovascular outcomes,including acute coronary syndromes, heart failure and death from plaque erosion, and impaired vasoreactivity with ensuing myocardial ischemia[ 37 , 39 - 41A ]s. a result, testing for SIHD, especially in women, is now moving beyond testing for the presence or absence of obstructive epicardial CAD, which is only one of several possible contributors to myocardial ischemia. Over the last decade, the clinical integration of advanced diagnostic imaging tools is helping to redef ne ischemic heart disease and highlight the importance of nonobstructive CAD and CMD. Specif cally, noninvasive approaches using CCTA, PET, and CMRI have provided very sensitive assessments for the evaluation of anatomic atherosclerotic plaque, functional ischemia, and myocardial f brosis, respectively.
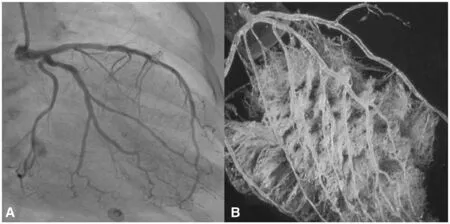
Figure 2 The coronary macrocirculation and microcirculation.
Detection of Atherosclerotic Plaque and Nonobstructive Coronary Artery Disease
Noninvasive CCTA has dramatically increased test sensitivity for the diagnosis of CAD and also enabled early characterization of plaque morphology.Recent observational studies have revealed stepwise incremental risk of adverse events in patients along a continuum of both severity (i.e., mild, moderate, or severe stenosis) and extent (i.e., number of involved segments or vessels) of atherosclerotic plaque [ 10,49 - 51A ]c. celerated by the growth of CCTA, mounting evidence now supports that (1) the presence of any atherosclerotic plaque, obstructive or not, portends increased risk of events and (2) the higher the overall plaque burden that is present, the higher the risk. From the Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry (CONFIRM), in which 23,854 consecutive patients without known CAD (33% asymptomatic)underwent CCT A between 2005 and 2009, per-patient nonobstructive and obstructive CAD conferred increased risk of death in a stepwise manner as compared with absence of CAD, which was associated with very low risk [ 51]. In a subsequent sexspecif c subgroup analysis of patients with no or nonobstructive CAD ( n = 18,158), nonobstructive CAD was associated with a modest adjusted risk of MACE s(composite of death and nonfatal myocardial infarction) that was similarly increased in both women and men [ 52]. Also, in a smaller subset of patients with longer follow-up ( n = 5632 followed up for 5 years,30% with nonobstructive CAD), there was no effect of sex on the association between per-vessel extent of obstructive CAD and MACEs ( Figure 3 ) , and the study authors concluded that exploratory analyses of atherosclerotic burden did not identify sex-specif c patterns predictive of MACEs [ 53]. Thus, women and men with comparable risk and extent of CAD had comparable prognosis. Nonetheless, prevalent patterns of disease do differ between women and men, with more symptomatic women than symptomatic men manifesting nonobstructive CAD rather than obstructive CAD [ 10, 36, 42], with important implications for diagnosis and management.
