3D打印石墨烯制备技术及其在储能领域的应用研究进展
王 楠,燕绍九,彭思侃,陈 翔,戴圣龙
(中国航发北京航空材料研究院 石墨烯及应用研究中心,北京 100095)
3D打印石墨烯制备技术及其在储能领域的应用研究进展
王 楠,燕绍九,彭思侃,陈 翔,戴圣龙
(中国航发北京航空材料研究院 石墨烯及应用研究中心,北京 100095)
石墨烯优异的力学和物理性能使其成为理想的储能材料。因结构精确可控,易实现规模化制备,3D打印石墨烯材料有望在储能领域得到广泛应用。本文全面综述了3D打印石墨烯制备技术及其在储能领域的应用研究进展。石墨烯墨水的黏度和可打印性是实现石墨烯3D打印的制约因素。实现工艺简单、浓度可控、无黏结剂石墨烯墨水的规模化打印将成为3D打印石墨烯制备技术未来的研究热点。石墨烯超级电容器、锂硫电池、锂离子电池等储能元件一体化打印成型是3D打印石墨烯在储能领域应用的发展方向。
3D打印;石墨烯;储能;超级电容器;锂离子电池
石墨烯纳米片是从石墨材料中剥离出来、由碳原子组成的单层原子厚度的二维晶体,6个碳原子以sp2杂化方式构成蜂窝状晶格[1,2]。石墨烯展现出超高的强度,优异的热导率、透光率以及柔性轻质特性[3]。此外,石墨烯具有巨大的比表面积和超高的电导率,其理论比表面积>2500m2·g-1[4],电子迁移率高达200000cm2·V-1·s-1[5]。凭借独特的物理、化学性能,石墨烯在纳米电子、传感器、复合材料、生物支架,尤其是储能元件方面展现出广阔的应用前景[6-12]。
然而,研究发现二维石墨烯倾向于团聚或堆叠以降低表面能,这极大削弱了石墨烯的优异性能[13]。但三维石墨烯同时具备多孔结构和石墨烯优异的固有特性,这不仅为电荷储存提供额外离子可接触表面,同时有利于离子在其中传输[14]。从而使得由三维石墨烯构成的器件有望在储能领域表现更为突出。三维石墨烯制备技术存在的最大挑战是在保留石墨烯固有优异性能的同时,实现结构可设计的规模化制备[15]。目前三维石墨烯的制备方法主要包括模板法和非模板法(表1)。模板法包括CVD法[16-20]、水热/溶剂热合成法[21,22]、电沉积法[18]、溶胶-凝胶合成法[23]。模板法可控制三维石墨烯内部孔径尺寸,但很难实现规模化制备。同时模板法制备出的三维石墨烯的力学性能通常并不理想,表现出可压缩性差的特点[15]。非模板法包括真空抽滤法[24]、水热凝胶合成法[25]、真空离心蒸发法[26]。非模板法虽能实现规模化制备,但三维石墨烯的形状仍然存在随机性。因此,以上三维石墨烯制备方法都无法同时满足形状精确可设计和可规模化制备的要求。3D打印技术具有打印结构可设计,易实现快速、规模化制造的优点。将3D打印技术应用到三维石墨烯制备中,有望解决上述问题。
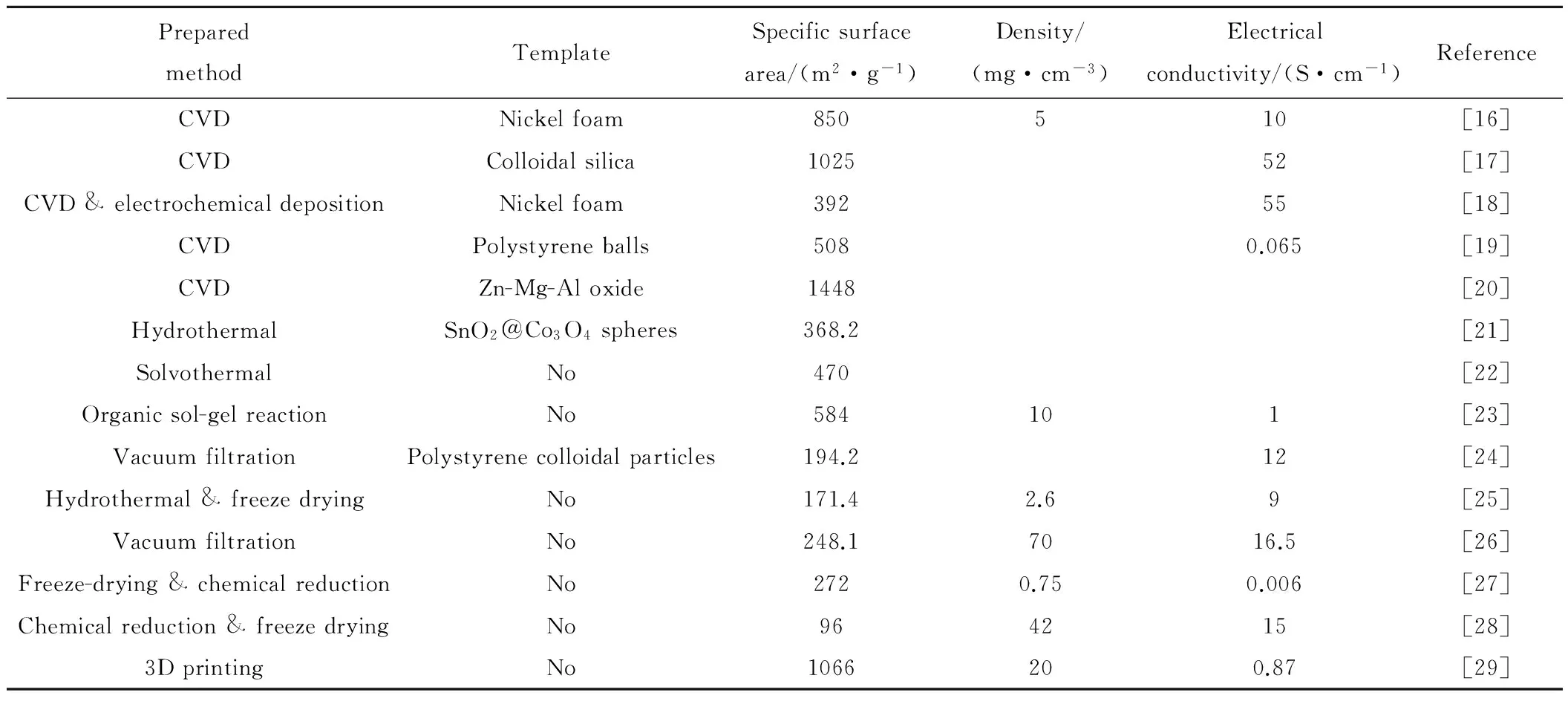
