功能化石墨烯四溴双酚A的电化学传感研究
吴生丽,刘 忆,孙艳娟,曹 媛,邹 菁
武汉工程大学化学与环境工程学院,湖北 武汉 430205
功能化石墨烯四溴双酚A的电化学传感研究
吴生丽,刘 忆,孙艳娟,曹 媛,邹 菁*
武汉工程大学化学与环境工程学院,湖北 武汉 430205
以N,N-二甲基甲酰胺为剥离溶剂,柠檬酸钠为剥离助剂,通过超声剥离得到了功能化石墨烯纳米片,并构建出高性能的电化学传感器.对所得产物进行透射电子显微镜表征,结果发现与原块体材料相比,所得产物为单层或少数几层的石墨烯纳米片.通过优化超声时间、石墨烯质量浓度和溶液的pH值,构建了对四溴双酚A(TBBPA)具有高灵敏度的功能化石墨烯电化学传感器.该传感器的电化学阻抗结果表明,剥离后的石墨烯具有更快的电子传递速率.更重要的是,所构建的功能化石墨烯电化学传感器对TBBPA呈现出的线性范围为 0.1 μmol/L~14 μmol/L和较低的检出限[5×10-8mol/L(S/N=3)],并且成功地将其应用于实际水样的检测,其加标回收率在98%~101%之间.该电化学传感器不仅表现出较高的准确性和灵敏度,而且还具有较好的选择性和稳定性.
石墨烯;剥离;N,N-二甲基甲酰胺;四溴双酚A;电化学传感器
石墨烯是由平面单层碳原子紧密堆叠成的蜂巢状的二维晶格,受到科学界的广泛关注[1].二维结构对电子的约束使其具有独特的电学和光学性质[2],它被广泛应用于太阳能电池[3]、晶体管[4]、电化学生物传感器[5-6]等中.2008年,Shang等首次在硅上生长阵列垂直排列的石墨烯薄片(graphene nanosheets,GNSS),并用于制备多巴胺传感器[7].文献[8-14]采用由化学还原氧化石墨烯制备的石墨烯纳米片修饰电极,文献[15-17]采用液相剥离石墨的方法制备石墨烯.由于液相剥离法制备石墨烯过程温和,操作简单,所制备石墨烯电化学性能良好,特别适合作为电极修饰材料以制备电化学传感器.
四溴双酚 A(tetrabromobisphenol A,TBBPA),是一种溴化阻燃剂,广泛用于聚合反应中的反应添加剂[18].然而,树脂中的TBBPA很容易泄露到环境中,它对原代培养的小脑颗粒神经元具有毒性[19].在沉积物、下水污泥、野生动植物和人类血清中都曾发现有 TBBPA[20].检测 TBBPA的方法有多种,如表面增强拉曼光谱法(surface enhancement of Raman scattering,SERS)[21]、气相色谱-质谱联用法(gas chromatography tandem mass spectrometry,GC-MS)[22]、液相色谱-质谱联用技术(high-performance liquid chromatography tandem mass spectrometry,HPLC-MS)[23]、高 效 液 相 色 谱 -串 联 质 谱 法(high-performanceliquidchromatographyspectrometry tandem mass spectrometry tandem mass spectrometry,HPLC-MS-MS)[24]和其它复杂的技术[25-26].然而,这些方法受限于样品预处理繁琐费时,设备昂贵.由于电化学传感器灵敏快速,操作简单,价格低廉,使其受到广泛关注.然而,目前,有关超声剥离功能化石墨烯修饰电极应用于TBBPA传感研究还未见报道.
石墨烯化学稳定性高,表面呈惰性状态,与其他介质(如溶剂等)的相互作用较弱,并且石墨烯片与片之间有较强的范德华力,容易产生聚集,使其难溶于水及常用的有机溶剂,这给石墨烯的进一步研究和应用造成了极大的困难.为了充分发挥其优良性能,并改善其成型加工性(如提高溶解性、在溶剂中分散性等),必须对石墨烯进行有效的功能化.本文以N,N-二甲基甲酰胺(N,N-dimethylformamide,DMF)为剥离溶剂,表面活性剂柠檬酸钠为助剂,液相超声剥离石墨烯,获得了柠檬酸钠-石墨烯复合纳米材料,改进了石墨烯的分散性,并制备了功能化石墨烯修饰电极.将该修饰电极用于电化学检测TBBPA,方法简单,检测灵敏度高,具有良好的稳定性和选择性.