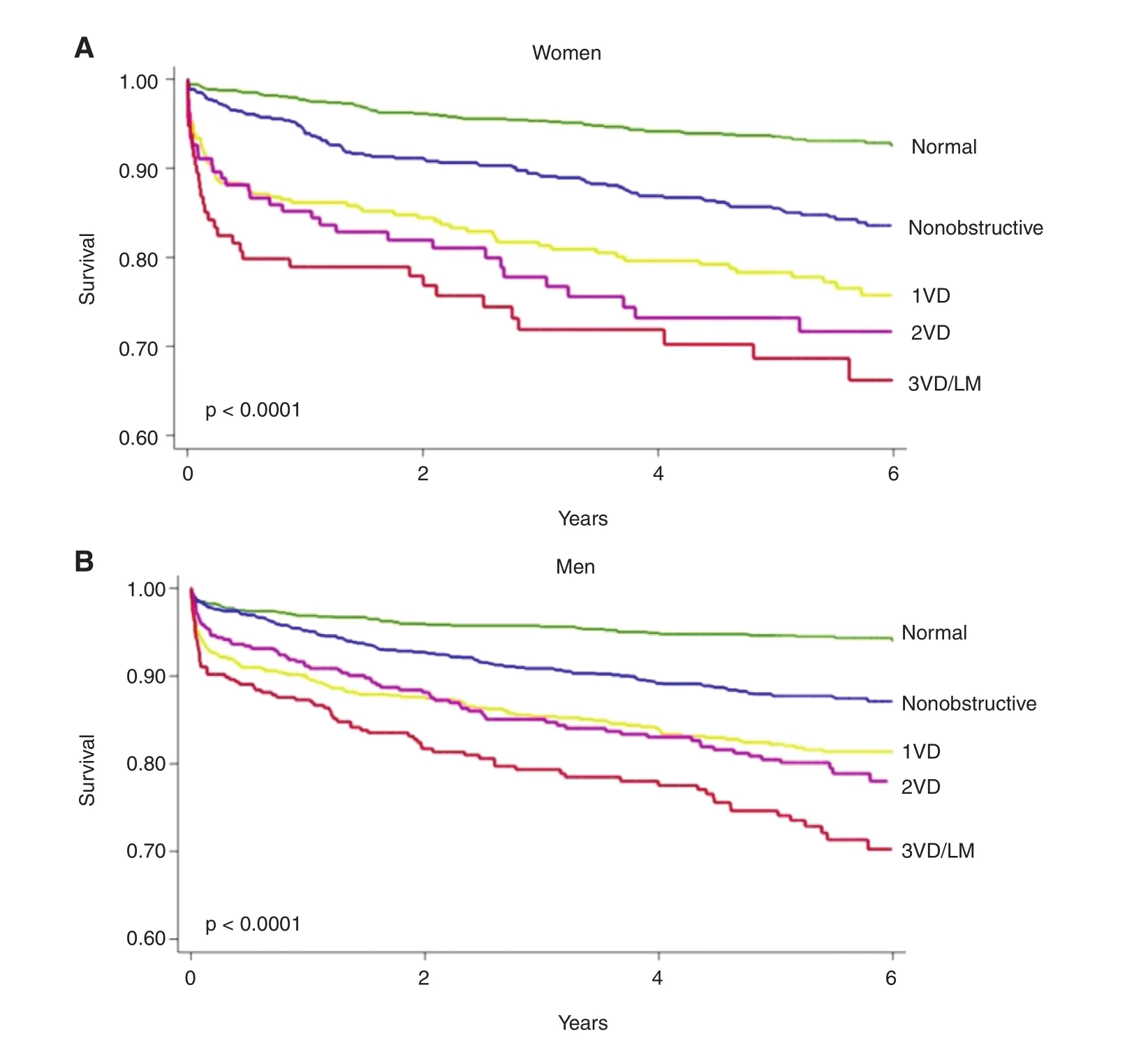
Figure 3 Unadjusted rate of major adverse cardiovascular events (death or myocardial infarction) by sex and coronaryartery disease (CAD) extent in a subset of patients from the Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry (CONFIRM) followed up for 5 years.
Among nonobstructive plaques, CCTA also allows characterization of high-risk plaque features,including positive remodeling, low CT attenuation,or napkin-ring sign consisting of ringlike peripheral higher CT attenuation with central low attenuation.In an observational substudy [ 54] of the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) including 4415 symptomatic outpatients (52% women) who underwent CCT A and were followed up for a median of 25 months for a composite of MACEs (death, myocardial infarction, and hospitalization for unstable angina), the presence of high-risk plaque in patients with nonobstructive CAD increased the adjusted risk of adverse events 1.6-fold, and high-risk plaque was more strongly associated with events in women(adjusted hazard ratio 2.41, 95% conf dence interval 1.25- 4.64) than in men (adjusted hazard ratio 1.40,95% conf dence interval 0.81 - 2.39). Nonetheless,the positive predictive value for MACEs of highrisk plaque detection in these patients was low (only 4.8% of patients with high-risk plaque experienced MACEs), limiting its current clinical applicability.