表1 三维石墨烯材料主要制备方法Table 1 Main methods to synthesize three dimensional graphene materials
3D打印技术,又称增材制造(additive manufacturing)技术。根据美国材料与试验协会(ASTM)公布的定义,增材制造是一种与传统的材料加工方法截然相反的、基于三维CAD 模型数据、通过增加材料逐层制造的方式[30]。现有3D打印技术种类众多,主要包括熔融沉积成型(Fused Deposition Modeling,FDM)、直接喷墨打印技术(Direct Ink Writing,DIW)、立体平板印刷(Stereolithography,SLA)和选择性激光熔化技术(Selective Laser Melting,SLM)等。同时,3D打印材料是3D打印技术发展的重要“物质基础”,决定3D打印技术能否有更广泛的应用。不同3D打印材料的特点不同,但打印材料的力学性能、加工性能、耐热性、耐腐蚀性、化学稳定性通常是制约3D打印技术发展的因素。目前,3D打印材料主要包括热塑性塑料、光硬化树脂、陶瓷材料、橡胶材料、金属材料以及石墨烯等。3D打印技术及打印材料归纳于表2。
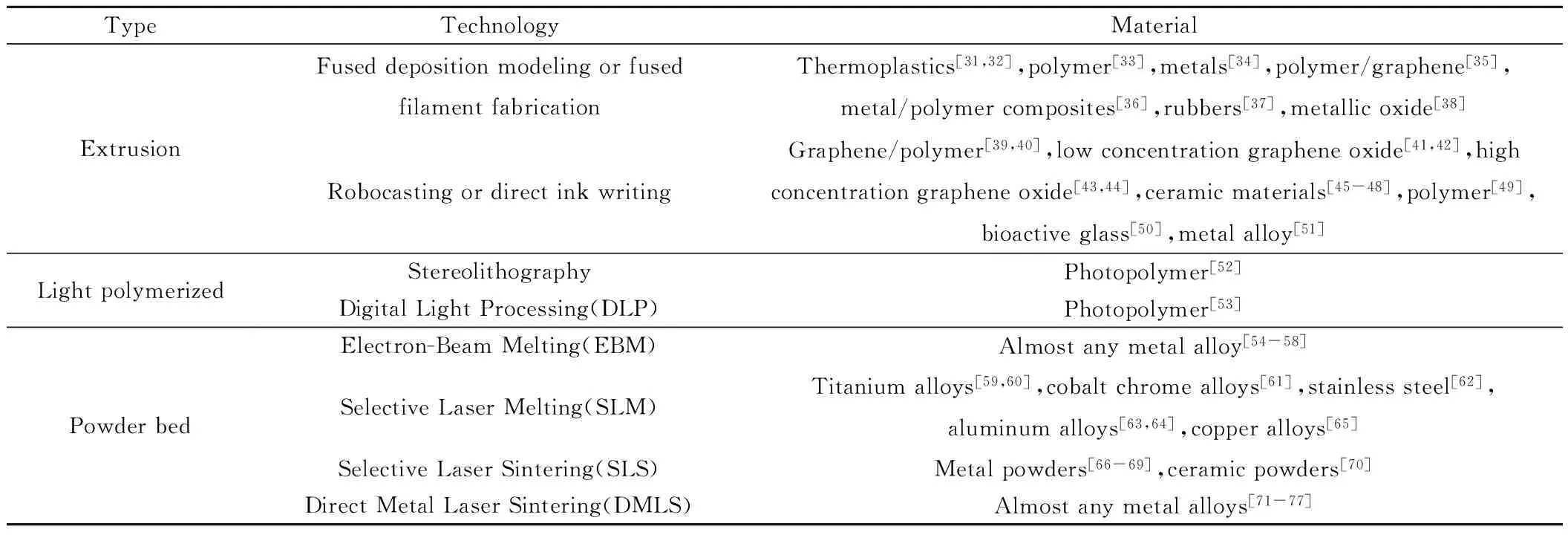
表2 3D打印技术及打印材料Table 2 3D printing technologies and materials
以热塑性材料/石墨烯混合物作为打印材料的熔融沉积成型3D打印技术可被视为石墨烯3D打印技术的雏形。随后,有研究者采用直接喷墨打印技术,以氧化石墨烯(GO)/聚合物或氧化石墨烯为墨水,通过逐层堆叠方式制备出多种结构三维石墨烯。直接喷墨打印技术是最常用的石墨烯3D打印技术,该技术是将墨水装入3D打印机喷墨打印腔室,对腔室内的墨水施加一定压力,墨水被从腔室前端喷头挤出,层层堆叠,同时在三维空间移动喷头即可打印出经结构设计的三维石墨烯。石墨烯3D打印技术不仅能实现三维石墨烯的规模化制备,还能精确控制三维石墨烯的形状,使其在储能领域的工程应用成为可能。随着石墨烯3D打印技术的不断进步,今后有望实现储能元件的整体、快速及规模化生产。
1 3D打印石墨烯研究进展
3D打印石墨烯研究以解决墨水可打印性的研究历程为主线。起初,研究者们尝试将石墨烯添加到热塑性聚合物中以提高其导电性。随着石墨烯3D打印技术的发展,直接喷墨打印技术成为石墨烯3D打印技术主流。其中,石墨烯墨水的黏度和可打印性是该打印技术的关键因素。然而,因石墨烯墨水黏度不足,可打印性差,随后的研究工作都是围绕解决石墨烯墨水可打印性展开的。导电聚合物以黏结剂的形式与石墨烯混合以增加墨水黏度。另外,在加入导电聚合物的同时,控制墨水的pH值也能提高墨水的可打印性。随着打印技术的改进,纳米3D打印技术可将无黏结剂的氧化石墨烯墨水打印成纳米尺度三维石墨烯。同时,冷冻铸造方法也可实现石墨烯3D打印,从而实现低浓度(<13.35mg/mL)氧化石墨烯3D打印。研究发现,高浓度(>13.35mg/mL)氧化石墨烯墨水具有可打印性,这为纯石墨烯打印提供了基础。随后,高浓度氧化石墨烯成功实现3D打印。高浓度氧化石墨烯3D打印被视为较具应用潜力的石墨烯3D打印技术。
