Nonobstructive Coronary Artery Disease in Women: Risk Factors and Noninvasive Diagnostic Assessment
Keith C. Ferdinand, MD and Rohan Samson, MD
1 Tulane University Heart and Vascular Institute, Tulane University School of Medicine, 1430 Tulane Avenue, SL-8548,New Orleans, LA 70112, USA
Abstract Sex-specif c differences in the epidemiology and pathophysiology of coronary artery disease and ischemic heardt i sease are now well recognized. Women with angina more often have nonobstructive coronary artery disease( NOCAD) compared with men. This patient population carries a signif cant risk of future cardiovascular events that is not commonly appreciated, often leading to delayed diagnosis and treatment. While coronary microvascular dysfunction plays a central role in the pathophysiology of NOCAD in women, other mechanisms of myocardial ischemia are now recognized.Risk factors such as hypertension and obesity disparately affect women and are likely to account for a signif cant proportion of NOCAD in the coming years. Vascular inf ammation is an important pathophysiologic pathway in NOCAD and is a potential therapeutic tar get. Coronary CT angiography provides a comprehensive assessment of coronary anatomy and plaque morphology and is a reasonable screening test of choice for NOCAD.
Keywords: nonobstructive coronary artery disease; hypertension; obesity; inf ammation
Introduction
Although the rates of coronary heart disease (CHD)have reduced in the past decade, it continues to contribute signif cantly to overall morbidity and mortality in women worldwide.
While it is true that rates of CHD have declined in both sexes, the rates in females aged 55 years or younger have not [ 1]. The prevalence of CHD in adult American women between 2011 and 2014 was 7.5 million (5.3%) [ 2]. In 2015, CHD contributed to nearly 150,000 deaths in women, accounting for 43% all CHD-related deaths [ 2]. The epidemiology and pathophysiology of coronary artery disease(CAD) in women are unique when compared with those in men because of a number of factors, some that are still not well understood and are the focus of intense current investigation. Women have a higher burden of traditional risk factors such as hypertension and obesity and a higher prevalence of vascular inf ammation and microvascular dysfunction relative to men. We will f rst discuss the def nition and pathophysiology of nonobstructive CAD (NOCAD)and sex differences in coronary anatomy. The impact of traditional risk factors - namely, hypertension and obesity - on the coronary circulation and the atherosclerotic process and thereby in women with ischemia and NOCAD will be reviewed. Thereafter,evidence supporting the role of inf ammation as a novel risk factor and therapeutic target will be addressed. Lastly, current diagnostic approaches to NOCAD will be discussed.
NOCAD: Definition and Pathophysiology
CAD has traditionally been assessed by visual estimation of stenoses during invasive coronary angiography (CAG). This visual estimate is based on lumenography with enhancement of the contour of a vessel with use of contrast media. Obstructive CAD as assessed by CAG is arbitrarily def ned as at least one epicardial stenosis greater than or equal to 50% [ 3]. However, there has been considerable variation in the def nition of obstructive CAD.Coronary stenosis in the range of 50 - 70% during CAG was previously labeled as being signif cant or obstructive [ 4 - 6T h]. is visual assessment of epicardial coronary stenosis during CAG is therefore inaccurate for the assessment of lesion severity.Functional assessment of angiographically inter-mediate coronary stenosis between 40 and 70% via fractional f ow reserve and instantaneous wave free ratio is currently used to assess the clinical signif -cance and guide revascularization [ 7, 8].
Epicardial coronary artery stenoses not signif cant enough to warrant revascularization are deemed nonobstructive. Obstructive CAD has been def ned as at least one stenosis greater than or equal to 20% but less than 50% [ 3]. The current treatment paradigm is to relieve obstructive disease, and thus NOCAD has received less attention and has been inherently felt to be associated with a benign prognosis, although this is increasingly recognized as inaccurate [ 3 ]. The term “ nonobstructive coronary artery disease” (NOCAD) is now used to represent a rather heterogeneous spectrum of CAD and coronary disease that occurs in the absence of f owlimiting stenosis that implicates the involvement of microvascular circulation, the endothelium, and anatomical and functional differences in the coronary vasculature. This was previously described as ischemic heart disease without obstructive CAD,open artery ischemic heart disease, myocardial infarction with no coronary artery obstruction,coronary microvascular dysfunction (CMD), and microvascular angina. The term “ cardiac syndrome X” is no longer used.
NOCAD is highly prevalent in women under going CAG to evaluate ischemic symptoms, and nearly 70% women but only 30% of men unde rgoing CAG to evaluate stable angina had NOCAD [ 9 -1 1].A female preponderance for NOCAD is surprising considering that symptomatic women usually present 10 - 15 years later than their male counterparts and have a higher prevalence of risk factors such as diabetes mellitus, hypertension, dyslipidemia, and tobacco use. Myocardial ischemia due to mechanisms other than f ow-limiting epicardial stenosis such as endothelial dysfunction and microvascular dysfunction is more frequently observed in women. CMD, def ned as epicardial, microvascular endothelial, or nonendothelial dysfunction that causes ischemia, is usually identif ed as reduced coronary f ow reserve (CFR) [ 12], and has come to play a central role in attempts to describe sex differences in ischemia. Coronary vasomotor abnormalities are known to portend poor cardiovascular outcomes, especially in women [ 13 - 17W ]. hile data on sex differences in the prevalence of CMD have been conf icting, CMD remains a key player in the evaluation of NOCAD [ 18, 19]. Contrary to previous reports of a signif cantly higher prevalence of CMD in women, Sara et al. [ 18] reported a clinically insignif cant sex dif ference in CMD prevalence. In patients with chest pain and NOCAD,coronary blood f ow and CFR were evaluated with acetylcholine and adenosine, respectively. While female sex was not signif cantly associated with CMD on multivariable analysis, a higher propor-tion of women was noted in patients with abnormal CMD. Recently, however, during CMD evaluation with acetylcholine provocation testing, a pathological f nding was noted in 70% of women compared with only 43% of men [ 19]. The acetylcholine dose needed to elicit an abnormal response was much lower in women than in men. Further, in a recent study, excess cardiovascular risk in women compared with men was attributed to severely impaired CFR, but not obstructive CAD [ 20]. Lack of standardized protocols for invasive CMD evaluation could account for the disparate f ndings noted above.