Diagnosing Coronary Microvascular Ischemia and Dysfunction

Figure 4 (A) Prevalence of coronary microvascular dysfunction, def ned by coronary f ow reserve (CFR) less than 2, in symptomatic women and men referred for cardiac positron emission tomography/computed tomography with normal myocardial perfusion. (B) Adjusted cumulative rate of major adverse cardiovascular events by sex and CFR in patients with normal myocardial perfusion. Adapted with permission from [ 11 ].
Neither conventional angiography nor CCT A can detect CMD, which is def ned not anatomically but functionally as a reduced coronary f ow reserve(CFR) in the absence of f ow-limiting CAD [ 45].Global CFR, calculated as the ratio of hyperemic to rest absolute myocardial blood f ow averaged over the left ventricle, is an integrated marker of coronary vasomotor dysfunction that measures the hemodynamic effects of focal, dif fuse, and smallvessel CAD on myocardial tissue perfusion 5[ 5, 56],and has emerged as an important prognostic imaging marker of cardiovascular risk. Observational data have shown that CFR measurements obtained with cardiac PET distinguish patients at low or high risk of MACEs, including cardiac death [ 57 - 60 ],beyond comprehensive clinical assessment, myocardial perfusion defects, left ventricular ejection fraction (LVEF), low-level troponin elevation [ 61],diastolic dysfunction [ 62], or plaque severity on invasive coronary angiography [ 63]. In parallel to f ndings with atherosclerotic plaque, evidence now supports that (1) the existence of impaired CFR,whether in the presence or in the absence of obstructive CAD, portends increased risk of cardiovascular events and (2) the more severely impaired the overall global CFR, the higher the risk. Whereas CFR of 2 or greater in patients without severe myocardial perfusion defects ef fectively excluded high-risk angiographic CAD and was associated with low rates of annualized cardiac death [ 64], event rates for those with CFR less than 2 increased exponentially as CFR decreased [ 59, 65, 66]. Because CFR is a measure of not only the effects of epicardial obstructive CAD but also of the effects diffuse atherosclerosis and CMD on myocardial tissue perfusion, worse prognosis in patients with CFR less than 2 may be related to coronary vasomotor dysfunction arising from a mix of pathophysiologic CAD phenotypes. As such, it may be particularly useful for diagnosis and prognosis of symptomatic women with suspected SIHD.
CMD often coexists with diffuse, nonobstructive atherosclerosis [ 44, 45, 63]. Similarly to CCTA f ndings for nonobstructive CAD, cardiac PET studies support that CMD is prevalent in symptomatic patients, affecting approximately 50% of patients who were found to have normal myocardial perfusion imaging and LVEF following referral for noninvasive evaluation ( Figure 4A )[ 1 1 ]. When present,CMD was associated with adverse cardiovascular events independently of sex ( Figure 4B ), but was more common in women, who accounted for twothirds of these patients. Although most patients had some atherosclerotic plaque, the results were consistent even in patients with a coronary artery calcium (CAC) score of 0, and global CFR, but not CAC score, provided signif cant incremental risk stratif cation over clinical risk score for prediction of MACEs. Thus, symptomatic patients who do not demonstrate regional ischemia associated with f ow-limiting CAD may have diffuse atherosclerosis and CMD for which a more sensitive, quantitative assessment of ischemia may better diagnose abnormalities and identify novel targets for systemic therapies. Although not a uniquely female disorder, this pattern of abnormalities may be more prognostically useful in women. Smaller epicardial coronary arteries in women [ 67 - 6c9o ] upled with differences in shear stress [ 70] and inf ammatory mediators over the lifespan may modify the development of CAD into a dif fuse pattern with more contribution from CMD than focal obstruction.Such an effect would be consistent with previously described clinical and pathological observations of sex differences in the presentation of SIHD.
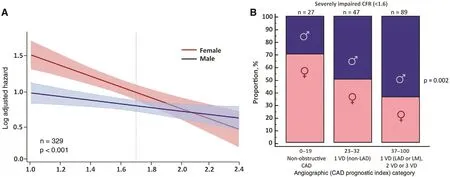
Figure 5 (A) Log adjusted hazard for major adverse cardiovascular events by sex and coronary f ow reserve (CFR). (B)Patients with severely impaired CFR ( < 1.6) by angiographic disease and sex categories. CAD, coronary artery disease; LAD,left anterior descending artery; LM, left main artery; 1VD, one-vessel disease; 2VD, two-vessel disease; 3VD, three-vessel disease. Adapted with permission from [ 36 ].
