Challenges in Cardiovascular Risk Prediction and Stratification in Women
Sonia Henry, MD , Rachel Bond, MD , Stacey Rosen, MD , Cindy Grines, MD and Jennifer Mieres, MD
1 Northwell Health, Department of Cardiology, Northshore University Hospital, Hofstra School of Medicine, 300 Community Drive, Manhasset, NY 11030, USA
2 Northwell Health, Department of Cardiology, Hofstra North Shore-LIJ School of Medicine, Lenox Hill Hospital, 110 E. 59th Street, Suite 8A, New York, NY 10022, USA
3 Northwell Health, Department of Cardiology, Hofstra School of Medicine, The Katz Institute for Women’ s Health, 1981 Marcus Avenue, Suite E110, Lake Success, NY 11042, USA
4 Hofstra School of Medicine, Northwell Health, 1979 Marcus Avenue Suite 236, Lake Success, NY 11042, USA
Abstract There has been an appropriate focus, since the turn of the 21 st century, on sex- and gender-specif c cardiovascular disease (CVD) as increasing evidence suggests that there are substantial dif ferences in the risk factor prof le, social and environmental factors, clinical presentation, diagnosis, and treatment of ischemic heart disease in women compared with men. As a result of increased awareness, detection, and treatment of ischemic heart disease in women, there has been signif cant reduction (greater than 30%) in cardiovascular mortality, and in 2013, more US men than US women died of CVD. Nevertheless, continued ef forts are required as CVD remains the leading cause of cardiovascular morbidity and death of women in the Western world, and in women younger than 55 years there has been a rise in cardiovascular mortality. In this article, we review several of the contributing factors that continue to cause challenges in accurate risk prediction and risk stratif cation in women.
Keywords: sex and gender disparity; risk prediction; risk stratif cation; ischemic heart disease
Introduction
During the last two decades there has been an appropriate focus on women ’ s cardiovascular heath as increasing evidence suggests that there are substantial differences in the risk factor prof le, presentation, diagnosis, and treatment of ischemic heart disease (IHD) in women compared with men.The landmark 2010 Institute of Medicine (IOM)publication Women’ s Health Research: Progress,Pitfalls, and Promise highlights the fact that women’ s health involves two aspects: (1) sex dif ferences from biological factors and (2) gender dif ferences- those affected by broader social, environmental,and community factors [ 1]. The emphasis on sexand gender-specif c cardiovascular disease (CVD)research and our enhanced understanding of sexspecif c pathophysiology for coronary disease in women have resulted in the expansion of the spectrum of IHD in women to include obstructive and nonobstructive coronary artery disease (CAD) with supply-demand mismatch as well as dysfunction of the coronary microvasculature and endothelium[ 2 , 3 ]. Signif cant reduction (greater than 30%) in cardiovascular mortality in women has occurred for the f rst time this decade as a result of increased awareness and detection, sex- and gender-specif c cardiovascular research, and the improved application of evidence-based treatments of IHD [ 4 - 6 ].In 2013, for the f rst time, more US men than US women died of CVD [ 5].
Despite progress, CVD remains the leading cause of morbidity and death of women in the Western world. Mortality associated with CVD is declining in women, but at a slower rate than in men.Furthermore, women with IHD have worse outcomes than men. Data from the VIRGO study and other studies demonstrate that there has been a rise in CVD mortality in female patients younger than 55 years [ 3]. The IOM report highlighted,although major progress had been made in reducing cardiovascular mortality in women, that many subgroups of women def ned by age, race, ethnicity, gender, socioeconomic status, and educational level still show striking disparities in cardiovascular health [ 1 ].
In this article we review several of the factors contributing to the sex and gender disparity seen in IHD - factors that continue to cause challenges in accurate risk prediction and risk stratif cation in women. Gaps in knowledge with risk awareness, f awed CVD risk scores, and inequalities in sex-specif c coronary heart disease (CHD) research are reviewed. We review traditional and nontraditional risk factors and the role of current imaging techniques for diagnosis and risk stratif cation in at-risk women. Lastly, we discuss a contemporary approach to focus on “ well-women” visits and high-risk groups and the requirements needed to implement this approach to prevent and reduce IHD in women.
Overarching Issues with CVD Risk Prediction in Women
Substandard Risk Awareness in Women
Accurate perception of risks is critical to inf uence behavioral change and adherence to recommendations for the prevention of CVD. There is a great “ perception gap ” between the 10-year or lifetime risk and the risk awareness of the general population [ 7], which impedes the attainment of improved CVD outcomes. This phenomenon of risk misperception is more prominent among women. In 1997, a national survey conducted by the American Heart Association (AHA) found that only one in three women correctly identif ed heart disease as the leading cause of death for women [ 8]. Over the past few decades, health awareness campaigns have made progress in increasing heart disease awareness in women, which has resulted in better care,better use of resources, and improved cardiovascular outcomes. Currently, the rate of awareness among women that heart disease is their number one threat has doubled to 55% [ 9] since the 1997 AHA survey; however, it is still suboptimal and has been stagnant since 2006. Furthermore, awareness was and is still lower in women with lower education, with lower income, and from an ethnic minority (Latinos and Blacks) [ 10, 11]. McDonnell et al.[ 12] found that women have strikingly low levels of awareness regarding the traditional risk factors and their association with heart disease. In 2014,the Women’ s Heart Alliance (WHA) conducted surveys to determine current knowledge, attitudes, and beliefs regarding women ’ s cardiovascular health.The results highlighted knowledge gaps for women.Nearly 71% never raised the issue of heart disease with their physician [ 13]. Few women understood the relationships between heart disease and autoimmune disorders, pregnancy complications, early menopause, and irregular periods [ 13]. Further work is needed to raise awareness regarding the risk of heart disease in women so as to continue to impact outcomes.
Substandard Risk Awareness in Physicians
Awareness among physicians also appears to be suboptimal. Studies demonstrate that physicians are more likely to assign a lower CVD risk to female patients compared with risk-matched male patients, are more likely to underestimate the probability of CVD in women [ 14], and are less likely to refer women and people from ethnic minorities for diagnostic cardiac catheterization [ 15]. Women often receive suboptimal cardiac preventative care[ 3 , 14 , 16 ]. In a recent WHA survey [ 13 ] among primary care physicians (PCPs), heart disease was not their top priority in women. PCPs ranked heart disease after weight issues and breast health. Only 49% of PCPs and 59% of cardiologists reported that their medical training had prepared them to assess the cardiac risk in their female patients. Although more than 90% of PCPs and cardiologists over-whelmingly agreed with the statement “ women can present with different signs and symptoms of heart disease than men, ” only 49% of PCPs and 52% of cardiologists agreed that women ’ s and men ’ s hearts are physiologically dif ferent. Continued advocacy of national action campaigns and improved physician education are needed, starting from medical school.