While our current understanding of the pathophysiology of NOCAD is limited, several novel pathophysiological mechanisms of myocardial ischemia have now been described (Figure 1) [ 21].Vascular causes include endothelial dysfunction,hypercoagulable states, inf ammatory disorders(lupus, polyarteritis, etc.), spontaneous coronary artery dissection, and inf ammation/raref cation of the coronary microcirculation, while nonvascular causes include disorders of the cardiomyocytes(transcellular, intracellular, and mitochondrial) and the adventitia. The clinical implications of these novel mechanisms of ischemia are presently unclear and need to be evaluated further.
Sex Differences in Coronary Anatomy
Coronary circulation is signif cantly dif ferent in women compared with men. In women the coronary macrovasculature and the coronary microvasculature are both smaller in diameter with stiffer vessels than in men [ 22, 23]. Smaller epicardial vessel luminal areas in women compered with men have been consistently demonstrated, with the exception of the left main coronary artery, which was not affected by sex in the Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) study [ 24]. The difference in epicardial coronary artery diameter persists even after adjustment for body size or left ventricular mass [ 25].
Women have increased coronary microvasculature tone and are particularly prone to endothelial and smooth muscle dysfunction. Impaired CFR in response to adenosine occurs more frequently in women than in men and predicts future cardiovascular events [ 26]. Evaluation of the retinal arterial caliber, an indirect marker of myocardial blood f ow,also suggests an increased CMD prevalence and increased cardiovascular events in women [ 27, 28].A smaller retinal arterial caliber (representing lower hyperemic myocardial blood f ow) predicts incident coronary events in women but not in men [ 27, 28].Further, vasospastic vascular disorders such as coronary vasospasms, Raynaud phenomenon, and vasculitis occur more frequently in women [ 29].
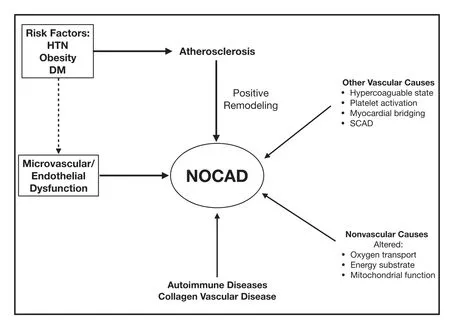
Figure 1 Mechanisms for Nonobstructive Coronary Artery Disease (NOCAD) in Women.
Sex-specif c characteristics and differences in the coronary atherosclerotic process are being increasingly recognized in women with stable and/or acute coronary syndromes. Dif ferences include the “ spread-out” nature of atherosclerotic plaque buildup in women rather than focal manifestations of discrete lesions as seen by the Women’ s Ischemia Syndrome Evaluation (WISE) investigators in women presenting with ischemic symptoms and who underwent CAG [ 17]. Similarly, the mechanisms of plaque disruption in women with acute co-r onary syndromes show a sex predilection for plaque erosion versus plaque rupture. Culprit lesions in men predominantly display plaque rupture, thin-cap f broatheroma, and a high plaque burden [ 30 - 32].In women, plaque erosion appears to be unrelated to the presence of thin-cap f broatheroma or plaque burden [ 33 - 36N ]e. arly 50% of women with stable ischemic heart disease have atherosclerotic plaque features associated with acute coronary syndromes[ 37, 38]. Patterns of coronary artery remodeling in response to atherosclerosis may also differ between men and women. Evidence from subanalyses of the WISE study suggests that positive or compensatory remodeling of coronary arteries is prevalent in women with NOCAD [ 39, 40]. Positive remodeling is a well-recognized feature of the vulnerable plaque phenotype; vulnerable plaques have a lar ger lipid content, a necrotic core, and macrophage inf ltration and are more frequently associated with unstable coronary syndromes compared with plaques with negative remodeling [ 41 - 43 ].
Role of Risk Factors
Hypertension: A Major Modifiable CAD Risk Factor in Women
Nearly one in three adult women has hypertension,and approximately 20% of all deaths in women are related to hypertension [ 2, 44]. Hypertension is a major contributor to cardiovascular disease in both men and women, with a higher burden of CAD, heart failure, stroke, atrial f brillation, and chronic kidney disease in women than in men[ 45]. Hypertension is a much stronger risk factor for CAD in women than in men, in part because of the association of aging and postmenopausal status with hypertension, particularly in women older than 65 years. Sex hormones (namely, estrogens) have a direct ef fect on the vascular pathophysiology,and regulate vascular reactivity, angiogenesis, and atherosclerosis [ 46]. Estrogens modulate endothelial function and vascular tone by increasing the activity of endothelium-derived vasodilator factors while simultaneously decreasing the activity of vasoconstrictor factors such as endothelin 1, angiotensin-converting enzyme, and angiotensin I receptor [ 47]. Further, estrogen withdrawal results in an increase in peripheral sympathetic neuronal activity and circulating levels of norepinephrine, causing elevated blood pressure [ 48, 49]. The cardiovascular benef ts of hormone replacement in clinical studies has been inconsistent, however, and require further investigation [ 50 - 52 ].
Ethnic differences are also well recognized among women. The burden of myocardial infarction and fatal CAD is higher for African-American women than for other race/ethnic groups [ 53]. Data from Heron [ 54] demonstrate higher CAD death rates among African Americans than among whites, with a greater disproportional rate in African-American women than in white women, such that not only the CAD death rates but also the life expectancy of African-American women more signif cantly ref ect values seen in white men versus white women[ 54]. Among African-American men, CAD prevalence was lower than in white men (7.2 vs. 7.8%);however, this is reversed in women (7.0 vs. 4.6%).Despite the lower prevalence, the rates of death from CAD remain higher in African Americans than in the white population.
In the latest landmark trial, the Systolic Blood Pressure Intervention Trial (SPRINT), the proportion of women represented was less than ideal in consideration that elderly women are the predominant population of persons with isolated systolic hypertension [ 55, 56]. Only 28% of the entire SPRINT cohort were aged 75 years or older, and the upper limit was 80 years, limiting the ability to apply the mortality and morbidity benef ts of the SPRINT data to women [ 56]. Furthermore, younger women, who may have hypertensive disorders related to pregnancy and systemic autoimmune diseases, were not represented in SPRINT.