1.1 3D打印聚合物/石墨烯复合材料
热塑性聚合物常被用于熔融沉积成型3D打印。为提高热塑性材料电导率,将适量石墨烯以导电添加剂形式与热塑性材料混合,打印出聚合物/石墨烯复合材料。Wei等[35]采用熔融沉积成型打印技术,打印出丙烯腈-丁二烯-苯乙烯共聚物(ABS)/石墨烯复合材料。复合材料中石墨烯的质量分数最高为5.6%。为避免团聚,采用氧化石墨烯作为复合材料导电添加剂的前驱体,并在混合后采用肼将氧化石墨烯还原。
3D打印材料制备过程:首先将氧化石墨烯和ABS分别溶于N-甲基吡咯烷酮(NMP)中,随后将两种溶液混合搅拌。氧化石墨烯经肼还原后,放入水中以使ABS/还原氧化石墨烯复合材料与NMP分离。最后,经真空干燥得到ABS/还原氧化石墨烯复合材料,后经挤压成细丝。打印过程中复合材料细丝被加热到玻璃化温度以上,经喷嘴挤出,以堆叠方式进行3D打印。研究发现,石墨烯的质量分数在5.6%以下时,复合材料可从喷嘴平滑地挤出。但当石墨烯的质量分数增加至7.4%时,石墨烯发生团聚,从而堵塞喷嘴。如采用高能量分散技术,可使石墨烯分散更均匀,石墨烯含量也可进一步提高。电导率测试结果表明,复合材料的电导率随石墨烯含量的增加而显著增加。复合材料的导电机理可以用渗流模型[78]解释,当石墨烯联结成导电网络后,电导率出现突变。石墨烯的分散性直接影响复合材料的可打印性和其他物理性能。为此,改进石墨烯纳米片在复合材料中的分散性是后续研究工作重点。以石墨烯作为导电添加剂的3D打印聚合物/石墨烯复合材料受制于石墨烯难以分散的问题,石墨烯含量难以提高,严重影响复合材料导电性,同时也未充分发挥石墨烯本身优异特性。除大功率超声分散技术外,采用石墨烯表面化学修饰以及使用表面活性剂等方法也可解决石墨烯难以分散的问题。
除将石墨烯作为导电添加剂外,还希望用高石墨烯含量墨水打印三维石墨烯,以最大限度利用三维石墨烯本身高比表面积、低密度、高电导率等优异性能。然而,要实现这一目标,3D打印石墨烯墨水需要满足如下要求:(1)墨水应具有一定黏弹性,以满足墨水持续流动性,并确保打印后能保持打印形状;(2)墨水具有一定机械强度,以承受后续堆叠墨水的质量而不发生变形,同时还能附着在前一层石墨烯上。由于石墨烯/氧化石墨烯墨水黏弹性不足等问题,纯石墨烯/氧化石墨烯墨水打印在成型上还存在很多困难。研究者们通常在石墨烯/氧化石墨烯墨水中加入聚合物作为黏结剂以提高墨水黏弹性。