1 实验部分
1.1 实验仪器与试剂
透射电子显微镜(transmission electron microscope,TEM):JSM-2100,日本电子;台式高速离心机:TG-16,长沙平凡仪器仪表有限公司;电子分析天平:JA1003N,北京赛多丽斯仪器系统有限公司;超纯水器:PGUV-10-AS,武汉器冠仪器设备有限公司;超声波器:QC31200,湖北鼎泰恒胜科技设备有限公司;电化学工作站:CHI660E,上海辰华仪器有限公司.
石墨烯:德阳烯碳科技有限公司;柠檬酸钠,AR,国药集团化学试剂有限公司;DMF,AR,国药集团化学试剂有限公司;磷酸氢二钠及磷酸二氢钠,AR,国药集团化学试剂有限公司.实验用水均为超纯水.
1.2 修饰电极的制备
典型制备方法:称取适量的石墨烯和1 mg柠檬酸钠于小烧杯中,再加入5 mL DMF,超声一定时间,即得剥离石墨烯修饰液.同样,称取1 mg柠檬酸钠于小烧杯中,再加入5 mL DMF,超声一定时间,即得DMF修饰液.
将玻碳电极(glassy carbon electrode,GCE)用0.05 μm的Al2O3抛光粉打磨,用无水乙醇、超纯水超声清洗5 min,再用氮气吹干备用.
取5 μL上述修饰液分别均匀滴涂在预处理好的GCE电极表面上,在红外灯下烘干即得相应的功能化石墨烯修饰电极及DMF修饰电极.
2 结果与讨论
2.1 石墨烯的TEM表征
图1为剥离前和超声剥离3 h后的石墨烯(G@3h)的TEM图,由图1可以看出,剥离后的石墨烯的厚度明显变薄,形成了单层或几层结构的石墨烯.
2.2 TBBPA在G@3h/DMF/GCE上的电化学行为
图2为GCE、DMF/GCE和G@3h/DMF/GCE在5 mmol/L 的[Fe(CN)6]3-/4-和 0.1 mol/L 的 KCl溶液中的阻抗图.G@3h/DMF/GCE的交流阻抗明显小于GCE和DMF/GCE,说明G@3h/DMF/GCE具有更快的电子转移速率.
图3为TBBPA在不同电极上的循环伏安图.缓冲溶液为pH=6.5的0.1 mol/L磷酸缓冲溶液,曲线Ⅰ、曲线Ⅱ、曲线Ⅲ分别对应TBBPA在GCE、DMF/GCE和G@3h/DMF/GCE上的循环伏安曲线.与GCE相比,G@3h/DMF/GCE上TBBPA的氧化峰负移了约34 mV,峰电流明显增加,说明功能化石墨烯加速了TBBPA和电极间的电子传递速率,G@3h/DMF/GCE对TBBPA具有较高的电化学活性.TBBPA的氧化峰对应为-OH键氧化为C=O,TBBPA电化学氧化过程如图4所示.

图1 石墨烯的TEM图:(a)未剥离,(b)剥离后Fig.1 TEM images of graphene:(a)before exfoliation and(b)after exfoliation

图2 不同电极在含有0.1mol/L KCl的5 mmol/L[Fe(CN)6]3-/4-溶液中的阻抗图Fig.2 Nyquist plots of different electrodes in 5 mmol/L[Fe(CN)6]3-/4-solution containing 0.1mol/L KCl

图3 不同电极在10-5mol/L TBBPA溶液中的循环伏安图(扫描速度为100 mV/s)Fig.3 Cyclic voltammograms of different electrodes in 10-5mol/L TBBPA with a scan rate of 100 mV/s

图4 TBBPA的电化学氧化过程Fig.4 Electrochemical oxidation progress of TBBPA
2.3 电化学分析条件的优化
2.3.1 超声时间 石墨烯在DMF溶液中分别超声剥离1 h、3 h、6 h和9 h,将所得到的石墨烯溶液分别修饰GCE,得到不同的修饰电极用于检测TBBPA.图5比较了10-5mol/L TBBPA溶液在不同修饰电极上的循环伏安图,缓冲溶液为pH=6.5的0.1 mol/L磷酸缓冲溶液,由图5可以看出,超声3 h的石墨烯修饰电极(G@3h/DMF/GCE)对TBBPA的峰电流响应最高,因此,本实验选择石墨烯超声剥离时间为3 h.