Obesity
Over the past several decades there has been an alarming rise in the rates of obesity worldwide[ 57]. In the United States, data from the National Health and Nutrition Examination Survey indicate that women have a higher prevalence of obesity compared with men [ 58]. Among women, non-Hispanic African-American women had the highest rate of obesity (55%), followed by Hispanic women (50%) [ 59]. Obesity and overweight are major risk factors for CHD and its associated risk factors such as hypertension [ 2]. Since obesity is a well-established risk factor for CAD, it is expected that the rates of CAD (and possibly in women) will increase over the next few decades [ 60]. Obesityassociated comorbidities such as hypertension, diabetes, dyslipidemia, and metabolic syndrome are all independent risk factors for CAD. Correlation between obesity and CAD was established early on in the Framingham Heart Study and Nurses’ Health Study cohorts [ 61 - 63]. The risk of CAD in the Nurses’ Health Study increased nearly threefold as BMI increased from less than 21 kg/m2to more than 29 kg/m2. In the Pathological Determinants of Atherosclerosis in Youth study, it was noted that obesity in adolescents and young adults acceler-ates the progression of CAD decades before clinical manifestation of CAD, and that a high BMI correlates with more complex CAD lesions [ 64].Abdominal obesity or visceral fat signif cantly contributes to a chronic, low-grade systemic and vascular inf ammation through the production proinf ammatory adipokines (leptin and resistin) and cytokines (interleukin [IL]-6, tumor necrosis factor [TNF]- α) [ 65, 66]. Compared with BMI, waisthip ratio is a stronger predictor of cardiovascular events and atherosclerotic burden, highlighting the role of regional fat distribution in promoting atherosclerosis [ 67 - 69T ]. here is also growing evidence that adipose-derived proinf ammatory substances and epicardial adipose tissue alter coronary vasomotor function and contribute to CMD[ 69, 70]. Eroglu et al. [ 71] evaluated the association between serum adiponectin, an adiposederived protein hormone, and CFR in women with normal coronary arteries. Lower adiponectin levels were associated with impaired CFR in women with normal epicardial coronary arteries, suggesting that hypoadiponectinemia may promote CMD in women. Further, in a recent study, Dou et al.[ 72] demonstrated that aging and obesity increase the expression of a vascular endothelial metalloproteinase, ADAM17, which regulates soluble TNF- α release in adipose tissue and contributes to the development of remote CMD in obese patients aged 69 years or older.
Inflammation: A New Pathway to Decreasing Cardiovascular Disease
The inf ammatory response to the presence of atherosclerosis is considered a hallmark and potential leading pathway to the identif cation and treatment of CAD [ 73, 74]. Sex-based variation in immune responses to atherosclerosis are being increasingly recognized [ 75]. Immune cells within atherosclerotic plaque express sex steroid hormone receptors and are thereby regulated by sex hormones, in particular, by estrogen [ 76]. Estrogen inhibits TNF, IL-1, and IL-6 by downregulating nuclear factor κ B [ 77 ]. Estrogen is also reported to decrease the levels of oxidized low-density lipoproteins and increase apolipoprotein E level, thereby promoting an antiatherosclerotic phenotype in macrophage [ 77]. Altered immune responses in women are not limited only to atherosclerosis; women are known to develop an exaggerated systemic antibody and autoantibody response to immunological stress and are thus predisposed to autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis [ 75]. High cardiovascular mortality rates have been observed in women with autoimmune disease [ 75 , 78 - 8 0 ].
Sex-based immune responses could result in unique biomarkers that could be used to monitor endothelial dysfunction and inf ammation in women. Sex-specif c inf ammatory biomarkers may improve the predictive ability of cardiovascular risk models and traditional risk factors that typically underestimate the risk in women [ 81, 82].Inf ammatory biomarkers such as high-sensitivity C-reactive protein (CRP), f brinogen, IL-6, matrix metalloproteinase 9 and E-selectin and markers of hemostasis such as f brinogen, D-dimer, and plasminogen activator inhibitor 1 were found to improve cardiovascular risk prediction in postmenopausal women [ 83]. Combination of the CRP level with the Framingham risk score increased the accuracy of cardiovascular risk prediction in women [ 84]. Other biomarkers, such as high-sensitivity troponin, natriuretic peptides, and other lipid measures such as the levels of lipoprotein A and lipoprotein-associated phospholipase A2, have also been evaluated to improve cardiovascular risk prediction [ 81 , 82 ].
T argeting inf ammation unrelated to lipid level lowering or blood pressure reduction led to decreased cardiovascular events in the recently concluded Canakinumab Anti-inf ammatory Thrombosis Outcome Study (CANT OS) [ 85]. In stable patients with previous myocardial infarction and a persistent proinf ammatory response(def ned as high-sensitivity CRP level of 2 mg/L or more), IL-1 β inhibition with a monoclonal antibody(canakinumab) reduced the risk of the primary end point of nonfatal myocardial infarction, nonfatal stroke, and cardiovascular death by 15% compared with placebo. The CANTOS results suggest that other anti-inf ammatory pathways such as those that alter inhibit inf ammosome function or downstream IL-6 signaling may also serve as atheroprotection targets. The clinical utility of ta rgeting inf ammation in women with NOCAD remains to be elucidated.
Diagnosis
The vast majority of investigations in patients with NOCAD have used CAG to assess epicardial coronary anatomy and invasive coronary vasoreactivity testing to evaluate patients for CMD. Several protocols for coronary vasoreactivity testing have been described [ 86]. However, their use as reported in the literature remains inconsistent, often yielding conf icting results regarding the prevalence, extent,and cause of CMD. While invasive testing has a low complication rate [ 86], a serious complication such as a coronary dissection or myocardial infarction can occur. Some authors have questioned the effectiveness of an invasive strategy for detection of CMD, since its presence or absence does not alter treatment of these patients, even though its presence does offer an explanation for their symptoms [ 87].Noninvasive techniques now offer a comprehensive assessment of epicardial coronary anatomy and coronary microvasculature and may soon replace CAG as the gold standard.