Flawed Risk Scores Can Result in Risk Miscalculation for Women
Risk equations are a cornerstone of prevention.Several risk tools are currently available that predict cardiovascular outcome. The Framingham Risk Score (FRS) is the most commonly used global model for risk prediction [ 17]. The FRS uses age,gender, cholesterol prof le, smoking, and blood pressure to predict one ’ s chance of having a cardiac event in 10 years. The FRS was adopted in 2001 into the National Cholesterol Education Program/Adult Treatment Panel III (A TP III) cholesterol guidelines. One problem of this short-term risk estimation is that women traditionally have a lower short-term cardiovascular risk but a higher lifetime risk. Thus the FRS characteristically underestimates cardiovascular risk for women. FRS underestimation of CHD in women was also recognized in the Multi-Ethnic Study (MESA.) In MESA, women in the highest quartile of coronary artery calcium(CAC) scores were characterized as being at low risk by the FRS [ 18]. Additionally, ATP III predicts future cardiovascular events but not angina or revascularization, which are two important end points for women [ 19].The Reynolds Risk Score (RRS) was initially developed specif cally for women, and incorporates family history, inf ammatory biomarkers (highsensitivity C-reactive protein), and hemoglobin A1C, in addition to the FRS risk factors [ 20]. The RRS reclassif ed 40 - 50% of women at intermediate risk into higher or lower categories in a cohort from the Women’ s Health Study [ 20]. The RSS also improves discrimination in multiethnic populations in comparison with the FRS. The RRS is a more accurate short-term predictor but the overall gains are modest [ 17]. As with the FRS, the RRS fails to include many of the risk factors unique in women.
A major limitation of the FRS and RRS is the narrow focus on short-term risk and the extrapolation of data from very high risk or healthy patients,which do not apply to the general population [ 21].To address these limitations, AHA guidelines on prevention of CVD in women classif ed women into “ high risk, ” “ at risk, ” or ‘ ideal cardiovascular health” and was inclusive of novel CVD risk factors in women. This simplif ed classif cation emphasizes the concept of increased lifetime risk for women and is easily accessible to patients and providers. In a validation cohort, the model proposed by the AHA identif ed cardiac risk with an accuracy similar to the FRS [ 22].
In 2013, the American College of Cardiology and AHA published a guideline on assessment of cardiovascular risk [ 23] and a new tool for the estimation of cardiovascular events, namely, a pooled cohort equation. The pooled cohort equation is gender specif c and provides specif c information for Caucasians and African Americans. It provides both a 10-year atherosclerotic cardiovascular risk and a lifetime risk. The benef t for women is that their higher lifetime cardiovascular risk is included in this calculation. A concern is the overestimation of cardiovascular risk with advanced age [ 24, 25].
Disparities of CHD Research in Women
Despite advances in cardiovascular outcomes for women, most of the current treatment directing prevention and management of IHD is from studies of predominantly male participants. Female representation in research was evaluated in clinical trials from 1997 to 2006, and was noted to be a meager 27% [ 26]. Another analysis showed that the 156 IHD trials included only 25% women [ 27].Recognizing these inequalities, the IOM called for ongoing ef forts to enhance inclusion of women in clinical trials [ 1]. Additionally, in 2015 the National Institutes of Health announced that “ sex”is a biological variable that should be included in basic sciences as well as clinical research design and analysis [ 28, 29]. The Research for All Act was passed by the 114thCongress (2015- 2016) and supports equal inclusion of women in research and further subgroup analysis based on sex [ 30]. Continued responsibility and enforcement of sex- and genderspecif c research is needed by government, industry, professional bodies, and journal publishers.
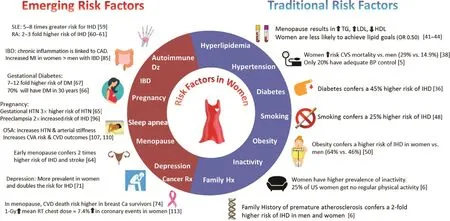
Figure 1: Emerging and Traditional Risk Factors.
Gender Disparities in CHD Risk Factors
Although men and women share similar modif able risk factors for CHD (diabetes, smoking, elevated cholesterol level, hypertension, sedentary lifestyle,obesity), several of these traditional risk factors are more potent in women. Also, certain novel sex-specif c risk factors, such as early menopause, inf ammatory diseases [systemic lupus erythematosus(SLE) and rheumatoid arthritis (RA)], and complications of pregnancy (preeclampsia and gestational diabetes) are associated with an increased risk of heart disease. An additional cardiovascular risk for women is breast cancer treatment. Breast cancer overwhelming affects women versus men, and CVD mortality is higher in postmenopausal women who have received cardiotoxic chemotherapy and/or radiation therapy ( Figure 1 ) [ 31]. Fifty percent of the reduction in CVD mortality is attributable to reducing risk factors [ 32 ].
Challenges of Risk Prediction with Traditional Risk Factors
Diabetes
Data from the Framingham Study highlighted the greater relative impact of diabetes on women compared with men [ 33], with diabetic women having a relative risk for fatal CHD 50% higher than men [ 34]. A gender-specif c analysis from the INTERHEART study [ 35], which highlights the nine factors responsible for 90% of all myocardial infarction (MI), found that several risk factors,including diabetes mellitus (DM), are more potent in women than men, particularly below the age of 60 years, and are more strongly associated with MI. Diabetic women have a 45% greater risk of incident CHD and a 25% greater stroke risk. Also of concern are data demonstrating that women are less likely to be treated aggressively with regard to glucose control [ 36]. The Rancho Bernado Study,a single-site, 40-year cohort trial studying gender differences in heart disease, showed that women with DM were more likely to have additional classic cardiac risk factors than men; it is suggested that this “ excess risk factor clustering ” in diabetic women may partially explain why DM eliminates any “ cardioprotection” women are felt to have before menopause [ 37].