There is emer ging evidence that women with very low CFR may be at an especially elevated risk of adverse cardiovascular events ( Figure 5A ). In symptomatic patients referred for invasive coronary angiography after cardiac PET, women had a lower pretest probability of CAD and a lower burden of obstructive CAD relative to men, but were not protected from MACEs [ 36], consistent with previously described epidemiologic trends. Although outcomes for men were closely associated with the presence or absence of severely obstructive CAD, in women only those with impaired CFR demonstrated a signif cantly increased adjusted risk of MACEs.The excess cardiovascular risk observed in women relative to men referred for coronary angiography was independently associated with and mediated by impaired CFR, not obstructive CAD (P for interaction 0.04). Whereas most men with severely impaired CFR were found to have one-vessel,two-vessel, or three-vessel CAD on coronary angiography, most women with similarly impaired CFR demonstrated nonobstructive or one-vessel CAD( Figure 5B ). In adjusted analysis, approximately 40% of this observed dif ferential effect of sex on outcomes was mediated by CFR [ 36].
A very low CFR may represent a critical link to understanding the hidden biological risk of SIHD among women. When present, this abnormality is associated with increased cardiovascular risk in both women and men, but may constitute an especially malignant phenotype in a subset of severely af fected women [ 36], with important implications for their treatment. Data [ 63] support that revascularization,especially by coronary arterial bypass grafting, in individuals with severely impaired CFR may be benef cial. That the ef fects of sex dif ferences on outcomes of cardiovascular events are amplif ed in those with severely impaired CFR further suggests that certain patients (e.g., with very low CFR and less obstructive CAD, a phenotype more prevalent in women and less amenable to focal revascularization)may be at especially high risk F( igure 6 ) [ 36]. Instead of being interpreted as demonstrating a “ false positive” (or in some cases negative) traditional ischemic evaluation, patients with impaired CFR and less obstructive CAD may be at signif cantly increased CVD risk despite having access to revascularization, which is fundamentally tar geted at management of obstructive CAD. Thus, although providing optimal, equitable guideline-directed care remains a critical goal for treating women with SIHD, doing so according to current paradigms may be insuff cient to address what is likely a combination of biological and environmental determinants of their prognosis.Additional research is needed to determine precisely what is optimal care for this subset of vulnerable patients ( Figure 7 ). These f ndings may be especially relevant in women with risk factors including obesity [ 71], diabetes, and/or metabolic syndrome, and autoimmune inf ammatory disease, as well as those with HFpEF and ischemia with no obstructive CAD(INOCA) [ 72 ] .
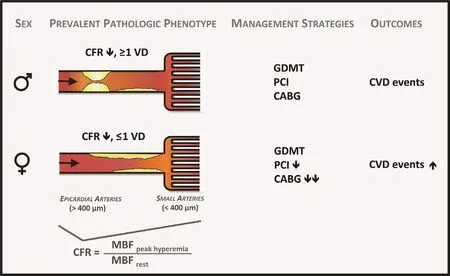
Figure 6 Model of prevalent pathological phenotypes in women and men with ischemic heart disease with possible impact on cardiovascular management strategies and outcomes.