While CAG is the diagnostic gold standard, it visualizes only the lumen. Quantifying the degree of epicardial coronary obstruction by only measurement of the reduction of the lumen diameter can be misleading. Coronary computed tomography angiography (CCTA) is less prone to error in assessment of lesion severity compared with the lumenogram obtained with CAG and does not expose the patient to complications from an invasive procedure. In stable chest pain patients with a low burden of obstructive disease, CCT A provides better prognostic information than functional stress testing (i.e., exercise treadmill testing, nuclear stress test, or stress echocardiography) [ 88]. Compared with men, women derive a greater prognostic benef t from CCTA than from noninvasive testing [ 89].CCTA with or without calcium scoring and physiological testing is now the screening test of choice for CAD [ 90]. Current criteria to assess the severity of epicardial stenosis by CCTA are listed in Table 1[ 91]. NOCAD diagnosed by CCTA is def ned as less than 50% luminal narrowing in at least one coronary artery segment [ 92]. CCTA can accurately detect CAD and coronary plaque when compared with intravascular ultrasonography [ 93 , 94 ]. Further,there is robust correlation between CAD diagnosed by CCTA and cardiovascular event rates. NOCAD diagnosed by CCTA was associated with an increase risk of death in the CONFIRM registry [ 95]. Other large CCTA registries demonstrate an escalating risk of death with more vessels or coronary segments with NOCAD [ 96 - 98I n] . addition to angiography,CCTA can also provide a thorough assessment of plaque morphology and burden [ 99]. High-risk features of coronary plaque such as positive remodeling, low attenuation, or napkin-ring sign can be detected on CCTA [ 43, 100]. In a lar ge contemporary cohort of outpatients with stable chest pain,Ferencik et al. [ 101] recently demonstrated a 70%increase in the risk of future major adverse cardiac events (MACEs) in patients with high-risk plaque on CCTA, independent of obstructive disease. In patients with NOCAD, the high-risk plaque doubled the risk of MACEs. Further, the presence of highrisk plaque conferred the strongest predictive value for MACEs among patients with NOCAD and those with lower atherosclerosis burden such as women and young patients.

Table 1 Guideline-Recommended Grades of Epicardial Coronary Artery Disease (CAD) Based on Qualitative and Quantitative Assessment of Lesion Severity [ 91].
Sex-based dif ferences in plaque morphology have also been observed with a qualitative plaque assessment on CCTA [ 102]. Nonobstructive plaque in women was associated with death in women,an association not observed in men [ 103]. In the CONFIRM registry, NOCAD conferred a nearly two times higher risk of adverse cardiovascular events in women [ 102]. Dynamic CT can now be used to evaluate the intramyocardial blood volume and the microcirculation as well [ 104]. Modern CT scanners are able to accurately di fferentiate between attenuation dif ferences between the endocardium and epicardium. Recently, Kuhl et al. [ 105] demonstrated that in patients with NOCAD, a worsening CT-derived myocardial perfusion reserve from epicardium to the endocardium was suggestive of CMD. Computational modeling techniques evaluating f ow of contrast medium in epicardial vessels are also being used to estimate regional myocardial perfusion [ 106, 107]. As technology improves in the coming years, a reduction in radiation exposure and artifacts may promote a broader clinical application of CCTA.
Magnetic resonance (MR) techniques have also progressed rapidly in the past decade [ 108].Dobutamine and vasodilator stress MRI have superior accuracy in identifying CAD compared with single-photon emission CT (SPECT) [ 109,110]. The diagnostic yield and prognostic value of stress MRI is well established in women [ 111, 112].Increased spatial resolution and diagnostic accuracy coupled with lack of radiation exposure and body habitus limitations makes stress MRI particularly suited to evaluate ischemia in women [ 113]. Apart from perfusion imaging, MRI is also an excellent tool for myocardial blood f ow assessment. Good correlation has been established between blood f ow measured by MRI and microsphere-derived myocardial blood f ow in animal models [ 114].Hays et al. [ 115] demonstrated abnormal endothelial function with MR-based coronary blood f ow measurements in a predominantly female population with NOCAD compared with controls.Metabolic imaging is also feasible by MR spectroscopy using phosphorous-31. In women with chest pain but no epicardial CAD, a subset of patients was found to have a decrease in phosphocreatine to adenosine triphosphate ratio on hand grip exercise[ 116]. Abnormal hand-grip test f ndings on phosphorous-31 MR spectroscopy predicted cardiovascular events during a 3-year follow-up period [ 117].Coronary MR angiography is currently not used to evaluate CAD.
Unlike CCT A, positron-emission tomography(PET) does not directly assess coronary anatomy;however, it is an excellent tool to assess myocar-dial perfusion. Attenuation correction with PET/CT scanning reduces breast tissue attenuation, signif -cantly increasing the diagnostic accuracy compared with SPECT. Stress perfusion with PET has a higher diagnostic and prognostic capability than SPECT[ 118, 119]. In addition to perfusion imaging, PET has been widely studied for quantif cation of myocardial blood f ow and CFR. However, because of inconsistent def nition of NOCAD across various studies,f ow impairment in the coronary microvasculature in the absence of epicardial CAD has never been clearly established. Geltman et al. [ 120] showed that approximately 50% of patients with chest pain and NOCAD have an abnormal CFR, highlighting the heterogeneity in the pathophysiology of NOCAD patients [ 120]. Elevated CRP levels were associated with reduced PET-derived CFR in NOCAD patients,underscoring the role of inf ammation in this patient population [ 121]. PET has also been used to monitor changes in CFR following therapy in CMD patients.Patients treated with a sodium channel inhibitor,ranolazine, for 1 month showed signif cant improvement in PET-derived CFR [ 122].
Conclusion
The burden of ischemic heart disease and CAD is the leading cause of death in women as well as men. NOCAD in women has unique pathobiology, necessitating novel diagnostic and therapeutic approaches, beyond conventional vascular inter-ventions for stenotic lesions. Hypertension, exacerbated by increasing rates of obesity, is a highly prevalent and potent risk factor for cardiovascular events, especially in women. Specif c characteristics of coronary circulation in women, compared with men, and the presentation of CAD with microvascular disease strongly support newer diagnostic technologies to unmask myocardial ischemia.Diagnostic approaches using CT, MRI and PET are rapidly developing. Clinicians will need to recognize the emerging concepts of NOCAD in women to appropriately diagnose and treat this previously underrecognized patient population.
Conflict of Interest
The authors declare that they have no conf icts of interest.
ThS
1. Safdar B, Spatz ES, Dreyer RP,Beltrame JF, Lichtman JH, Spertus JA, et al. Presentation, clinical prof le, and prognosis of young patients with myocardial infarction with nonobstructive coronary arteries (MINOCA): results from the VIRGO study. J Am Heart Assoc 2018;7(13):e009174.
2. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke statistics - 2018 update: a report from the American Heart Association. Circulation 2018;137(12):e67- 492.