Hypertension
Hypertension is the leading cause of cardiovascular death worldwide, with an increased population-adjusted cardiovascular mortality for women compared with men, 29 versus 15% 3[ 8]. The prevalence of hypertension in men younger than 45 years exceeds that in women, the prevalence in men and women equalizes from 45 to 64 years of age, and for age greater than 65 years the prevalence of women with hypertension exceeds that of men [ 1]. Estrogen inf uences the vasculature, maintaining endothelial function through nitric oxide production and reduction in sympathetic nervous system activity. This may help explain the higher rates of hypertension seen in postmenopausal women. While women are more likely to have hypertension and benef t from drug therapy similarly to men, women are less likely than men to achieve adequate blood pressure control [ 39]. In the United States, 20% of elderly women have adequate blood pressure control in contrast to the 41% elderly men [ 5]. Women are at higher risk of left ventricular hypertrophy and symptomatic heart failure (HF) with preserved ejection fraction [ 40 ].
Hyperlipidemia
Premenopausal women tend to have lower lowdensity lipoprotein (LDL) cholesterol levels and higher high-density lipoprotein (HDL) cholesterol and apolipoprotein A-I levels than men or their postmenopausal counterparts. A meta-analysis of 17 prospective population-based studies revealed a 37% increased risk of CVD events in women with higher concentrations of triglycerides (relative risk[RR] 1.8) compared with a 14% increased risk in men (RR 1.3) [ 41, 42]. Despite comparable lipidlowering benef t, women are less likely to be treated with statins than men after MI [ 43, 44]. Crosssectional data from a lar ge-scale population study suggest that around the time of menopause, LDL cholesterol levels increase by approximately 15 -25% [ 45, 46]. Because this increase is lar ger than that observed in men over the same age span and closely approximates that observed in women after oophorectomy, it is likely that reduced circulating estrogen levels associated with menopause play a role in the adverse changes in both blood lipid levels and CHD incidence.
Tobacco
Smokers are two to four times more likely to develop heart disease than nonsmokers [ 5].Seventeen percent of women in the United States smoke, and younger women are more likely than younger men to start smoking [ 47]. There is a 25% additional increase in cardiovascular risk for women smokers as compared with men smokers,with cigarette smoking tripling the risk of MI in women [ 48 ] .
Obesity and Physical Inactivity
Early data from the Nurses ’ Health Study conf rmed that being even mild (BMI 25 - 26.9 kg/m2) or moderately (BMI 27 - 29.9 kg/m 2) overweight increases the risk of CAD (RR 1.6 and RR 1.9, respectively)in women aged 40 - 74 years [ 49]. The most recent data from the AHA reveal that 65% of US women are overweight or obese, including 82% of African-American women [ 5]. Obesity increases coronary risk more for women than for men, 64 versus 46%[ 50]. Body weight may increase during the f rst years after menopause, and body fat distribution
shifts from a gynoid to an android (central) pattern.Central obesity with increase in visceral fat occurs with a higher presence of comorbid risk factors and components of metabolic syndrome in women compared with aging men, including a higher prevalence of DM [ 51].
Inactivity has been shown to be an important risk factor for women and compounds the risk related to being obese. Physical inactivity is the most prevalent risk factor for US women, with 25% of US women reporting no regular physical activity, and 34%reporting less than the recommended amount of weekly activity (150 min/week). The EPIC-Norfolk study showed that women with abdominal obesity and features of metabolic syndrome who were physically active had a 50% lower risk of CHD than those with abdominal obesity who were sedentary [ 52]. In the INTERHEAR T study, the protective effects of exercise appeared greater for women than for men [ 53]. Benef cial physical activity data,specif c to women, derive from the Nurses ’ Health Study, where there was a decreased development of DM among women who exercised regularly; also,among diabetic women, physical activity decreased the risk of cardiovascular events [ 54].
Metabolic Syndrome
Metabolic syndrome is characterized by abdominal obesity, insulin resistance, atherogenic dyslipidemia, and hypertension. In the general population,women with metabolic syndrome have a two-fold increased risk of major adverse cardiovascular events and death. Although studies have shown that the prevalence of obesity and metabolic syndrome is similar between men and women, a meta-analysis of prospective studies concluded that metabolic syndrome confers a greater over-all CVD risk in women, resulting in a 30% higher relative risk compared with men [ 41]. In women,obesity, low HDL level, increased waist circumference, and hyperglycemia were signif cantly larger contributors to metabolic syndrome, while in men,hypertension and elevated levels of triglycerides contributed most [ 55].
An additional risk for metabolic syndrome in women is polycystic ovarian syndrome (PCOS).Forty percent of women with PCOS have metabolic syndrome, and the risk is higher for black women [ 56]. The association between PCOS and cardiovascular comorbidities is well recognized,with a higher incidence of obesity, a f ve-fold risk of DM, and dyslipidemia seen in 70% of PCOS patients [ 57]. Furthermore, women with PCOS have an increased prevalence of subclinical atherosclerosis, and endothelial dysfunction, increased carotid intima media thickness, and increased CAC scores [ 58 ].
Challenges of Risk Prediction with Nontraditional Risk Factors ( Table 1)
Inflammatory Diseases
Many population studies have found a sex-specif c association between inf ammatory diseases and increased mortality, mainly secondary to CVD [ 75,76]. Evidence suggests that traditional risk factors alone do not explain the increase in the incidence of CHD and that chronic inf ammation with endothelial cell injury and antiphospholipid antibodies also contribute [ 77 ]. Autoimmune inf ammatory diseases are far more prevalent in women, with RA present in 4% of women versus 2% of men and SLE having a 10:1 female predominance [ 78, 79]. Women with SLE have a f ve to eight times greater risk of heart disease [ 59]. A longitudinal study of women with SLE showed progressive atherosclerotic changes in the carotid arteries, with a 10% annual progression [ 80 ]. Thirty-f ve percent of patients with SLE have evidence of atherosclerosis, and the risk of MI in these patients is signif cant, with the overall prevalence of clinical CHD in SLE patients ranging from 6 to 10% in many cohorts [ 81]. Women in the Framingham Offspring Study aged 34 - 44 years with SLE were more than 50 times more likely to experience an acute MI than similarly aged women without SLE [ 82]. RA also increases the risk of IHD; the risk is doubled for developing HF and 1.5-fold to two-fold risk of CAD. There is a two-fold to threefold increase in the risk of MI and cardiovascular death in women with RA [ 60, 61]. It has been recognized that CVD risk scoring systems underestimate the burden of CVD risk in patients with RA and SLE, and an empirical European League Against Rheumatism (EULAR) multiplier of 1.5 has been suggested [ 83 , 84 ].