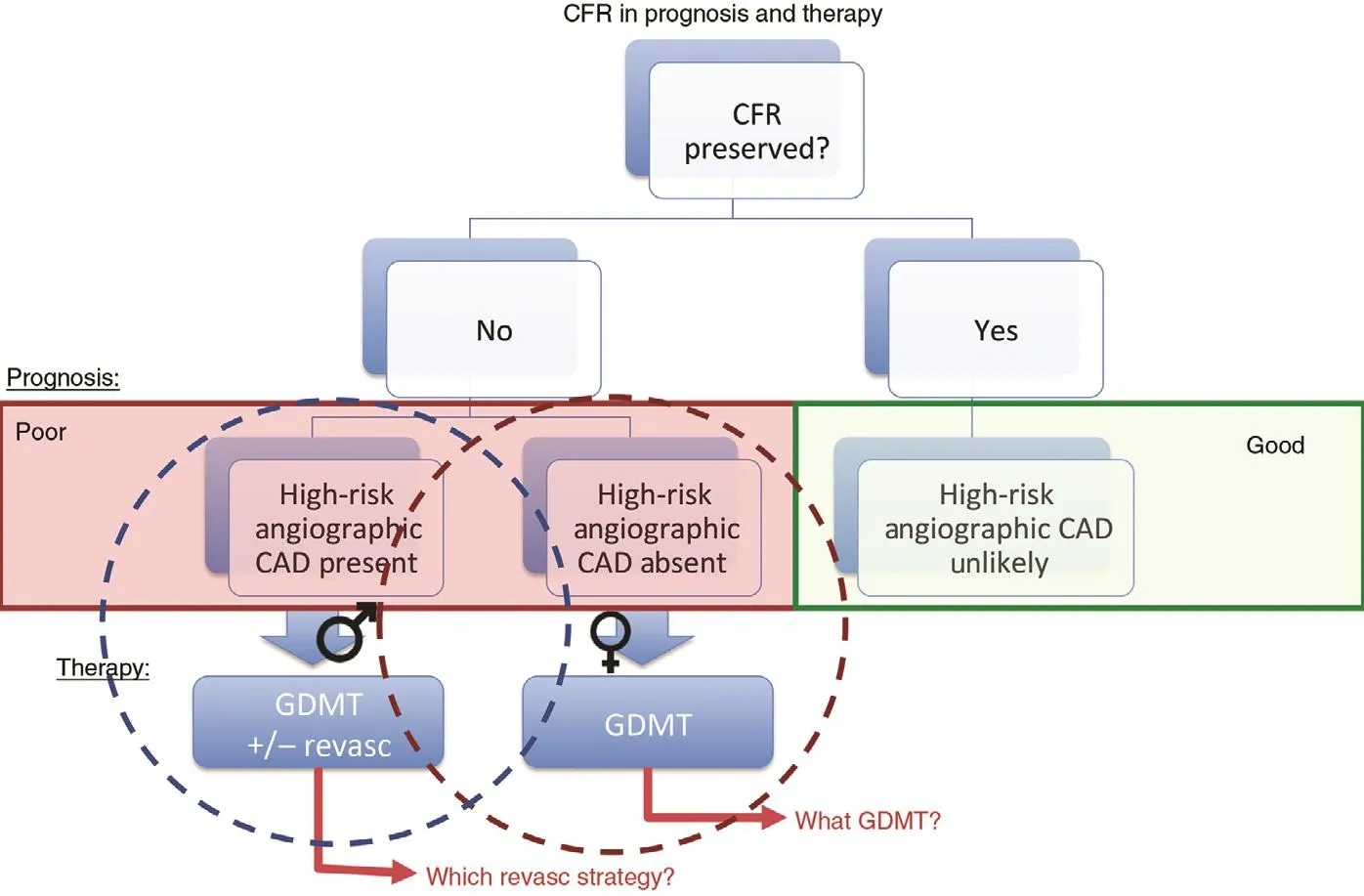
Figure 7 Conceptual summary of coronary f ow reserve (CFR) in prognosis of and therapy for patients with suspected ischemic heart disease.