3. Pepine CJ, Ferdinand KC, Shaw LJ, Light-McGroary KA, Shah RU, Gulati M, et al. Emergence of nonobstructive coronary artery disease: a woman ’ s problem and need for change in def nition on angiography. J Am Coll Cardiol 2015;66(17):1918- 33.
4. Boden WE, O ’ Rourke RA, Teo KK,Hartigan PM, Maron DJ, Kostuk WJ, et al. Optimal medical ther-apy with or without PCI for stable coronary disease. N Engl J Med 2007;356(15):1503- 16.
5. Maddox TM, Plomondon ME,Petrich M, Tsai TT, Gethof fer H,Noonan G, et al. A national clinical quality program for Veterans Affairs catheterization laboratories (from the Veterans Affairs clinical assessment, reporting, and tracking program). Am J Cardiol 2014;114(11):1750- 7.
6. Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM,Maron DJ, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease:a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology,Society for Cardiovascular Angiography and Interventions,Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol 2017;69(17):2212- 41.
7. Pijls NH, Fearon WF, Tonino PA,Siebert U, Ikeno F, Bornschein B, et al. Fractional f ow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study.J Am Coll Cardiol 2010;56(3):177 - 84 .
8. Davies JE, Sen S, Dehbi HM,Al-Lamee R, Petraco R, Nijjer SS, et al. Use of the instantaneous wave-free ratio or fractional f ow reserve in PCI. N Engl J Med 2017;376(19):1824- 34.
9. Jespersen L, Hvelplund A,Abildstr ø m SZ, Pedersen F, Galatius S, Madsen JK, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012;33(6):734- 44.
10. Sharaf BL, Pepine CJ, Kerensky RA, Reis SE, Reichek N, Rogers WJ, et al. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women ’ s Ischemia Syndrome Evaluation [WISE] study angiographic core laboratory). Am J Cardiol 2001;87(8):937- 41.
11. Shaw LJ, Shaw RE, Merz CN,Brindis RG, Klein L W, Nallamothu B, et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry.Circulation 2008;117(14):1787 - 801.
12. Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007;356(8):830- 40.
13. Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM,et al. Impaired myocardial f ow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol 2011;58(7):740- 8.
14. Murthy VL, Naya M, Foster CR,Hainer J, Gaber M, Di Carli G,et al. Improved cardiac risk assessment with noninvasive measures of coronary f ow reserve. Circulation 2011;124(20):2215- 24.
15. Fukushima K, Javadi MS, Higuchi T, Lautam ä ki R, Merrill J, Nekolla SG, et al. Prediction of short-term cardiovascular events using quantif cation of global myocardial f ow reserve in patients referred for clinical82Rb PET perfusion imaging. J Nucl Med 2011;52(5):726- 32.
16. Britten MB, Zeiher AM, Sch ä chinger V. Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular longterm outcome. Coron Artery Dis 2004;15(5):259 - 64 .
17. Pepine CJ, Anderson RD, Sharaf BL,Reis SE, Smith KM, Handberg EM,et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women ’ s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol 2010;55(25):2825- 32.
18. Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv 2015;8(11):1445- 53.
19. Aziz A, Hansen HS, Sechtem U,Prescott E, Ong P. Sex-related differences in vasomotor function in patients with angina and unobstructed coronary arteries. J Am Coll Cardiol 2017;70(19):2349 - 58 .
20. T aqueti VR, Shaw LJ, Cook NR,Murthy VL, Shah NR, Foster CR,et al. Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary f ow reserve, not obstructive disease. Circulation 2017;135(6):566 - 77 .
21. Pepine CJ. Multiple causes for ischemia without obstructive coronary artery disease: not a short list.Circulation 2015;131(12):1044- 6.
22. Pepine CJ, Kerensky RA, Lambert CR, Smith KM, von Mering GO,Sopko G, et al. Some thoughts on the vasculopathy of women with ischemic heart disease. J Am Coll Cardiol 2006;47(3 Suppl):S30- 5.
23. Jacobs AK. Coronary intervention in 2009: are women no different than men ? Circ Cardiovasc Interv 2009;2(1):69- 78.
24. Chandrasekhar J, Mehran R. Sexbased differences in acute coronary syndromes: insights from invasive and noninvasive coronary technologies. JACC Cardiovasc Imaging 2016;9(4):451- 64.
25. Hiteshi AK, Li D, Gao Y, Chen A,Flores F, Mao SS, et al. Gender differences in coronary artery diameter are not related to body habitus or left ventricular mass. Clin Cardiol 2014;37(10):605- 9.
26. Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease:results from the NHLBI WISE study.Am Heart J 2001;141(5):735- 41.
27. W ang L, Wong TY, Sharrett AR,Klein R, Folsom AR, Jerosch-Herold M. Relationship between retinal arteriolar narrowing and myocar-dial perfusion: multi-ethnic study of atherosclerosis. Hypertension 2008;51(1):119 - 26.
28. W ong TY, Klein R, Sharrett AR,Duncan BB, Couper DJ, Tielsch JM,et al. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA 2002;287(9):1153 - 9.
29. Stock EO, Redberg R. Cardiovascular disease in women. Curr Probl Cardiol 2012;37(11):450- 526.
30. Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, et al. In vivo diagnosis of plaque erosion and calcif ed nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol 2013;62(19):1748- 58.
31. Hong MK, Mintz GS, Lee CW, Lee BK, Yang TH, Kim YH, et al. The site of plaque rupture in native coronary arteries: a three-vessel intravascular ultrasound analysis. J Am Coll Cardiol 2005;46(2):261- 5.
32. V irmani R, Burke AP, Farb A,Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol 2006;47(8 Suppl):C13- 8.
33. Lafont A. Basic aspects of plaque vulnerability. Heart 2003;89(10):1262- 7.
34. Sheifer SE, Canos MR, Weinfurt KP, Arora UK, Mendelsohn FO,Gersh BJ, et al. Sex dif ferences in coronary artery size assessed by intravascular ultrasound. Am Heart J 2000;139(4):649- 53.
35. Han SH, Bae JH, Holmes DR, Lennon RJ, Eeckhout E, Barsness GW, et al.Sex differences in atheroma burden and endothelial function in patients with early coronary atherosclerosis.Eur Heart J 2008;29(11):1359- 69.
36. V accarino V. Ischemic heart disease in women: many questions,few facts. Circ Cardiovasc Qual Outcomes 2010;3(2):111 - 5.