Inf ammatory bowel disease (IBD), ulcerative colitis, and Crohn disease also causes signif -cant systemic inf ammation and increase the risk of MI compared with the general population. In a recent study [ 85], where 17.5 million patients were assessed from electronic medical records, MIs were almost twice as common in patients with IBD. After adjustment for age, race, sex, and traditional heart disease risk factors, it was found that patients with IBD had about 23% higher odds of having an MI.Females among other subgroups (age 20 - 24 years,black) were at highest risk.
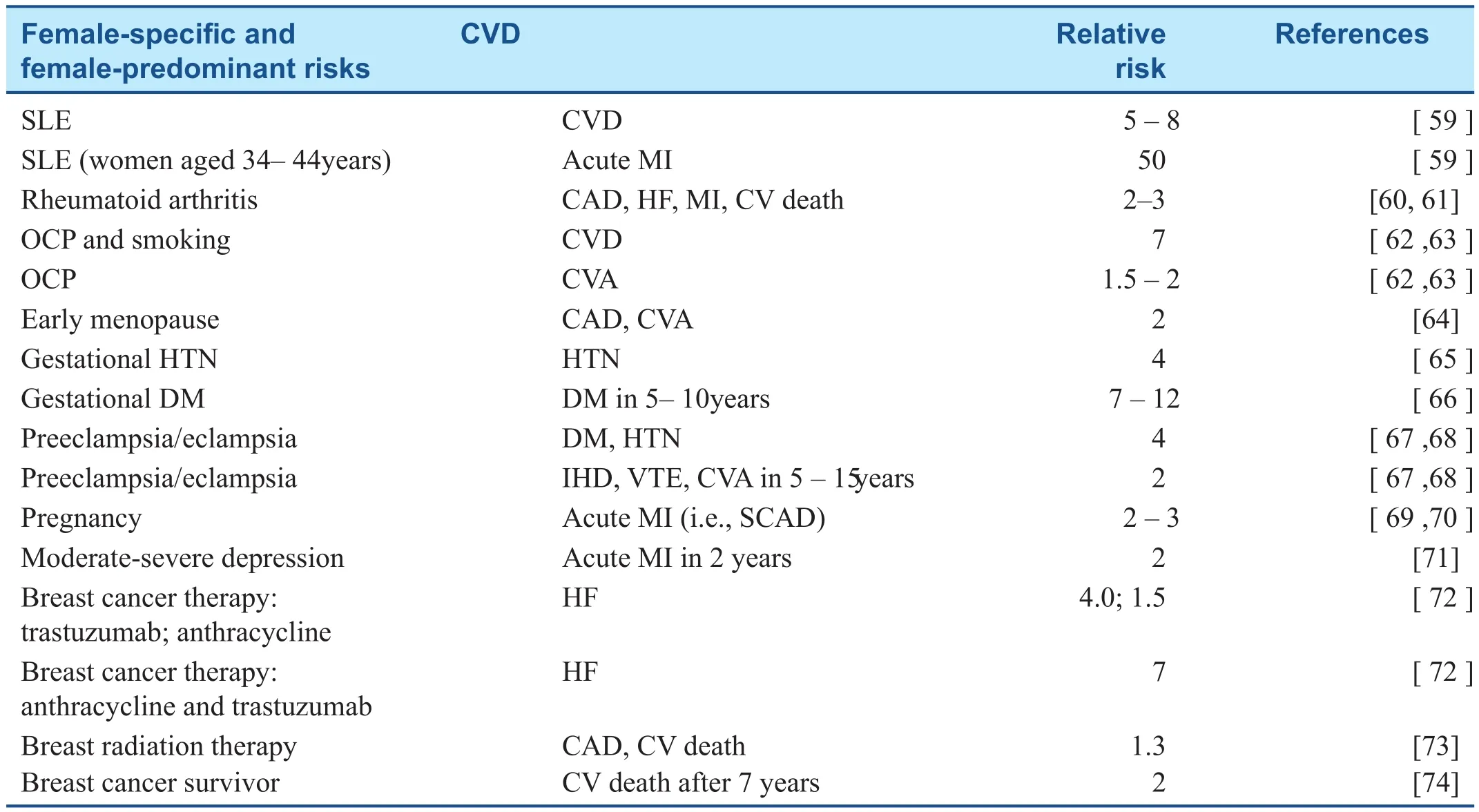
Table 1 Female-Speci fc and Female-Predominant Cardiac Risks and Associated Relative Risk of Cardiovascular Disease (CVD).
Impact of Exogenous and Natural Estrogen over the Lifespan
Natural estrogen is “ cardioprotective, ” maintaining endothelial function through nitric oxide production, and has some regulatory effect on the sympathetic nervous system. Conversely, oral contraceptive pills (OCPs) have ef fects on the reninangiotensin system and increase blood pressure in most women [ 86]. Smoking and OCPs are associated with a seven-fold increase in cardiovascular risk. There is a 1.5 - 2.0 times increase in the risk of stroke, which increases with the duration of OCP therapy [ 62 , 63 ].
The effects of hormonal changes over a women ’ s lifetime have important implications for the risk of CHD, and at the time of natural menopause,women are more likely to develop lipid abnormalities (increase in LDL levels of 10 - 25%), changes in blood pressure, and increased BMI. Several lar gescale studies have shown an association between early menopause or primary ovarian failure and a two-fold increased risk of CAD and cerebrovascular accident [ 64]. Some controversy still remains,but the Heart and Estrogen/Progestin Replacement Study (HERS) and the Women’ s Health Initiative provided evidence that postmenopausal hormone replacement therapy is not benef cial for the prevention of CVD [ 87 - 89] and is not recommended for primary or secondary prevention of CVD.Coronary risk increases about 10 years after the onset of menopause, coinciding with the onset of coronary artery calcif cation on cardiac computed tomography [ 90 , 91 ].