图5 10-5mol/L TBBPA溶液在不同超声时间剥离石墨烯的修饰电极上循环伏安图Fig.5 Cyclic voltammograms of GCEs modified electrodes at different ultrasonic times in 10-5mol/L TBBPA
2.3.2 石墨烯质量浓度 为研究功能化石墨烯质量的浓度对检测TBBPA的影响,将不同质量浓度(0.1mg/mL,0.2mg/mL,0.5mg/mL,1mg/mL,2mg/mL,3 mg/mL和4 mg/mL)功能化石墨烯的修饰电极(G@3h/DMF/GCE)在含有10-5mol/L的TBBPA溶液中进行循环伏安扫描,缓冲溶液为pH=6.5的0.1 mol/L磷酸缓冲溶液.实验结果如图6所示,氧化峰电流随功能化石墨烯修饰浓度增加而先增大后减小,最佳修饰浓度为2 mg/mL.因此,本实验选择的功能化石墨烯浓度为2 mg/mL.

图6 剥离石墨烯浓度和TBBPA氧化峰电流的关系Fig.6 Relationship between oxidation peak current and concentration of exfoliated graphene
2.3.3 缓冲液的pH 介质溶液的pH值对TBBPA在修饰电极表面的电化学氧化有影响,采用循环伏安法研究了TBBPA在G@3h/DMF/GCE上于不同pH值(5.8,6.5,7.0,7.4和8.0)的磷酸缓冲溶液介质中的电化学行为.由图7可知,随pH值增加,TBBPA的氧化峰逐渐负移,这说明其氧化过程伴随有质子的转移.pH=6.5时TBBPA的峰形最好且峰电流最大,因此,本实验选择pH=6.5的磷酸缓冲溶液为介质.
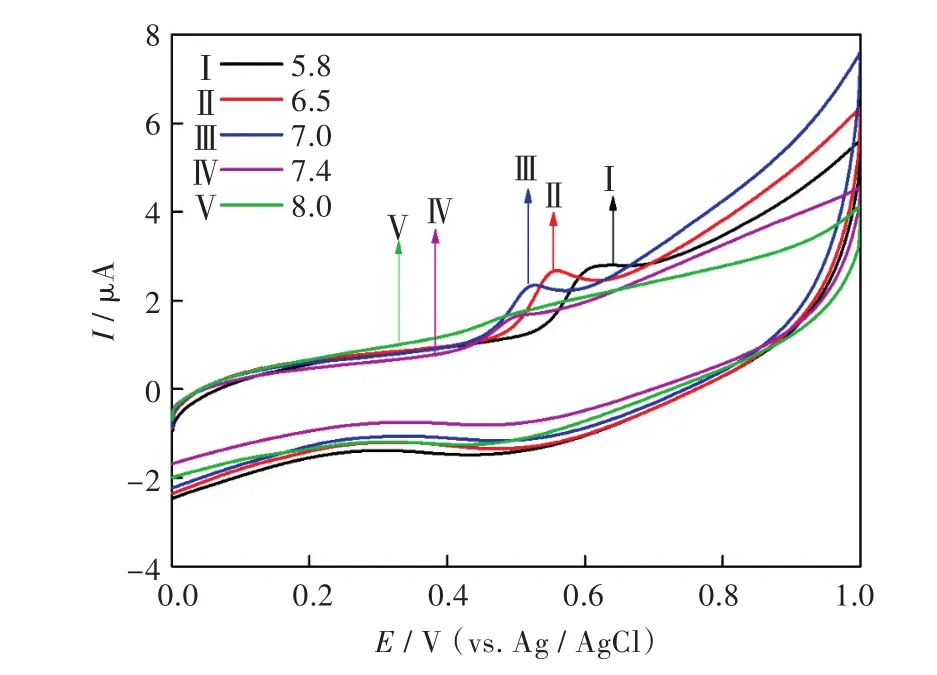
图7 在不同pH的磷酸缓冲液中10-5mol/LTBBPA在G@3h/DMF/GCE上的循环伏安图Fig.7 Cyclic voltammograms of G@3h/DMF/GCE in phosphate buffer solution(containing 10-5mol/L TBBPA)with different pH
2.4 扫描速度
通过改变循环伏安的扫速对TBBPA在G@3h/DMF/GCE电极表面的反应过程进行了探讨.研究了不同扫描速度(20 mV·s-1、40 mV·s-1、60 mV·s-1、80 mV·s-1、100 mV·s-1和 120 mV·s-1)下 TBBPA 在修饰电极上的电化学行为.结果如图8所示.由图可知,TBBPA的氧化峰电流值随扫速的增大而增大,且氧化峰电流与扫描速度呈线性关系,线性方程为:Ipa=0.007 9v+0.147 6(R=0.997).说明TBBPA在G@3h/DMF/GCE上的氧化还原过程是吸附控制过程.