Identification of Subclinical Myocardial Scar
In addition to cardiac PET, CMR has been used to evaluate selected patients for CMD [ 73, 74]. Its unique strength lies in its superior spatial resolution,allowing the comprehensive assessment of cardiovascular structure and function, including the sensitive quantif cation of myocardial scar. In myocardial infarction, necrotic myocardium undergoes replacement f brosis in which collagen f ber deposition results in expansion of the extracellular space. CMR techniques can be used to evaluate focal changes at the tissue level, including edema, microvascular obstruction, and the progression of replacement f brosis to fully developed regional scar using late gadolinium enhancement (LGE) [ 75]. Gadolinium,an extracellular contrast agent, demonstrates slow washout from areas of f brosis relative to normal myocardium, which allows visualization of subcentimeter lesions on delayed images on CMR, with distinction between subendocardium, midmyocardium, and subepicardium involvement. Among symptomatic women ( n = 341) with suspected ischemia but no obstructive CAD and preserved LVEF who underwent LGE CMR in the Women’ s Ischemia Syndrome Evaluation - Coronary Vascular Dysfunction (WISE-CVD) study, the prevalence of LGE was 8%, and one-third of affected women did not have a prior diagnosis of myocardial infarction despite most patients demonstrating a scar pattern typical of CAD [ 76]. An emerging technique for assessment of tissue remodeling by CMR involves T1 mapping with and without gadolinium to measure the extracellular volume fraction and native myocardial T1, respectively. Extracellular volume fraction has been correlated with diastolic dysfunction in HFpEF patients [ 77] and may be a biomarker of diffuse myocardial f brosis. Native T1 mapping has recently been proposed for diagnosis of myocardial ischemia with and without obstructive CAD[ 78]. In a recent pilot study involving participants in the WISE-CVD study, native T1 was signif cantly increased in symptomatic INOCA patients relative to controls, and was associated with reduced myocardial perfusion reserve index [ 79].
Putting It All Together: Patient Selection for Testing
The role of any imaging procedure, in women or men,is to provide information that ref nes the clinician ’ s decision-making process with the goal of relieving patient symptoms and bettering clinical outcomes. In the setting of chest pain or ischemic equivalent symptoms, the prevalence of CAD differs depending on patient characteristics, including not only sex but also age, coronary risk factors, and the nature of the presenting symptoms. Low-risk individuals are unlikely to benef t from the addition of imaging for the pur-poses of CAD diagnosis or risk stratif cation regardless of the imaging modality used [ 1, 80, 81]. This concept was illustrated in PROMISE, which randomized 10,003 symptomatic patients to a strategy of initial anatomic testing (using CCT A) or functional testing(using exercise electrocardiography, nuclear stress testing, or stress echocardiography) [ 82]. Although the approximately 3% event rate over a median of approximately 2 years of follow-up was lower than predicted by the study investigators, this f nding is less surprising when one considers that 78% of enrolled patients with angina had atypical symptoms, and in the CCTA arm, more than one-third of patients demonstrated a CAC score of 0 [ 83]. This has led some to question not whether an anatomic versus functional strategy was superior but whether any testing versus a deferred testing strategy would have been appropriate. Indeed,over the last two decades, we have witnessed falling diagnostic yields for noninvasive stress testing [ 84]and invasive coronary angiography [ 10, 85], ref ecting the referral of patients with lower risk and less prevalent obstructive CAD.
This phenomenon has been particularly problematic for women, for whom continued use of traditional risk tables based on decades-old approaches to estimate the pretest probability of obstructive CAD has led to systematic overestimation of risk [ 86].However, although an initial testing strategy is not recommended in low-risk women with stable chest pain, it has become unclear how to best def ne who is at low risk. The 2016 guidelines from the UK National Institute for Health and Care Excellence(NICE) [ 87], which favor f rst-line use of CCT A for the evaluation of patients with new-onset chest pain due to suspected CAD, have led to controversy about whether assessment of patient pretest probability remains a sound clinical strategy. In considering this question, however, one must recognize two major issues. The f rst is that the updated NICE recommendations apply only to patients without known CAD who present with certain characteristic anginal symptoms and a normal ECG, all of which does, in effect, still require consideration of a patient ’ s likelihood of having disease, and which still depends on accurate classif cation of sometimes vague symptoms. Secondly, in determining the accuracy of risk assessments, one must also consider pretest risk of what exactly ? As already discussed, a symptomatic patient’ s risk of obstructive CAD versus f ow-limiting CAD versus microvascular ischemia and CMD may be very dif ferent and cannot be assessed with a one-size-f ts-all approach. Although many lowrisk women may benef t from a deferred testing or no imaging strategy, some women may require additional specialized testing with advanced imaging techniques ( Figure 8 ).