CVD Complications of Pregnancy
Complications in pregnancy such as pregnancyinduced hypertension, gestational diabetes, preeclampsia/eclampsia, preterm delivery, and small size for gestational age add to the cardiac risk prof le of a woman [ 92 - 94]. Mosca et al. [ 21] referred to these complications of pregnancy as representing a “ failed stress test, ” unmasking endovascular or metabolic dysfunction. Robbins et al. [ 95] evaluated 301 women with gestational hypertension and found higher rates of obesity, CVD, and DM than in women without a history of hypertension.Furthermore, there is a four times greater risk of subsequent hypertension [ 65]. In a lar ge metaanalysis, up to 70% of women with gestational diabetes will develop overt DM within three decades after pregnancy [ 66, 67]. Women with preeclampsia or eclampsia, often called the “ metabolic syndrome of pregnancy,” are more than four times more likely to develop diabetes and hypertension requiring drug treatment [ 68]. In a recent large study, preeclampsia/eclampsia doubled the risk of subsequent IHD, stroke, and venous thromboembolic events over the 5 - 15 years after pregnancy[ 96 , 97 ] .
Acute MI in women of childbearing age is rare.Pregnancy, however, has been shown to increase the risk of acute MI three-fold to four-fold [ 69]. The most common cause (43%) of peripartum-related MI is spontaneous coronary artery dissection [ 70].The cause of pregnancy-associated spontaneous coronary artery dissection is not fully understood.One hypothesis is that estrogen and progesterone receptors on the coronary arteries may arbitrate changes that weaken the vessel wall and culminate in arterial wall rupture [ 98, 99].
Acute Stress, Anxiety, and Depression
Depression is associated with a 70% increased risk of heart disease, and is twice as prevalent in women as in men [ 100]. Elevated levels of atherosclerotic and inf ammatory markers are seen with depression and impair vascular function and also cause impaired platelet activation. Depression leads to nonadherence to medication and follow-up, apathy,poor diet, increased smoking, and physical inactivity. Collectively, the physiological and behavioral responses of depression contribute to negative outcomes.
Psychosocial issues, particularly depression, preferentially disadvantage women. In the INTERHEART study, psychosocial factors, including stress at work/home, f nancial stress, and major life events, were associated with greater cardiovascular mortality for women than for men, 45 ver-sus 29% [ 101]. Depression is signif cantly linked to CVD in women older than 55 years. A Johns Hopkins study of more than 7000 patients showed a signif cantly high risk of CHD in women aged 17- 39 years with depression [ 102]. Shah et al. [ 71]looked at 3237 women with established or suspected CVD and found young women with moderate to severe depression had double the risk of MI in the next 2 years and increased risk of death.Shah et al. [ 71] theorized that depression can interfere with normal ovulation and interrupt the normal hormonal cardioprotection of the premenopausal state. Similarly to any CVD risk, the identif cation and treatment of depression is particularly important in women, and therefore screening is paramount [ 103 ].
The effects of acute stress can induce myocardial ischemia in women but not men after MI. Anxiety has also been associated with an increased risk of fatal CHD in women [ 104]. The Myocardial Infarction and Mental Stress (MIMS) study compared middle-aged men and women after MI and showed dramatic differences in myocardial ischemia on nuclear perfusion imaging after mental stress in women younger than 50 years. Induced ischemia related to physical stress did not show a gender-based difference [ 105]. Acute stress can also cause a stress-induced myopathy (Takotsubo cardiomyopathy) that is more common in women. In the International Takotsubo Registry of 1750 patients with stress cardiomyopathy, 89% were women and the mean age was 66 years [ 106].
Sleep Disorders
Obstructive sleep apnea (OSA) and sleep disor-ders are now recognized as an important cardiac risk factor in women. Recent studies have shown OSA is independently associated with hypertension and arterial stif fness in perimenopausal women[ 107]. Sleep disorders, more reported in women than in men, are independently associated with atherosclerosis and heart remodeling [ 108]. Campos-Rodriguez et al. [ 109] showed that women younger than 65 years with severe untreated OSA have an increased incidence of serious cardiovascular outcomes, particularly stroke. Unfortunately, OSA and sleep disorders are underdiagnosed and undertreated in women [ 110]. Recent investigations described that predominately postmenopausal women with poor sleep have elevated levels of inf ammatory markers (C-reactive protein, f brinogen, and interleukin-6); this has not been noted in men [ 111]. One theory is that low estrogen level might play a role.Also, there was an increased association between poor sleep, resistant hypertension, and depression in women but not men [ 112].
Chemotherapy and Radiation Therapy
Both heart disease and breast cancer have a large public presence in women ’ s health. Common risk factors associated with these two conditions include obesity, smoking, older age, postmenopausal hormone replacement therapy, poor diet, and sedentary lifestyle. The risk of HF, IHD, and hypertension during and after cancer therapy is signif cant [ 1 13 -115]. In postmenopausal women, the risk of death attributable to CVD is about two times higher in breast cancer survivors than in women without a history of breast cancer. This greater risk manifests itself about 7 years after the diagnosis of breast cancer[ 74 ].
Anthracyclines are commonly used for breast cancer chemotherapy and are associated with early and late risk of left ventricular dysfunction, which may be irreversible. The risk of HF increases with increasing cumulative anthracycline doses and when underlying CVD risk factors exist [ 1 16 -1 18 ]. Trastuzumab is used for human epidermal growth factor receptor 2/neupositive breast cancer. Trastuzumab has revolutionized breast cancer treatment and reduced the risk of breast cancer recurrence by 50% and death by 33%. There was, however, progress at a price,as up to 29% of women develop some degree of cardiotoxicity [ 119]. Left ventricular dysfunction associated with trastuzumab has been well studied in the breast cancer population [ 120]. In contrast to the ef fects of anthracyclines, trastuzumabinduced cardiotoxicity is frequently reversible[ 121]. At highest risk of overall cardiotoxicity are those older than 50 years, patients with underlying heart disease or hypertension, those with baseline ejection fraction less than 50 - 55%, and those who have concomitant cardiotoxic chemotherapy[ 72 , 122 , 123 ] .
Exposure of the heart to ionizing radiation during radiation therapy for breast cancer increases the subsequent risk of CHD and cardiac death [ 73].Radiation therapy has signif cant effects on the macrovasculature and microvasculature and can cause inf ammation and oxidative damage that results in accelerated atherosclerosis, resulting in myocar-dial ischemia and injury [ 124, 125]. In a study of 2168 women with breast cancer, each increase in mean heart radiation dose by 1 Gy was associated with a 7.4% increase in coronary events, with no apparent threshold [ 113]. Cardiovascular ef fects can occur as early as 5 years after exposure among breast cancer survivors who receive left-sided thoracic radiation therapy, and persistence of risk remains for up to 30 years [ 126].