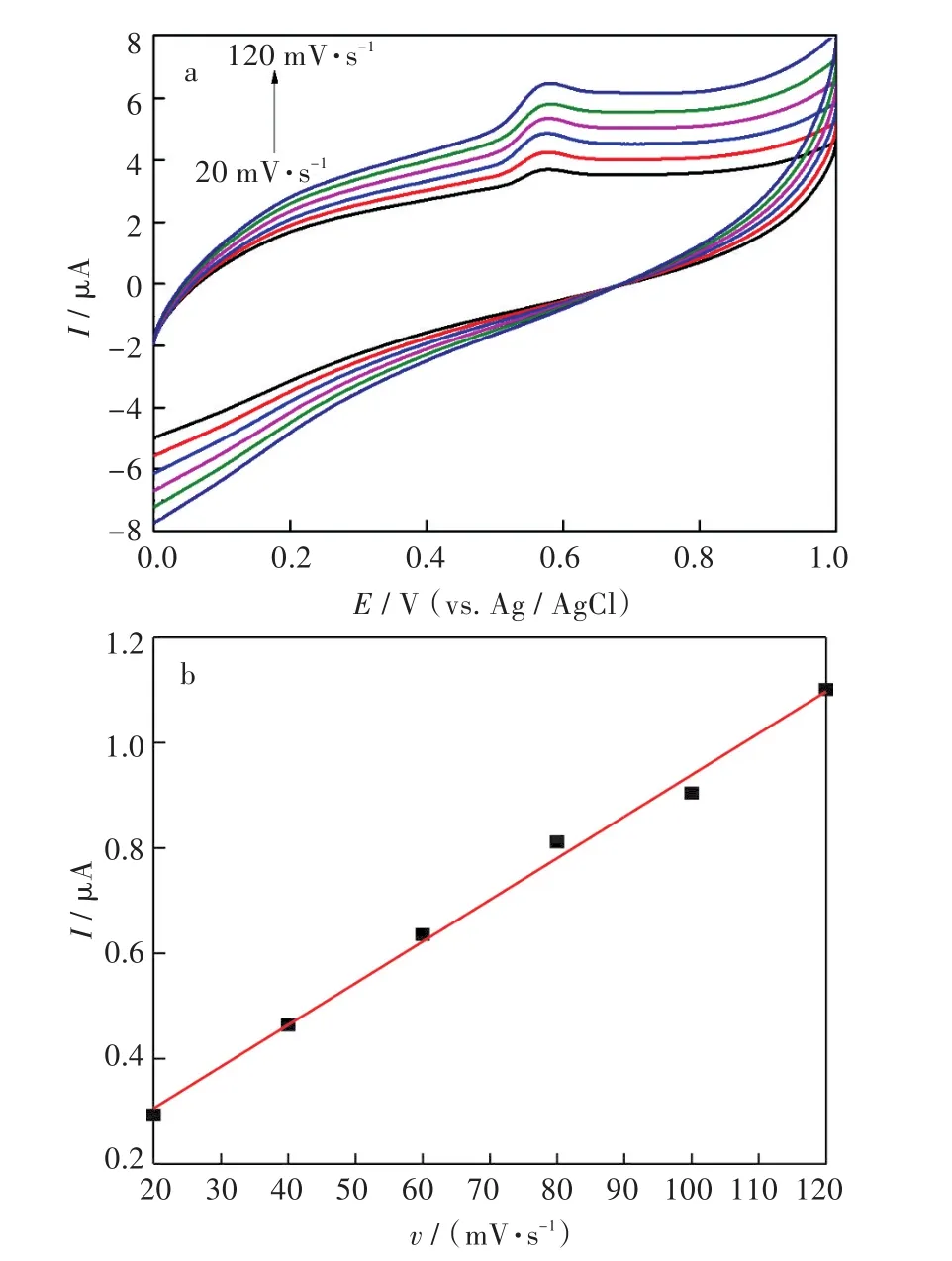
图8 (a)不同扫描速度下,10-5mol/L TBBPA的循环伏安图;(b)氧化峰电流与扫描速度的线性关系Fig.8 (a)Cyclic voltammograms of 10-5mol/LTBBPA at different scan rates;(b)Relationship between scan rate and anodic peak currents
2.5 检出限和线性范围
在最佳条件下,利用G@3h/DMF/GCE研究TBBPA的氧化峰电流与浓度关系,图9(a)是G@3h/DMF/GCE对不同浓度的多巴胺的差示脉冲伏安曲线.如图 9(b),在 0.1 μmol/L~14 μmol/L 浓度范围内,TBBPA的氧化峰电流与其浓度呈良好的线性关系,线性方程为Ipa=0.095 6+0.065 3 cTBBPA(Ipa:μA;cTBBPA:μmol/L,R=0.999 3),最低检出限为5×10-8mol/L(S/N=3).
2.6 电极的选择性
为了考查G@3h/DMF/GCE的选择性,采用计时电流法研究了几种常见金属离子(10-3mol·L-1的 NaCl,CuSO4)和有机物[2×10-5mol·L-1的对苯二酚(hydroquinone,HQ),五氯酚(pentachlorophenol,PCP),壬基酚(nonylphenol,NP),辛基酚(octylphenol,OP),双酚 A(bisphenol A,BPA)]对 TBBPA 测定的影响,如图10所示.图10结果说明NaCl、CuSO4、HQ、PCP、NP、OP和 BPA对 TBBPA的检测均不干扰,说明该修饰电极具有良好的选择性.