The limited accuracy of conventional stress testing for the diagnosis of CMD and its increased prevalence in women underscore the importance and complementary value of assessing both the macrocirculation and the microcirculation in symptomatic women with suspected SIHD. In women with refractory angina, CMD may be the culprit. Identif cation of no CAD or nonobstructive CAD in these patients,particularly in the setting of documented ischemia,should trigger consideration of CMD, with referral for specialized testing, such as with PET, to interrogate coronary microvascular function [ 72, 88]. In the future, additional assessments of atherosclerotic plaque burden and diffuse myocardial f brosis using CCTA and CMRI, respectively, may also further ref ne risk assessment and help focus management strategies in affected women ( Figure 9 ) .
Conclusion
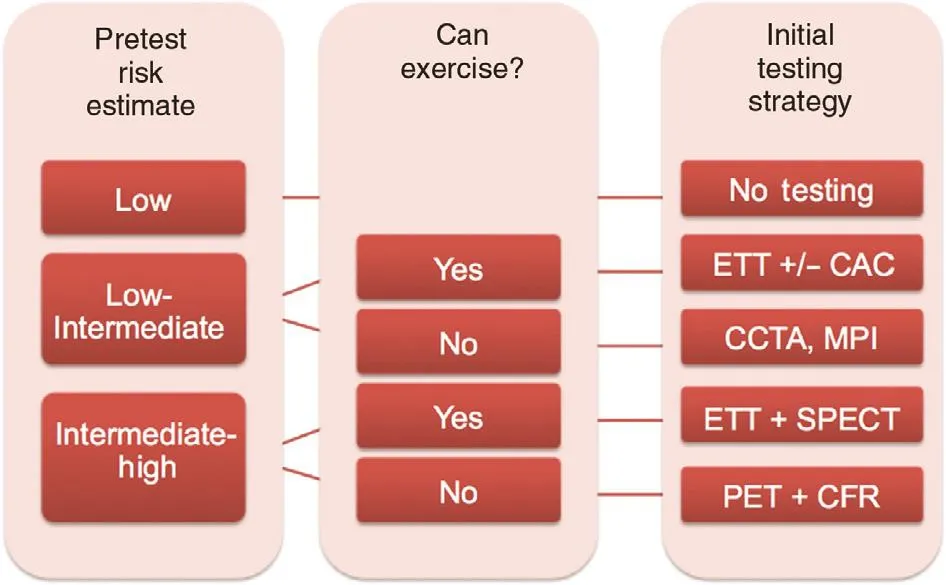
Figure 8 Proposed clinical algorithm for the preferred initial diagnostic evaluation of symptomatic women with suspected stable ischemic heart disease with or without obstructive coronary artery disease (CAD).
The evaluation of SIHD in women as compared with men can present unique challenges to clinicians. Designed primarily for the identif cation of obstructive CAD, traditional diagnostic testing approaches for suspected SIHD can lead to overtesting in women without dif ferentiating who is truly at risk. Moving forward, our collective goal must emphasize how to identify women at high risk of adverse cardiovascular events(not anatomically obstructive CAD per se) without overtesting those at low risk. More recently,the clinical integration of advanced diagnostic imaging tools such as CCT A, PET, and CMR is enabling very sensitive assessments of anatomic atherosclerotic plaque burden, macrovessel- and microvessel-related ischemia, and myocardial f brosis, respectively. Application of these novel technologies promises to broaden def nitions of CAD and ischemia to better ref ect the whole spectrum of pathological phenotypes in women,including nonobstructive CAD and CMD, and aid in the development of innovative and evidencebased strategies for their management.