Challenges in Risk Stratification:Imaging Modalities for Suspected Stable IHD
Early identif cation of women at risk of IHD is crucial, given that sudden cardiac death contributes substantially to mortality in women with and without obstructive CAD and is often the f rst manifestation of CAD in a signif cant proportion of women [ 127, 128]. Contemporary and novel diagnostic imaging techniques have now assumed an expanded role in the evaluation of symptomatic women to detect the full spectrum of IHD to include not only focal epicardial coronary stenosis but also nonobstructive atherosclerosis, along with the identif cation of ischemia resulting from endothelial and microvascular dysfunction [ 4].
Measurement of Coronary Artery Calcification
CAC measured by computed tomography is a subcomponent of atherosclerotic plaque and has emerged as one of the most powerful predictors of subclinical CHD, particularly in women [ 129].CAC scoring has been reported to ef fectively riskstratify women, particularly those with an intermediate FRS [ 130]. As previously noted, in MESA,CAC was present in 32% of women who had previously been classif ed as low CHD risk by the FRS.Furthermore, 4% had CAC ≥ 300 Agatston score,reclassifying them as high risk. Ethnicity was not signif cantly signif cant [ 18 ].
Evaluation of Symptomatic Women for IHD
The AHA released a consensus statement on the role of noninvasive testing for women with suspected IHD in 2014 [ 4] that provides gender-specif c evidence-based guidance ( Figure 2 ).
The use of exercise treadmill testing (ETT) is recommended as the initial diagnostic test for symptomatic women who are have an intermediate pretest risk of CAD, who are functionally capable of exercising, and who have an interpretable resting electrocardiogram [ 4]. In patients with a low to intermediate pretest probability, normal ETT has a very high negative predictive value. If functional capacity is unclear, the use of the 12-item Duke Activity Status Index is encouraged to estimate the metabolic equivalents. For women unable to achieve at least f ve metabolic equivalents with their activities of daily living, pharmacological testing may be considered.
For symptomatic patients with intermediate pretest risk of IHD with poor functional capacity or an abnormal resting electrocardiogram precluding ST segment interpretation during exercise, the addition of noninvasive imaging is suggested with functional stress imaging (i.e., echocardiography,single-photon-emission computerized tomography[SPECT], myocardial perfusion imaging [MPI],magnetic resonance imaging [MRI], positron emission tomography [PET]) to assess the patient for myocardial ischemia and/or an anatomical approach such as coronary computed tomographic angiography (CCTA) to visualize the extent, severity, and composition characteristics of coronary plaque [ 4 ].
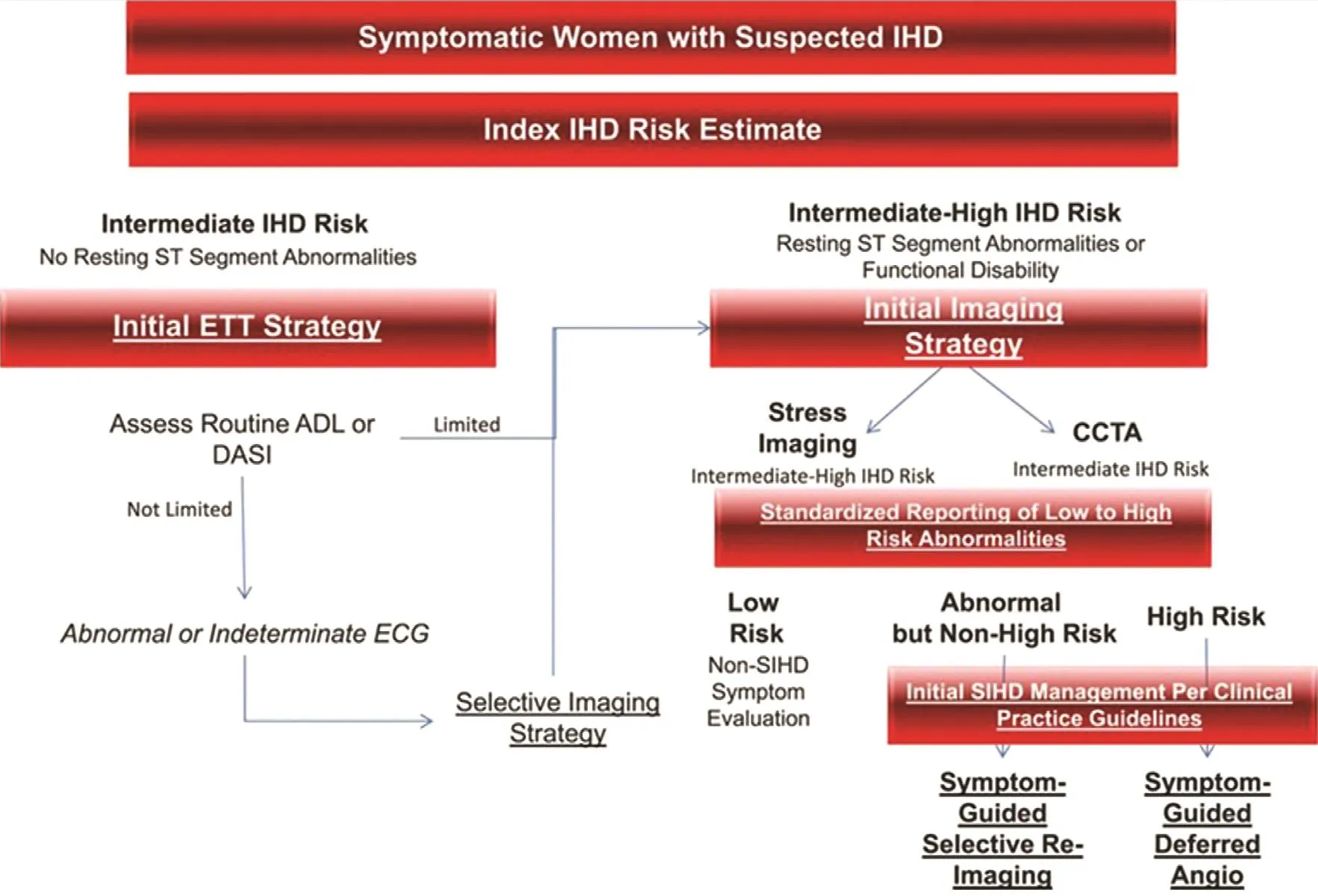
Figure 2: Diagnostic Evaluation Algorithm for Women Presenting with Suspected Ischemic Heart Disease Symptoms and Intermediate IHD Risk and Intermediate-High IHD Risk.