37. Maehara A, Mintz GS, Bui AB,Walter OR, Castagna MT, Canos D, et al. Morphologic and angiographic features of coronary plaque rupture detected by intravascular ultrasound. J Am Coll Cardiol 2002;40(5):904- 10.
38. S á nchez-Elvira G, Coma-Canella I, Artaiz M, P á ramo JA, Barba J,Calabuig J. Characterization of coronary plaques with combined use of intravascular ultrasound, virtual histology and optical coherence tomography. Heart Int 2010;5(2):e12.
39. Khuddus MA, Pepine CJ, Handberg EM, Bairey Merz CN, Sopko G,Bavry AA, et al. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease:a substudy from the National Heart,Lung and Blood Institute-Sponsored Women ’ s Ischemia Syndrome Evaluation (WISE). J Interv Cardiol 2010;23(6):511 - 9.
40. Nicholls SJ, Tuzcu EM, Wolski K,Johnson BD, Sopko G, Sharaf BL,et al. Extent of coronary atherosclerosis and arterial remodelling in women: the NHLBI-sponsored Women ’ s Ischemia Syndrome Evaluation. Cardiovasc Diagn Ther 2018;8(4):405- 13.
41. Schoenhagen P, Ziada KM, Vince DG, Nissen SE, Tuzcu EM. Arterial remodeling and coronary artery disease: the concept of “ dilated ”versus “ obstructive ”c oronary atherosclerosis. J Am Coll Cardiol 2001;38(2):297- 306.
42. V arnava AM, Mills PG, Davies MJ. Relationship between coronary artery remodeling and plaque vulnerability. Circulation 2002;105(8):939- 43.
43. Puchner SB, Liu T, Mayrhofer T, Truong QA, Lee H, Fleg JL,et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of signif cant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol 2014;64(7):684- 92.
44. Murphy SL, Xu J, Kochanek KD,Curtin SC, Arias E. Deaths: f nal data for 2015. Natl Vital Stat Rep 2017;66(6):1- 75.
45. Krako ff LR, Gillespie RL, Ferdinand KC, Fer gus IV, Akinboboye O,Williams KA, et al. 2014 hyper-tension recommendations from the eighth joint national committee panel members raise concerns for elderly black and female populations. J Am Coll Cardiol 2014;64(4):394- 402.
46. Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev 2008;60(2):210- 41.
47. Miller VM, Mulvagh SL. Sex steroids and endothelial function:translating basic science to clinical practice. Trends Pharmacol Sci 2007;28(6):263- 70.
48. Liu CC, Kuo TB, Yang CC. Effects of estrogen on gender-related autonomic differences in humans.Am J Physiol Heart Circ Physiol 2003;285(5):H2188- 93.
49. V ongpatanasin W, Tuncel M,Mansour Y, Arbique D, Victor RG.Transdermal estrogen replacement therapy decreases sympathetic activity in postmenopausal women.Circulation 2001;103(24):2903- 8.
50. Rubanyi GM, Johns A, Kauser K.Effect of estrogen on endothelial function and angiogenesis. Vascul Pharmacol 2002;38(2):89- 98.
51. Alexander KP, Newby LK,Hellkamp AS, Harrington RA,Peterson ED, Kopecky S, et al.Initiation of hormone replacement therapy after acute myocardial infarction is associated with more cardiac events during follow-up. J Am Coll Cardiol 2001;38(1):1- 7.
52. Heckbert SR, Kaplan RC, Weiss NS, Psaty BM, Lin D, Furberg CD, et al. Risk of recurrent coronary events in relation to use and recent initiation of postmenopausal hormone therapy. Arch Intern Med 2001;161(14):1709- 13.
53. Leigh JA, Alvarez M, Rodriguez CJ. Ethnic minorities and coronary heart disease: an update and future directions. Curr Atheroscler Rep 2016;18(2):9.
54. Heron M. Deaths: leading causes for 2015. Natl Vital Stat Rep 2017;66(5):1- 76.
55. W right JT, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. A randomized trial of intensive versus standard bloodpressure control. N Engl J Med 2015;373(22):2103- 16.
56. W enger NK, Ferdinand KC, Bairey Merz CN, Walsh MN, Gulati M,Pepine CJ, et al. Women, hypertension, and the Systolic Blood Pressure Intervention Trial. Am J Med 2016;129(10):1030- 6.
57. Afshin A, Forouzanfar MH,Reitsma MB, Sur P, Estep K, Lee A,et al. Health ef fects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377(1):13 - 27.
58. Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL.Trends in obesity and severe obesity prevalence in US youth and adults by sex and age,2007 - 2008 to 2015 - 2016. JAMA 2018;319(16):1723 - 5 .
59. Hales CM, Carroll MD, Fryar CD,Ogden CL. Prevalence of Obesity among adults and youth: United States, 2015 - 2016. NCHS Data Brief 2017;(288):1- 8.
60. W ang YC, McPherson K, Marsh T, Gortmaker SL, Brown M.Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2011;378(9793):815- 25.
61. Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983;67(5):968- 77.
62. Manson JE, Colditz GA, Stampfer MJ, Willett WC, Rosner B, Monson RR, et al. A prospective study of obesity and risk of coronary heart disease in women. N Engl J Med 1990;322(13):882- 9.
63. Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ,Hankinson SE, et al. Body weight and mortality among women. N Engl J Med 1995;333(11):677- 85.
64. McGill HC, McMahan CA,Herderick EE, Zieske AW, Malcom GT, Tracy RE, et al. Obesity accelerates the progression of coronary atherosclerosis in young men.Circulation 2002;105(23):2712- 8.
65. W ang Z, Nakayama T. Inf ammation,a link between obesity and cardiovascular disease. Mediators Inf amm 2010;2010:535918.
66. Selthofer-Relati K, Bo š njakI , Kibel A. Obesity related coronary microvascular dysfunction: from basic to clinical practice. Cardiol Res Pract 2016;2016:8173816.
67. Y usuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F,et al. Ef fect of potentially modif able risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364(9438):937- 52.
68. Despr é s JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation 2012;126(10):1301- 13.
69. See R, Abdullah SM, McGuire DK,Khera A, Patel MJ, Lindsey JB,et al. The association of dif fering measures of overweight and obesity with prevalent atherosclerosis:the Dallas Heart Study. J Am Coll Cardiol 2007;50(8):752- 9.