Jakus等[39]采用基于喷出原理的直接喷墨打印技术,制备出高石墨烯含量的石墨烯/PLG(polyester polylactide-co-glycolide)复合材料。将不同比例石墨烯与PLG在二氯甲烷(DCM)中混合,同时溶液中还加入表面活性剂2-丁氧基乙醇以及塑化剂酞酸二丁酯。均匀搅拌溶液,直到静态剪切速率黏度达到30Pa·s左右。打印过程中石墨烯/聚合物墨水从直径100μm喷嘴,以大于40mm/s的速率喷出,层层堆叠出网格结构。力学性能测试结果表明,石墨烯含量超过20%(体积分数,下同)后,复合材料抗拉强度与石墨烯含量成反比。含20%石墨烯的复合材料抗拉强度较纯PLG材料有所提高,应变达210%。然而含60%石墨烯的复合材料抗拉强度出现显著下降,应变为81%。这一结果被解释为,拉伸载荷最初由PLG弹性体承担,石墨烯含量增加并超过40%后,PLG弹性体无法包覆并紧固周围的石墨烯片,从而导致复合材料的强度和弹性模量都大幅降低。研究表明,挤压过程中剪切力使得石墨烯片沿着流动方向重新定位和排列,导致打印出的复合材料具有各向异性结构与性能。电导率测试结果表明,复合材料沿挤出方向的电导率与喷嘴直径成反比。这一规律很可能与挤出细丝的微观结构有关,小口径喷嘴在墨水挤出过程中能提供更大的切变速率,使得石墨烯片排列更加整齐。另外,经测量复合材料电导率大于800S·m-1,且随石墨烯含量的增加而增加。该研究工作利用聚合物作为黏结剂,成功实现石墨烯/聚合物复合材料3D打印。同时,对挤出过程中剪切应力对复合材料力学性能和电学性能的探讨为后续研究提供可借鉴的依据。然而,该研究工作仍未能实现纯石墨烯的3D打印。
1.2 3D打印低浓度氧化石墨烯
虽然在墨水中添加黏结剂可增加墨水的黏性,保证三维石墨烯的良好成型性。然而,引入黏结剂会在一定程度上影响3D打印三维石墨烯的导电性。研究表明,将特殊成型工艺与直接喷墨打印技术相结合可打印出无黏结剂的三维石墨烯。Kim等[41]将直接喷墨打印技术与微打印技术相结合,成功实现无黏结剂石墨烯纳米尺度3D打印。氧化石墨烯墨水浓度为1g·L-1,氧化石墨烯片径尺寸为1,3,5μm。打印过程中,氧化石墨烯墨水被装入微吸液管中,室温下挤压石墨烯墨水,同时利用弯液面成型原理在滴管尖端形成纳米线,并进行局部堆叠。微吸液管尖端直径为1.3μm,石墨烯纳米线直径为150nm。石墨烯纳米线直径与墨水挤出速率直接相关,可通过控制墨水挤出速率来控制石墨烯纳米线直径。随后,将得到的氧化石墨烯进行热还原或化学还原以得到还原氧化石墨烯。电导率测试结果表明,室温下单根石墨烯纳米线的电导率为11.3S·cm-1,同时以单根石墨烯纳米线为导线连接两个金电极组成的电路可以点燃一个LED灯。该研究工作成功实现石墨烯微观尺度3D打印,同时还能精确控制纳米丝线直径。该打印技术能在微尺度上打印出多种结构三维石墨烯器件,这些器件有望应用在电子设备上。然而,如何将石墨烯纳米线直径控制在10nm以下仍然是一项挑战。要减小纳米线尺寸,3D打印过程中墨水流动性的细节还有待研究,如墨水黏度研究,喷嘴尺寸对纳米线直径的影响规律研究等。同时,该打印技术仅限于微观尺度打印,低浓度氧化石墨烯墨水宏观尺度打印还有待进一步研究。