The use of stress echocardiography or SPECT additional to the treadmill test increases the diagnostic accuracy of the test. Given the absence of radiation exposure, stress echocardiography should be considered, especially for women of childbearing age. With regard to the overall accuracy, exercise echocardiography has higher diagnostic sensitivity(79%) and specif city (83%) when compared with ETT in detecting obstructive CAD in women [ 131].Similar numbers seen with the use of pharmacologic-dobutamine stress echocardiogram, with sensitivity of 80% and specif city of 84% [ 132]. The sensitivity of contemporary SPECT MPI exercise techniques ranges from 78 to 88%, with specif city of 61 - 91% [ 133 - 135W ]. hen exercise is not an option, pharmacologic stress MPI, particularly vasodilator stress, plays a key role in the diagnosis and risk evaluation of women with suspected IHD[ 136], with sensitivity of 85 - 93% and specif city of 78% for detecting obstructive CAD [ 135, 137].
Stress PET increases the diagnostic accuracy and prognostic value by providing enhanced evaluation of the full spectrum of coronary atherosclerosis. It does this by quantif cation of absolute myocardial blood f ow and coronary f ow reserve to assess the patient for endothelial dysfunction [ 138 - 140M ]e. taanalyses have conf rmed incremental increases in diagnostic accuracy with PET relative to SPECT for the diagnosis of obstructive CAD, with average sensitivity of 90% and specif city of 89% [ 141, 142],particularly in women with suboptimal stress SPECT or poor windows for stress echocardiography.
Stress cardiac MRI has the advantages of avoiding ionizing radiation compared with stress SPECT or PET, with excellent soft tissue characterization secondary to superior temporal and spatial resolution and absolute quantif cation of myocardial blood f ow, making it ideal for the assessment of endothelial dysfunction. A meta-analysis of 2456 patients (31% women) reported sensitivity of 89%and specif city of 80% for detecting obstructive lesions with stress MRI compared with invasive coronary angiography (ICA) [ 143].
CCTA uniquely provides information on the burden of atherosclerosis, as well as information on luminal narrowing, plaque location, remodeling,and composition [ 144, 145]. Although there is high diagnostic and prognostic accuracy for the detection of CAD in women at risk of IHD with use of CCT A,this is limited by its inability to diagnose hemodynamically signif cant lesions easily. In comparison with fractional f ow reserve measurement by ICA,anatomical CCTA had lower specif city of 0.4 [ 146].
In patients with a high pretest risk of IHD or abnormal noninvasive testing results, ICA remains the gold standard and end point of a diagnostic evaluation. The use of intracoronary imaging with intravascular ultrasonography and/or optimal coherence tomography [ 128] along with invasive hemodynamic and f ow assessment with provocative testing should be considered and may improve overall diagnosis, particularly in women who present with signs and symptoms of ischemia but with evidence of “ nonobstructive” CAD (which could be diffuse coronary disease) [ 147 , 148 ].
Beyond the Traditional Risk Assessment: Contemporary Approach of Identifying Women At Risk of IHD
Despite progress in improving outcomes and reducing mortality, IHD continues to be the leading cause of death in women, with recent data suggesting some reversal of the positive impact seen over the past 20 years. Signif cant disparities in diagnosis, treatment, and outcomes still exist in women compared with men. Women experience delays in reperfusion[ 149 , 150 ]; less revascularization [ 151 ]; underuse of pharmacotherapy for primary prevention [ 152],acute coronary syndome [ 153, 154], and stable IHD[ 44 ]; and worse morbidity [ 155 - 157an ] d greater mortality associated with CVD [ 44 , 158 - 160A ]t.highest cardiovascular risk are black, Latino, and South Asian women [ 161 , 162 ]; those disadvantaged by low income and educational status; and young women. As mentioned, the death rate is rising in women younger than 55 years [ 3]. Young women were less likely to be prescribed guideline-recommended medication [ 152], they were more likely to exceed the door-to-balloon time for percutaneous coronary intervention during ST-elevation MI compared with age-matched men (67 vs. 32%) [ 163],they were less likely to have revascularization (28 vs. 13%), and they had two-fold higher postinfarct in-hospital and 1-year mortality [ 164]. To move the needle forward with regard to outcomes and mortality, equity in care for women is needed and a novel approach for early identif cation and treatment of women at risk of IHD is crucial ( Figure 3 ).
The Woman’ s Healthcare Team
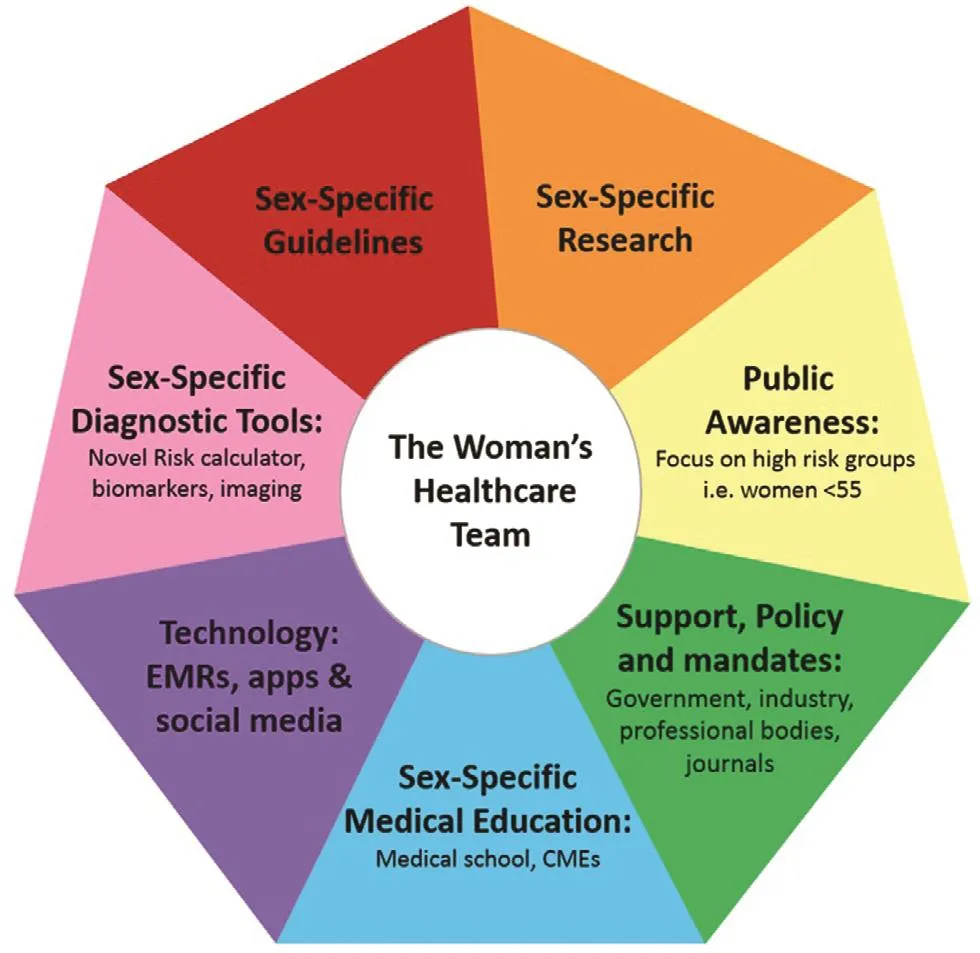
Figure 3: Contemporary Solution for Equitable Care and to Improve Cardiovascular Outcomes in Women.