70. Bagi Z, Broskova Z, Feher A.Obesity and coronary microvascular disease - implications for adipose tissue-mediated remote inf ammatory response. Curr Vasc Pharmacol 2014;12(3):453- 61.
71. Eroglu S, Sade LE, Bozbas H,Haberal A, Ozbicer S, Demir O,et al. Association of serum adiponectin levels and coronary f ow reserve in women with normal coronary angiography. Eur J Cardiovasc Prev Rehabil 2009;16(3):290- 6.
72. Dou H, Feher A, Davila AC,Romero MJ, Patel VS, Kamath VM, et al. Role of adipose tissue endothelial ADAM17 in age-related coronary microvascular dysfunction. Arterioscler Thromb Vasc Biol 2017;37(6):1180 - 93.
73. Libby P. Inf ammation in atherosclerosis. Arterioscler Thromb Vasc Biol 2012;32(9):2045- 51.
74. Golia E, Limongelli G, Natale F,Fimiani F, Maddaloni V, Pariggiano I, et al. Inf ammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr Atheroscler Rep 2014;16(9):435.
75. Whitacre CC. Sex differences in autoimmune disease. Nat Immunol 2001;2(9):777- 80.
76. Gilliver SC. Sex steroids as inf ammatory regulators. J Steroid Biochem Mol Biol 2010;120(2- 3):105 - 15.
77. Fairweather D. Sex differences in inf ammation during atherosclerosis. Clin Med Insights Cardiol 2014;8(Suppl 3):49- 59.
78. Jacobson DL, Gange SJ, Rose NR,Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol 1997;84(3):223- 43.
79. Gleicher N, Barad DH. Gender as risk factor for autoimmune diseases.J Autoimmun 2007;28(1):1 - 6.
80. Mosca L, Benjamin EJ, Berra K,Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectivenessbased guidelines for the prevention of cardiovascular disease in women- 201 1 update: a guideline from the American Heart Association.Circulation 2011;123(11):1243- 62.
81. Com ş a HI, Zdrenghea D, Man SC,Pop D. The role of novel atherosclerosis markers in peripheral artery disease: is there a gender di fference?Cardiovasc J Afr 2018;29:1- 10.
82. Paynter NP, Everett BM, Cook NR.Cardiovascular disease risk prediction in women: is there a role for novel biomarkers ? Clin Chem 2014;60(1):88- 97.
83. Kim HC, Greenland P, Rossouw JE,Manson JE, Cochrane BB, Lasser NL, et al. Multimarker prediction of coronary heart disease risk: the Women’ s Health Initiative. J Am Coll Cardiol 2010;55(19):2080- 91.
84. Cook NR, Buring JE, Ridker PM.The effect of including C-reactive protein in cardiovascular risk prediction models for women. Ann Intern Med 2006;145(1):21- 9.
85. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH,Ballantyne C, et al. Antiinf ammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377(12):1119 - 31.
86. Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease(INOCA): developing evidencebased therapies and research agenda for the next decade. Circulation 2017;135(11):1075- 92.
87. Bailey AL, Smyth SS. Invasive coronary vasoreactivity testing to diagnose microvascular dysfunction in women. JACC Cardiovasc Interv 2012;5(6):654- 5.
88. Ho ffmann U, Ferencik M, Udelson JE, Picard MH, Truong QA, Patel MR, et al. Prognostic value of noninvasive cardiovascular testing in patients with stable chest pain:insights from the PROMISE trial(Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017;135(24):2320 - 32 .
89. Pagidipati NJ, Hemal K, Coles A, Mark DB, Dolor RJ, Pellikka PA, et al. Sex dif ferences in functional and ct angiography testing in patients with suspected coronary artery disease. J Am Coll Cardiol 2016;67(22):2607- 16.
90. Moss AJ, Williams MC, Newby DE, Nicol ED. The updated NICE guidelines: cardiac CT as the f rstline test for coronary artery disease. Curr Cardiovasc Imaging Rep 2017;10(5):15.
91. Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, Mancini GJ,et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2014;8(5):342 - 58.
92. Emami H, Takx RAP, Mayrhofer T, Janjua S, Park J, Pursnani A,et al. Nonobstructive coronary artery disease by coronary CT angiography improves risk stratif cation and allocation of statin therapy. JACC Cardiovasc Imaging 2017;10(9):1031- 8.
93. Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H,Gottlieb I, et al. Diagnostic per-formance of coronary angiography by 64-row CT. N Engl J Med 2008;359(22):2324- 36.
94. Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, et al.Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY(Assessment by Coronary Computed Tomographic Angiography of Individuals Under going Invasive Coronary Angiography) trial. J Am Coll Cardiol 2008;52(21):1724 - 32 .
95. Min JK, Dunning A, Lin FY,Achenbach S, Al-Mallah M, Budoff MJ, et al. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography f ndings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry)of 23,854 patients without known coronary artery disease. J Am Coll Cardiol 2011;58(8):849- 60.
96. Lin FY, Shaw LJ, Dunning AM,Labounty TM, Choi JH, Weinsaft JW, et al. Mortality risk in symptomatic patients with nonobstructive coronary artery disease: a prospective 2-center study of 2,583 patients under going 64-detector row coronary computed tomographic angiography. J Am Coll Cardiol 2011;58(5):510- 9.
97. Ostrom MP, Gopal A, Ahmadi N,Nasir K, Yang E, Kakadiaris I,et al. Mortality incidence and the severity of coronary atherosclerosis assessed by computed tomography angiography. J Am Coll Cardiol 2008;52(16):1335- 43.
98. Pundziute G, Schuijf JD, Jukema JW, Boersma E, de Roos A, van der Wall EE, et al. Prognostic value of multislice computed tomography coronary angiography in patients with known or suspected coronary artery disease. J Am Coll Cardiol 2007;49(1):62- 70.
99. Voros S, Rinehart S, Qian Z, Joshi P, Vazquez G, Fischer C, et al.Coronary atherosclerosis imaging by coronary CT angiography:current status, correlation with intravascular interrogation and meta-analysis. JACC Cardiovasc Imaging 2011;4(5):537- 48.
100. Motoyama S, Ito H, Sarai M,Kondo T, Kawai H, Nagahara Y,et al. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in midterm follow-up. J Am Coll Cardiol 2015;66(4):337- 46.
101. Ferencik M, Mayrhofer T, Bittner DO, Emami H, Puchner SB, Lu MT, et al. Use of high-risk coronary atherosclerotic plaque detection for risk stratif cation of patients with stable chest pain: a secondary analysis of the PROMISE randomized clinical trial. JAMA Cardiol 2018;3(2):144- 52.