除纳米尺度3D打印,Zhang等[42]也成功实现无黏结剂宏观尺度石墨烯3D打印,如图1所示。与其他通过熔化或者室温下喷射的石墨烯3D打印方法不同,该方法通过纯氧化石墨烯溶液多管嘴喷墨和冷冻铸造技术来实现快速石墨烯3D打印。溶液中纯水结冰后起到支撑结构作用。随后,将三维石墨烯放入液氮中,冷冻干燥以去除水分。最后,经热还原得到3D打印超轻三维石墨烯。石墨烯墨水浓度范围为0.5~10mg·mL-1。

图1 3D打印与冷冻铸造法制备三维石墨烯示意图[42](a)3D打印装置;(b)3D打印冰支撑;(c)3D打印氧化石墨烯;(d)3D打印石墨烯冰结构液氮处理;(e)冷冻干燥;(f)热还原成超轻3D气凝胶Fig.1 Schematic diagrams of three dimensional graphene prepared by integrating process of 3D printing and freeze casting[42](a)3D printing setup;(b)3D printing of ice support;(c)3D printing of GO suspension;(d)immersing printed ice structure into liquid nitrogen;(e)freeze drying;(f)thermally reduced to 3D ultralight graphene aerogels
为研究3D打印石墨烯电子传输行为,对-192~400℃范围内3D打印石墨烯电阻变化进行测试。结果表明,电阻变化表现为负温度系数,并且出现5个不同区域。当实验温度降低到-192℃时,石墨烯电阻急剧增加了97%。当实验温度在-40~60℃之间时,石墨烯电阻保持稳定状态。但是当温度升高到350℃时,其电阻降低了48%。随后,当温度继续升高到400℃时,其电阻没有明显变化。实验结果表明,3D打印石墨烯电阻率和电导率随温度的变化规律为典型半导体行为。同时,3D打印三维石墨烯的电导率随三维石墨烯密度的增加而增加。当三维石墨烯密度从0.5mg·cm-3增加到10mg·cm-3,其电导率从2.2S·m-1增加到15.4S·m-1。
内面压缩测试和动态形变场测试结果表明,3D打印石墨烯具有高度可压缩性。6.21mg三维石墨烯可承重100g而不发生畸变,其载重比高达16100。三维石墨烯表现出非线性超弹性行为和可逆超大压缩性,并且最大应变高达50%。同时,三维石墨烯在50%应变下的应力与其密度呈线性关系。三维石墨烯的杨氏模量与密度的关系可以用方程E=Aρn表示[79],其中E为弹性模量,ρ为有效密度,A为常数,n为指数。3D打印石墨烯的杨氏模量与密度之间关系为:E∞ρ1.4,与通常由水热法或冷冻干燥法制备出的块体三维石墨烯指数(n=2.5)不同。方程指数减小与3D打印石墨烯独特的宏观中空结构有关。同时,该独特结构可使其在密度低于1mg·cm-3时仍然保持高杨氏模量。该研究巧妙地将喷墨打印与冷冻铸造技术相结合,实现无添加剂石墨烯3D打印。该方法可打印出不同密度三维石墨烯,甚至可低于1mg·cm-3。然而,打印过程所要求的低温环境将限制其向规模化制备方向的发展。