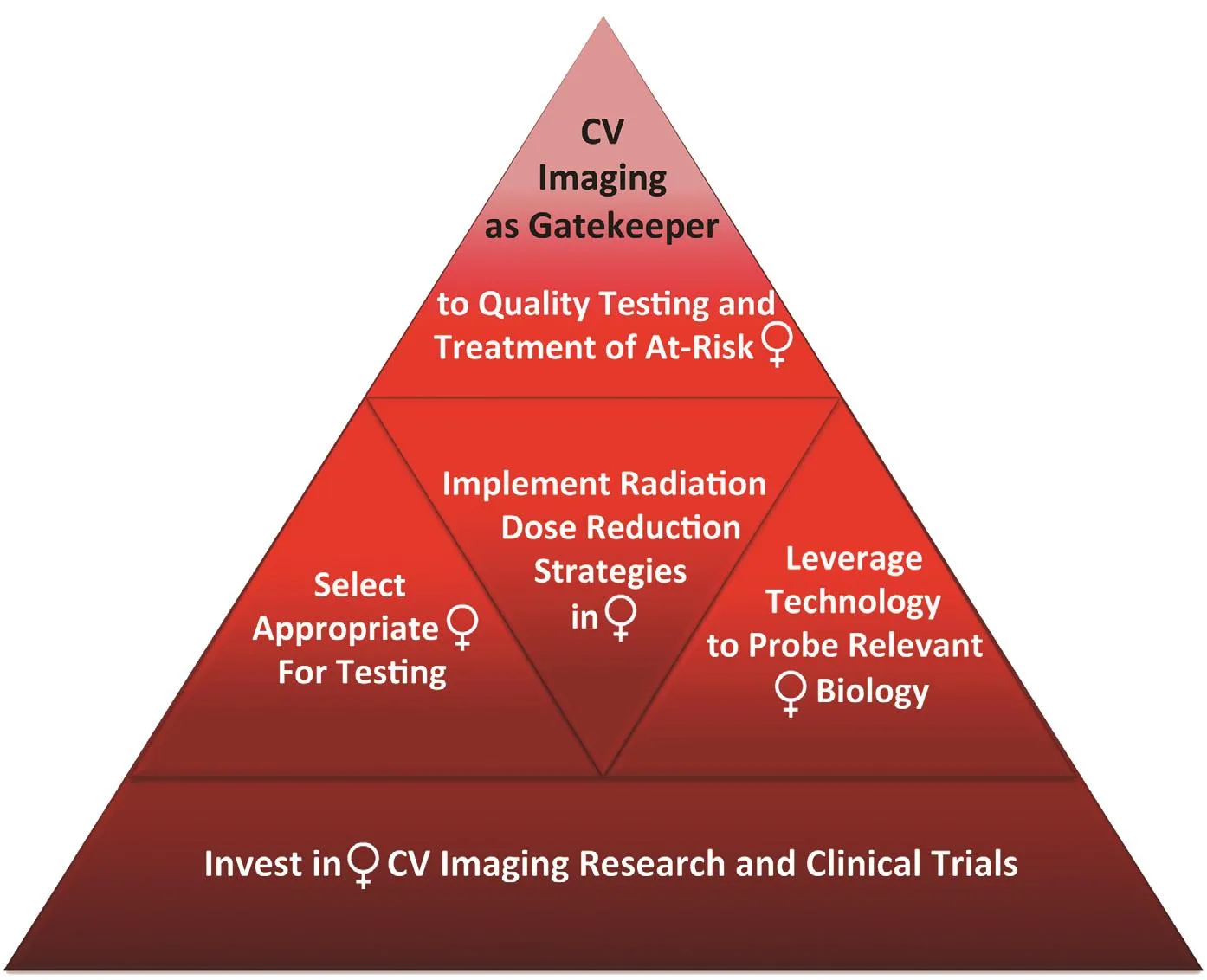
Figure 9 Top priorities for cardiovascular (CV) imaging in women.
Conflict of Interest
The author declares that she has no conf icts of interest.
 Cardiovascular Innovations and Applications2018年4期
Cardiovascular Innovations and Applications2018年4期
- Cardiovascular Innovations and Applications的其它文章
- Challenges in Cardiovascular Risk Prediction and Stratification in Women
- Nonobstructive Coronary Artery Disease in Women: Risk Factors and Noninvasive Diagnostic Assessment
- Antiplatelet Therapy Considerations in Women
- Psychosocial Stress, the Unpredictability Schema, and Cardiovascular Disease in Women
- Heart Disease in Pregnancy: A Special Look at Peripartum Cardiomyopathy
- Heart Failure with Preserved Ejection Fraction:Time to Revisit the Stiff Heart