All clinicians who care for women have an ur gent call to action to reduce IHD in women. Most women consider their obstetrician/gynecologist to be their sole physician, particularly during their childbearing years. The “ healthcare team for women ” approach,coined by Shaw et al. [ 165], is paramount in the care of women to reduce CVD, their number one health threat. The 2018 AHA/American College of Obstetricians and Gynecologists presidential advisory statement [ 166] recognizes the importance of the “ healthcare team. ” Furthermore, the obstetrician/gynecologist’ s role is essential since pregnancy and menopause impart unique cardiovascular effects in women. The annual obstetrician/gynecologist “ well-woman visit ” is a powerful opportunity to be comprehensive and counsel patients about maintaining a healthy lifestyle and minimizing health risks [ 167]. It should include family history and regularly screen the patient for traditional,female-predominant and female-specif c cardiac risk factors. Any signif cant f ndings should trigger referrals to appropriate services and/or a cardiologist. Also included in the “ healthcare team ” are a rheumatologist, an oncologist, a psychiatrist, pulmonologist, and a gastroenterologist, who directly manage and treat the female-predominant unique cardiovascular risk (SLE, RA, IBD, COPD, OSA,anxiety, depression, cancer), and thus should also routinely include heart risk assessment for early identif cation and treatment to prevent CVD. It is imperative to have an introduction to the“ healthcare team” approach in medical school paired with sexspecif c CVD education. This knowledge should be furthered with CMEs and/or scientif c sessions throughout a clinician’ s career.
Sex-Specific Research, Diagnostic Tools,and Guidelines Required
The Woman ’ s Ischemic Syndrome Evaluation(WISE) study and other sex-specif c research has established that the pathophysiology of IHD in women extends beyond epicardial stenosis to include diffuse atherosclerosis and dysfunction of coronary microvasculature and endothelium. Despite this knowledge, there is a lack of proven guidelinesdirected diagnostic approaches and treatment recommendations for women with signs and symptoms of ischemia with nonobstructive CAD [ 128, 147].Landmark sex-specif c clinical research is ur gently needed. Advanced research is also needed on predictive and diagnostic tools such as novel circulating, genetic and imaging biomarkers in women.Furthermore, a concise screening tool or risk calculator for women incorporating both traditional and contemporary (female-specif c and female-predominant) risk factors is compulsory, as the presence of nontraditional factors will almost certainly impact risk stratif cation and will impact the aggressiveness of preventative treatment in women. Despite mandates on inclusion of women in research, women represent only a quarter of the participants in clinical trials [ 27]. Government, policy, industry, and professional bodies must mandate inclusion of women and focused recruitment of women in trials. It is only from research on women that accurate sex-specif c guidelines can be generated.
Innovative Use of Technology to Impact IHD in Women
Electronic medical records could be used to improve cardiovascular health in women. Software algorithms should be designed and used to screen patients, calculate risk, and prompt patient education and referrals from electronic medical records.Social media and apps also have the wide potential to reach most populations, and are a valuable resource to educate, empower, and motivate women,especially younger women.
Sustained Efforts in Education and Awareness
Public awareness campaigns have been successful in increasing awareness in women that CVD is their number one killer, with the rate increasing from 30 to 56% [ 9]. Continued action and novel strategies to further advance awareness is vital. A new focus on those at highest cardiovascular risk(women younger than 55 years and black/Latino/South Asian women) is desperately needed. Other current, potential barriers to healthcare in women include social stigma (i.e., “ fat shaming ”), cultural/religious beliefs, caretaking responsibility, inadequate f nances, and insuff cient time [ 11]. New strategies to enhance public awareness should focus on these groups and use churches/cultural or ganizations, gyms, schools, workplaces, and community leaders to promote heart literacy and health behaviors that are culturally acceptable, easy, and affordable.
Conclusion
Substantial progress has been made to increase awareness and reduce IHD in women; however,we are still a long way from equitable care. IHD in women is multifaceted in that it goes beyond traditional risk factors to include female-specif c biological risk factors, unique pathophysiology and clinical presentation, and dif ferences in diagnosis and treatments, and also incorporates social and environmental factors. To improve risk prediction,risk stratif cation, and decrease morbidity and mortality associated with IHD in women, we must move forward fervently to embrace the “ healthcare team for women” approach. We are obligated to continue with new strategies in awareness, use technology,increase sex-specif c research, and mandate clinicians, researchers, professional bodies, journal publishers, and government to sustain these ef forts toward equitable care.
Conflict of Interest
The authors declare no conlict of interest.
 Cardiovascular Innovations and Applications2018年4期
Cardiovascular Innovations and Applications2018年4期
- Cardiovascular Innovations and Applications的其它文章
- Nonobstructive Coronary Artery Disease in Women: Risk Factors and Noninvasive Diagnostic Assessment
- Antiplatelet Therapy Considerations in Women
- Novel Imaging Approaches for the Diagnosis of Stable Ischemic Heart Disease in Women
- Psychosocial Stress, the Unpredictability Schema, and Cardiovascular Disease in Women
- Heart Disease in Pregnancy: A Special Look at Peripartum Cardiomyopathy
- Heart Failure with Preserved Ejection Fraction:Time to Revisit the Stiff Heart