1.3 3D打印高浓度氧化石墨烯
研究发现,低浓度氧化石墨烯溶液表现出类液晶性质[80]。当氧化石墨烯浓度超过13.35mg·mL-1[81]时,其独特的黏弹性行为使得氧化石墨烯溶液可被用于凝胶喷射打印工艺。2015年,Zhu等[29]采用直接喷墨打印技术,以高浓度氧化石墨烯为墨水打印出具有优异可压缩性的三维石墨烯宏观体。墨水流变性能可通过其表观黏度与剪切速率以及模量与剪切应力之间的关系进行表征(图2)。氧化石墨烯墨水浓度分别为20,40mg·mL-1,采用碳酸铵和R-F(间苯二酚-甲醛溶液)2种凝胶化添加剂。结果表明,氧化石墨烯浓度对墨水的流变性能影响巨大。当浓度从20mg·mL-1增加到40mg·mL-1时,墨水表观黏度增加近1个数量级(图2(a));同样地,其储能模量和损耗模量也分别增加1个数量级(图2(b))。为进一步增加石墨烯墨水黏性,改善流动性,还可向其中加入气相二氧化硅粉末。研究表明,加入后明显增强氧化石墨烯墨水的流变性能,其表观黏度、储能模量及损耗模量较纯氧化石墨烯墨水都增加1个数量级。
打印过程中氧化石墨烯墨水从喷嘴挤出,堆叠出三维结构,如图3所示[43]。打印出的宏观体被密封在玻璃瓶中,并在85℃环境下加热,以使其凝胶化。石墨烯中的二氧化硅可通过氢氟酸刻蚀法去除。随后,在1050℃高温下采用超临界二氧化碳干燥法干燥三维石墨烯宏观体。研究发现,通过改变墨水中凝胶化物质成分可调控三维石墨烯微观结构。3D打印得到的三维石墨烯的物理性能如表3所示[29],三维石墨烯的物理性能可以通过改变初始氧化石墨烯溶液中添加的R-F溶液浓度加以调节。力学性能测试结果表明,3D打印所得到的三维石墨烯具有优异的应变记忆效果,超强可压缩性,压缩变形量可达90%。2016年,Zhu等[43]在原有高浓度氧化石墨烯墨水中掺入不同比例的石墨烯纳米片,进一步提高三维石墨烯电导率。另外,该三维石墨烯作为电极材料被进一步应用在超级电容器中。
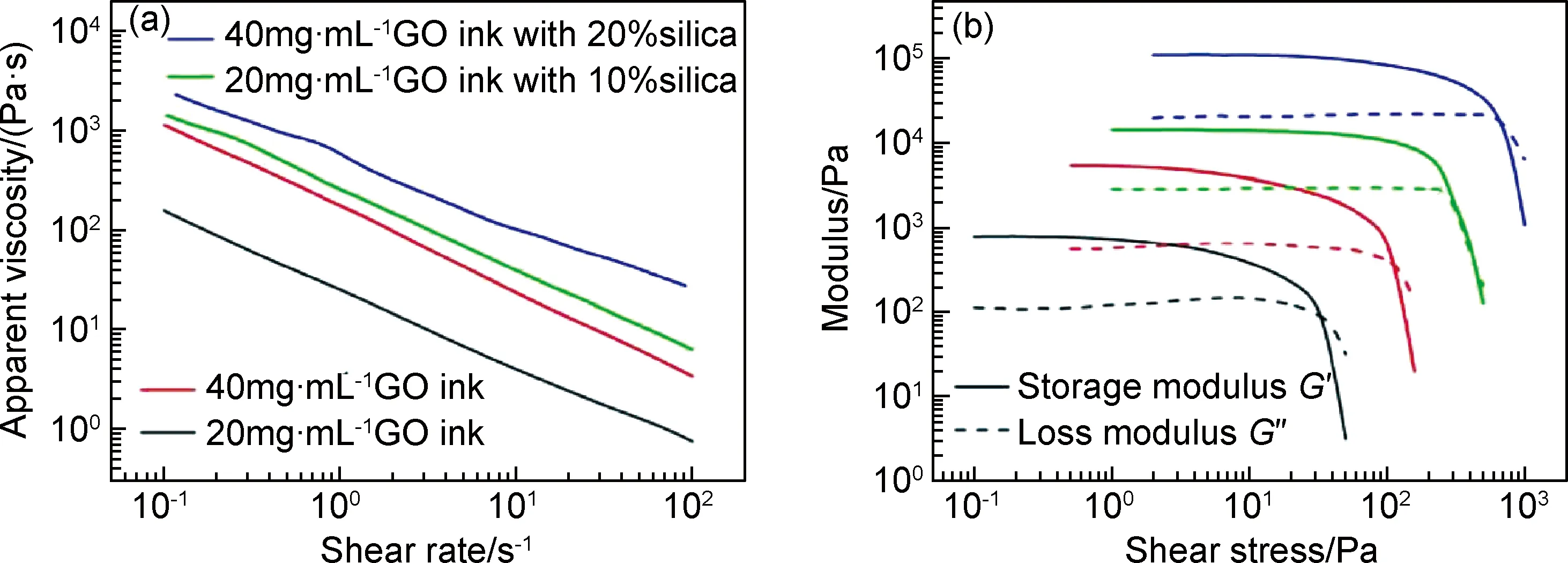
图2 添加与未添加二氧化硅时氧化石墨烯墨水的流变性能[29](a)剪切速率与表观黏度的关系;(b)剪切应力与储能模量和损耗模量的关系Fig.2 Rheological properties of GO ink with and without silica fillers[29](a)apparent viscosity as a function of shear rate;(b)storage modulus and loss modulus as a function of shear stress