图9 (a)TBBPA在G@3h/DMF/GCE上的差示脉冲伏安图;(b)TBBPA的氧化峰电流与浓度的关系Fig.9 (a)Differential pulse voltammetrys of G@3h/DMF/GCE in TBBPA with different concentrations;(b)Relationship between oxidation peak current and concentration of TBBPA

图10 TBBPA和几种干扰物在G@3h/DMF/GCE上的电流-时间曲线(应用电位为+0.533 V)Fig.10 I-t curves of G@3h/DMF/GCE in TBBPA and some interferents at+0.533 V
2.7 方法的准确度
为了检验方法的准确度,对TBBPA的模拟水样进行了加标回收实验.实验结果见表1,回收率在98.0%~101.0%之间,因此,G@3h/DMF/GCE电极的准确度高,可用于实际水样中TBBPA的检测.

表1 水样中TBBPA的回收率(n=3)Tab.1 Recoveries of TBBPA in water samples(n=3)
2.8 电极的稳定性和重现性
在相同条件下制备了5支G@3h/DMF/GCE,分别测定这5支电极对TBBPA的响应,测得5支修饰电极的氧化峰电流值的RSD为1.85%.再将电极放置30 d(4℃),该修饰电极的电流响应值仅衰减了7.39%.上述结果表明G@3h/DMF/GCE具有良好的重复性和稳定性.