102. Leipsic J, Taylor CM, Gransar H,Shaw LJ, Ahmadi A, Thompson A, et al. Sex-based prognostic implications of nonobstructive coronary artery disease: results from the international multicenter CONFIRM study. Radiology 2014;273(2):393- 400.
103. Shaw LJ, Min JK, Narula J, Lin F,Bairey-Merz CN, Callister TQ, et al.Sex differences in mortality associated with computed tomographic angiographic measurements of obstructive and nonobstructive coronary artery disease: an exploratory analysis. Circ Cardiovasc Imaging 2010;3(4):473 - 81 .
104. Feher A, Sinusas AJ. Quantitative assessment of coronary microvascular function: dynamic single-photon emission computed tomography,positron emission tomography, ultrasound, computed tomography, and magnetic resonance imaging. Circ Cardiovasc Imaging 2017;10(8).pii: e006427. doi:10.1161/CIRCIMAGING.117.006427.
105. K ü hlJ T, George RT, Mehra VC,Linde JJ, Chen M, Arai AE, et al.Endocardial-epicardial distribution of myocardial perfusion reserve assessed by multidetector computed tomography in symptomatic patients without signif cant coronary artery disease: insights from the CORE320 multicentre study.Eur Heart J Cardiovasc Imaging 2016;17(7):779- 87.
106. Nakazato R, Park HB, Berman DS, Gransar H, Koo BK, Erglis A, et al. Noninvasive fractional f ow reserve derived from computed tomography angiography for coronary lesions of intermediate stenosis severity: results from the DeFACTO study. Circ Cardiovasc Imaging 2013;6(6):881- 9.
107. Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, Kim HS, et al.Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional f ow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study.J Am Coll Cardiol 2011;58(19):1989- 97.
108. Lin K, Carr JC. MR imaging of the coronary vasculature: imaging the lumen, wall, and beyond. Radiol Clin North Am 2015;53(2):345- 53.
109. G reenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, Everett CC, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease(CE-MARC): a prospective trial.Lancet 2012;379(9814):453- 60.
110. Schwitter J, Wacker CM, Wilke N, Al-Saadi N, Sauer E, Huettle K, et al. Superior diagnostic per-formance of perfusion-cardiovascular magnetic resonance versus SPECT to detect coronary artery disease: The secondary endpoints of the multicenter multivendor MR-IMP ACT II (Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary Artery Disease Trial). J Cardiovasc Magn Reson 2012;14:61.
111. Klem I, Greulich S, Heitner JF,Kim H, Vogelsberg H, Kispert EM, et al. Value of cardiovascular magnetic resonance stress perfusion testing for the detection of coronary artery disease in women.JACC Cardiovasc Imaging 2008;1(4):436- 45.
112. Coelho-Filho OR, Seabra LF,Mongeon FP, Abdullah SM, Francis SA, Blankstein R, et al. Stress myocardial perfusion imaging by CMR provides strong prognostic value to cardiac events regardless of patient ’ s sex. JACC Cardiovasc Imaging 2011;4(8):850- 61.
113. Baldassarre LA, Raman SV, Min JK, Mieres JH, Gulati M, Wenger NK, et al. Noninvasive imaging to evaluate women with stable ischemic heart disease. JACC Cardiovasc Imaging 2016;9(4):421 - 35.
114. Christian TF, Rettmann DW,Aletras AH, Liao SL, Taylor JL, Balaban RS, et al. Absolute myocardial perfusion in canines measured by using dual-bolus f rst-pass MR imaging. Radiology 2004;232(3):677- 84.
115. Hays AG, Kelle S, Hirsch GA,Soleimanifard S, Yu J, Agarwal HK, et al. Regional coronary endothelial function is closely related to local early coronary atherosclerosis in patients with mild coronary artery disease: pilot study. Circ Cardiovasc Imaging 2012;5(3):341- 8.
116. Buchthal SD, den Hollander JA,Merz CN, Rogers WJ, Pepine CJ,Reichek N, et al. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med 2000;342(12):829- 35.
117. Johnson BD, Shaw LJ, Buchthal SD, Bairey Merz CN, Kim HW,Scott KN, et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women ’ s Ischemia Syndrome Evaluation(WISE). Circulation 2004;109(24):2993- 9.
118. Bateman TM, Heller GV, McGhie AI, Friedman JD, Case JA,Bryngelson JR, et al. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET:comparison with ECG-gated Tc-99m sestamibi SPECT. J Nucl Cardiol 2006;13(1):24- 33.
119. Kay J, Dorbala S, Goyal A, Fazel R, Di Carli MF, Einstein AJ, et al.Inf uence of sex on risk strat icfation with stress myocardial perfusion Rb-82 positron emission tomography: results from the PET (positron emission tomography) Prognosis Multicenter Registry. J Am Coll Cardiol 2013;62(20):1866- 76.
120. Geltman EM, Henes CG, Senneff MJ, Sobel BE, Bergmann SR.Increased myocardial perfusion at rest and diminished perfusion reserve in patients with angina and angiographically normal coronary arteries. J Am Coll Cardiol 1990;16(3):586- 95.
121. Recio-Mayoral A, Rimoldi OE, Camici PG, Kaski JC.Inf ammation and microvascular dysfunction in cardiac syndrome X patients without conventional risk factors for coronary artery disease. JACC Cardiovasc Imaging 2013;6(6):660- 7.
122. Safdar B, D ’ Onofrio G, Dziura J,Russell RR, Johnson C, Sinusas AJ. Ranolazine and microvascular angina by PET in the emer gency department: results from a pilot randomized controlled trial. Clin Ther 2017;39(1):55- 63.
 Cardiovascular Innovations and Applications2018年4期
Cardiovascular Innovations and Applications2018年4期
- Cardiovascular Innovations and Applications的其它文章
- Challenges in Cardiovascular Risk Prediction and Stratification in Women
- Antiplatelet Therapy Considerations in Women
- Novel Imaging Approaches for the Diagnosis of Stable Ischemic Heart Disease in Women
- Psychosocial Stress, the Unpredictability Schema, and Cardiovascular Disease in Women
- Heart Disease in Pregnancy: A Special Look at Peripartum Cardiomyopathy
- Heart Failure with Preserved Ejection Fraction:Time to Revisit the Stiff Heart