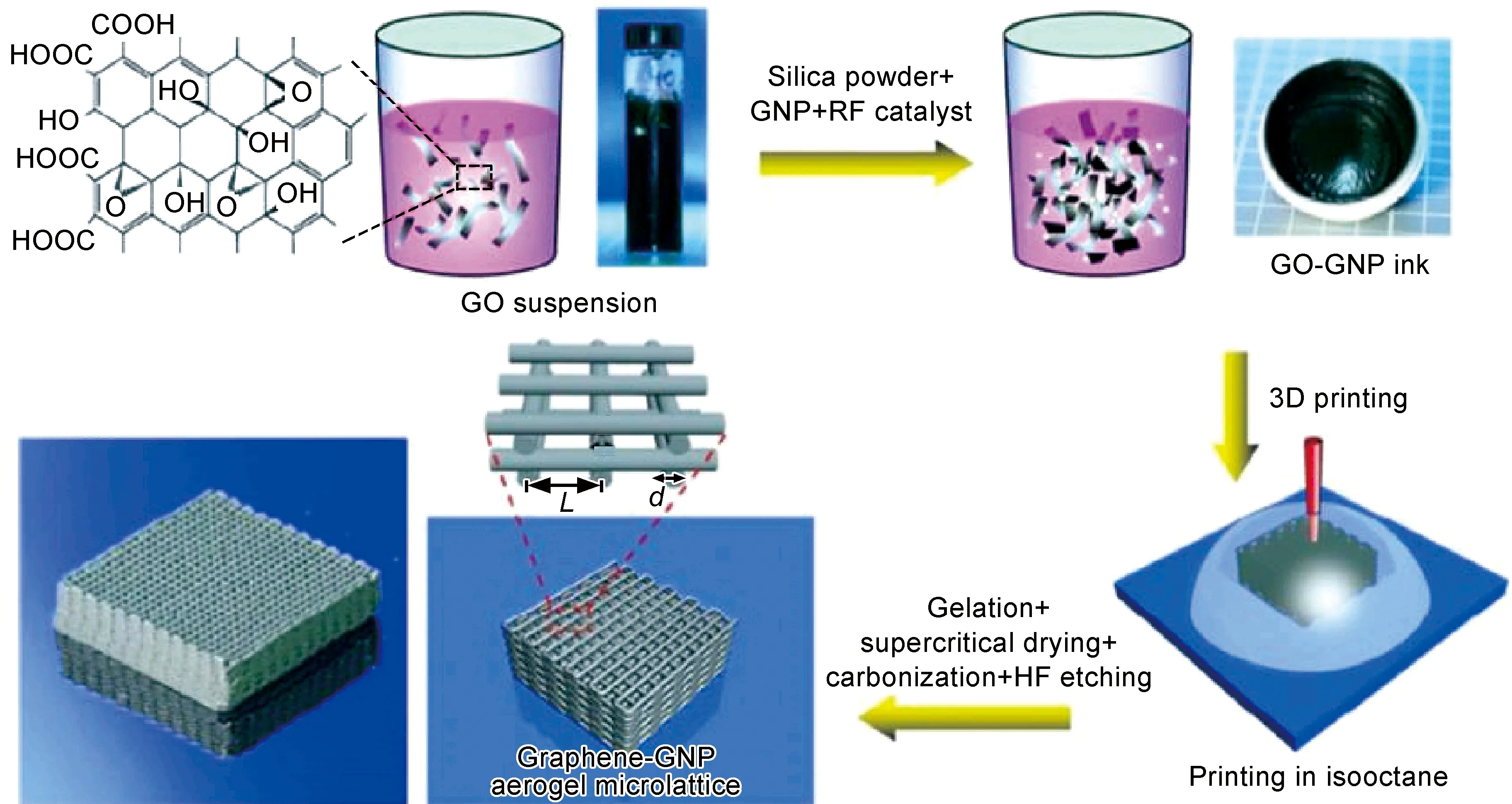
图3 高浓度氧化石墨烯3D打印示意图[43]Fig.3 Schematic diagrams of high concentration GO 3D printing[43]
目前,40mg·mL-1氧化石墨烯墨水已具备良好可打印性[29]。研究表明,提高氧化石墨烯墨水浓度会进一步增强墨水可打印性。Fu等[44]采用高浓度氧化石墨烯(85mg·mL-1),成功打印出锂离子正极材料磷酸铁锂(LiFePO4,LFP)/还原氧化石墨烯、负极材料钛酸锂(Li4Ti5O12,LTO)/还原氧化石墨烯、固态电解质及隔膜材料,实现锂离子电池主要部件整体打印。正极材料打印墨水为高浓度氧化石墨烯与磷酸铁锂混合物。负极材料打印墨水为高浓度氧化石墨烯与钛酸锂混合物。聚偏氟乙烯共联六氟丙烯(PVDF-co-HFP)与氧化铝颗粒混合物墨水兼具电解质及隔膜材料作用。研究表明,3种墨水表现出相似的剪切稀化行为,说明它们都是非牛顿流体,可被用作打印墨水。另外,3种墨水在1s-1切变速率下,表观黏度高达102~103Pa·s。高表观黏度保证了墨水的可打印性,也为复杂结构打印提供了基础。同时,3种墨水经长时间静置仍能保持稳定的表观黏度。墨水的储能模量(G′)和损耗模量(G″)分别表征其弹性和黏性性能。电极材料墨水的平台储能模量和损耗模量在104~105Pa和103~104Pa内。2种墨水屈服应力都高达103Pa。墨水的高平台模量和高屈服应力对打印过程中墨水成型性至关重要。打印过程如图4所示[44]。2种电极材料打印墨水分别贮存在2个注射器中,墨水通过压力从喷嘴中挤出,层层堆叠出打印结构。打印产物经过冷冻干燥去除水分并得到三维结构,随后经热还原处理将氧化石墨烯还原。热还原处理工序对提高3D打印三维石墨烯电导率起到至关重要的作用。电导率测试结果表明,经热还原后2种电极材料的电导率分别为31.6,6.1S·cm-1,明显大于未经热处理的电极材料的电导率(10-6~10-7S·cm-1)。
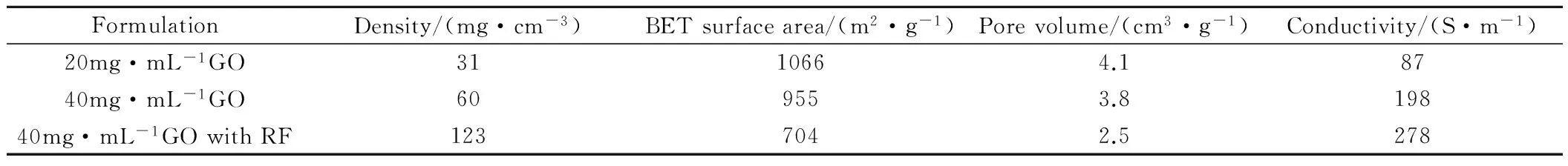
表3 不同配方3D打印石墨烯凝胶的物理性能[29]Table 3 Physical properties of different 3D printed graphene aerogel formulations[29]
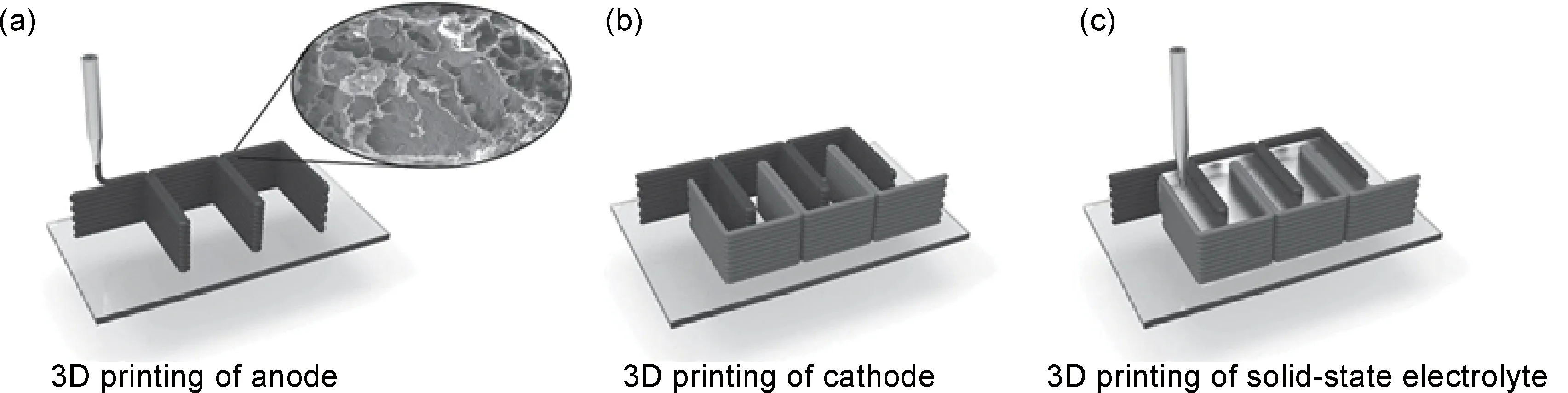