3 结 语
本文以DMF为剥离溶剂,以柠檬酸钠为助剂,采用液相超声剥离法制备了功能化石墨烯纳米片,并将其应用于修饰电极的制备.所制备的修饰电极对TBBPA的电催化氧化活性明显提高.所构建的新型TBBPA电化学传感器的氧化峰电流与其浓度在 0.1 μmol·L-1~14 μmol·L-1范围内呈良好的线性关系,其检出限为 5×10-8mol·L-1,建立了一种高灵敏度、快速简便的TBBPA电化学检测新方法,并用于实际样品分析,准确度高、实用性好.
[1] GEIM A K,NOVOSELOV K S.The rise of graphene[J].Nature,Materials,2007,6:183-191.
[2] NETO A H C,GUINEA F,PERES N M R,et al.The electronic of properties of graphene[J].Reviews of Modern Physics,2009,81(1):109-162.
[3] ZHANG L,DING Z C,TONG T,et al.Tuning the work functions of graphene quantum dot-modified electrodes for polymer solar cell applications[J].Nanoscale,2017,9(10):3524-3529.
[4] QU W,LIU W,LI X, etal.A triboelectric charge top-gated graphene transistor[J].Diamond and Related Materials,2017,73:33-38.
[5] SCHEDIN F,GEIN A K,MOROZOV SV,et al.Detection of individual gas molecules adsorbed on graphene[J].Nature Materials,2007,6(9):652-655.
[6] KIMMEL D W,LEBLANC G,MESCHIEVITZ M E,et al.Electrochemical sensors and biosensors [J].Analytical Chemistry,2011,84(2):685-707.
[7] SHANG N G,PAPAKONSTANTINOU P,MCMULLAN M,et al.Catalyst-free efficient growth,orientation and biosensing properties of multilayer graphene nanoflake films with sharp edge planes[J].Advance Functional Materials,2008,18(21):3506-3514.
[8] LIU H,GAO J,XUE M Q,et al.Processing of graphene for electrochemical application:noncovalently functionalize graphene sheets with water-soluble electroactive methylene green[J].Langmuir,the Acs JournalofSurfaces & Colloids,2009,25 (20) :12006-12010.
[9] TANG L,LI W Y,FENG H,et al.Preparation,structure,and electrochemical properties of reduced graphene sheet films [J]. Advance Functional Materials,2009,19(17):2782-2789.
[10] WU J F,XU M Q,ZHAO G C.Graphene-based modified electrode for the direct electron transfer of cytochrome c and biosensing[J].Electrochemistry Communications,2010,12(1):175-177.
[11] WANG Y,WAN Y,ZHANG D.Reduced graphene sheets modified glassy carbon electrode for electrocatalytic oxidation of hydrazine in alkaline media[J].Electrochemistry Communications,2010,12(2):187-190.
[12] SHAN C S,YANG H F,SONG J F,et al.Direct electrochemistry of glucose oxidase and biosensing for glucose based on graphene[J].Analytical Chemistry,2009,81(6):2378-2382.
[13] WANG Y,LI Y,TANG L,et al.Application of graphene-modified electrode for selective detection of dopamine [J]. Electrochemistry Communications,2009,11(4):889-892.
[14] ZHOU M,ZHAI Y,DONG S.Electrochemical sensing and biosensing platform based on chemically reduced graphene oxide[J].Analytical Chemistry,2009,81(14):5603-5613.
[15] COLEMAN J N.Liquid-phase exfoliation of nanotubes and graphene[J].Advanced Functional Materials,2009,19(23):3680-3695.
[16] NARAYAN R, LIM J, JEON T, et al.Perylenetetracarboxylate surfactant assisted liquid phase exfoliation of graphite into graphene nanosheets with facile re-dispersibility in aqueous/organic polar solvents[J].Carbon,2017,119:555-568.
[17] ARAO Y,MORI F,KUBOUCHI M.Efficient solvent systems for improving production of few-layer graphene inliquidphaseexfoliation[J].Carbon,2017,118:18-24.
[18] HYOETYLAEINEN T,HARTONEN K.Determination ofbrominated flame retardants in environmental samples[J].Trends in Analytical Chemistry,2002,21(1):13-20.
[19] GUO Y N,CHEN L,MA F Y,et al.Efficient degradation of tetrabromobisphenol A by heterostructured Ag/Bi5Nb3O15material under the simulated sunlight irradiation [J]. Journal of Hazardous Materials,2011,189(1/2):614-618.
[20] COVACI A,VOORPOEL S,ABDALLAH M A E,et al.Analytical and environmental aspects of the flame retardant tetrabromobisphenol-A and its derivatives[J].JournalofChromatographyA,2009,1216(3):346-363.
[21] KADASALA N R,WEIA.Trace detection of tetrabromobisphenol A by SERS with DMAP-modified magnetic gold nanoclusters[J].Nanoscale,2015,7(25):10931-10935.
[22] XIE Z Y,EBINGHAUS R,LOHMANN R,et al.Trace determination of the flame retardant tetrabromobisphenolA in the atmosphere by gas chromatography-massspectrometry [J]. Analytical Chimica Acta,2007,584(2):333-342.
[23] ZHAO R,X WANG ,YUAN J.Highly sensitive determination of tetrabromobisphenol A and bisphenol A in environmental water samples by solid-phase extraction and liquid chromatography-tandem mass spectrometry[J].Journal of Separation Science,2010,33(11):1652-1657.
[24] COVACI A,VOORSPOELS S,RAMOS L.Recent developments in the analysis of brominated flame retardants and brominated natural compounds [J].Journal of Chromatography A,2007,1153(1/2):145-171.
[25] CHU S G,HAFFER G D,LETCHER R J.Simultaneous determination of tetrabromobisphenol A,tetrachlorobisphenol A,bisphenol A and other halogenated analogues in sediment and sludge by high performance liquid chromatography-electrospray tandem mass spectrometry [J]. Journal of Chromatography A,2005,1097(1/2):25-32.
[26] HAYAMA H,YOSHIDA H,ONIMARU S,et al.Determination of tetrabromobisphenol A in human serum by liquid chromatography-electrospray ionization tandem mass spectrometry[J].Journal of Chromatography B,2004,809(1):131-136.
An Electrochemical Sensor for Tetrabromobisphenol A Determination Based on Functionalized Graphene
WU Shengli,LIU Yi,SUN Yanjuan,CAO Yuan,ZOU Jing*School of Chemistry and Environmental Engineering,Wuhan Institute of Technology,Wuhan 430205,China
An efficient electrochemical sensor based on the functionalized ultra-thin graphene nanosheets was fabricated,where the graphene nanosheets were prepared by an exfoliating technique with the exfoliation solvent(N,N-dimethylformamide)and the auxiliary agent(sodium citrate).The resulting samples were characterized by transmission electron microscopy.The result indicated that in comparison with bulk materials,the resulting samples were thinner graphene nanosheets with monolayer or few layers.Tetrabromobisphenol A(TBBPA)electrochemical sensor with high sensitivity was developed by using the functionalized graphene nanosheets after a series of optimization of the ultrasonic time,graphene mass concentration and solution pH.Electrochemical impedance spectroscopy showed that the electrochemical sensor exhibited a faster electron transfer rate.Moreover,the functionalized graphene nanosheets electrochemical sensor displayed linear range(0.1 μmol/L-14 μmol/L)and a lower limit of detection[5×10-8mol/L(S/N=3)]for TBBPA,respectively.And the fabricated electrochemical sensor was successfully applied in the detection of real water samples,and the recoveries were between 98%and 101%.In addition,this electrochemical sensor also exhibited good accuracy sensitivity,selectivity and stability.
graphene;exfoliation;N,N-dimethylformamide;tetrabromobisphenol A;electrochemical sensor
2017-08-28
国家自然科学基金(21471122);武汉工程大学研究生创新基金(2016171)
吴生丽,硕士研究生.E-mail:807886988@qq.com
*通讯作者:邹 菁,博士,教授,博士研究生导师.E-mail:jingzou@wit.edu.cn
吴生丽,刘忆,孙艳娟,等.功能化石墨烯四溴双酚A的电化学传感研究[J].武汉工程大学学报,2017,39(5):432-437.
WU S L,LIU Y,SUN Y J,et al.An electrochemical sensor for tetrabromobisphenol A determination based on functionalized graphene[J].Journal of Wuhan Institute of Technology,2017,39(5):432-437.
O0647
A
10.3969/j.issn.1674-2869.2017.05.005
1674-2869(2017)05-0432-06
苗 变