图4 3D打印锂离子电池石墨烯电极示意图[44](a)以LTO/GO为墨水3D打印电池负极;(b)以LFP/GO为墨水3D打印电池正极;(c)3D打印固态电解质Fig.4 Schematic diagrams of 3D printing graphene based electrodes for lithium ion batteries[44](a)LTO/GO ink used to print the anode via 3D printing;(b)LFP/GO ink used to print the cathode via 3D printing;(c)3D printing of solid-state electrolyte
值得一提的是,以氧化石墨烯作为墨水的打印工艺都需要经热还原处理将氧化石墨烯还原成石墨烯。为简化打印工艺,未来可在3D打印机上增加同步加热装置,进而实现3D打印氧化石墨烯同步还原技术。另外,储能元件各部件同时采用3D打印的高度集成化打印工艺,即一体化3D打印技术,也将为3D打印石墨烯规模化制备奠定基础。
2 3D打印石墨烯在储能领域中的应用研究
因兼具规模化制备、形状可控以及结构可设计的特点,3D打印技术已被用于锂离子电池和超级电容器电极材料的制备中。Sun等[82]采用磷酸铁锂LiFePO4(LFP)和钛酸锂Li4Ti5O12(LTO)作为打印墨水,设计并打印出锂离子电池的正负极。随后组装成微电池,并进行电化学性能测试。在2.7mW·cm-2功率密度下,得到的面能量密度为9.7J·cm-2,这使其在微电子和生物医疗设备上的应用潜力巨大。Zhao等[83]采用选择性激光熔化3D打印技术打印出三维钛金属电极,并在金属电极表面沉积聚吡咯。该电极被用作超级电容器极板,电化学测试结果表明,在37.4mA·cm-3电流密度下,电极材料体积比电容为2.4F·cm-3,体积能量密度为213.5Wh·m-3,体积功率密度为15.0kW·m-3。三维石墨烯具有大的比表面积、优异的化学稳定性,尤其是优异的导电性,已经被广泛应用于锂离子电池、锂硫电池、超级电容器等储能元件中。然而随着储能元件应用的发展,其对元件形状及尺寸精度也提出更高的要求。为此,研究者采用3D打印技术制备三维石墨烯,并将其应用到超级电容器和锂离子电池电极上。
Nathan-Walleser等[84]以高浓度还原氧化石墨烯为3D打印墨水,采用3D微喷出打印技术制备出超级电容器电极材料。与其他打印墨水制备工艺不同的是,氧化石墨烯首先在400℃高温下被部分还原成石墨烯,再经高压匀质机加工得到均匀分散的打印墨水。同时,由于得到的还原氧化石墨烯表面仍存在含氧官能团,使其仍然易分散于水、酒精及异丙醇等溶剂中形成打印墨水。该工艺无须向墨水中添加黏结剂和分散剂,从而避免了因黏结剂等非导电物质的残留对墨水导电性造成的影响。浓度为15g·L-1的石墨烯墨水电导率高达16S·cm-1。石墨烯墨水经3D微喷出打印机打印成圆盘状电极材料,并组装成超级电容器电极。电化学测试结果表明,电容器表现出典型的双电层电容特性。在全范围扫速下,其电压-电流曲线都几乎保持规则的矩形形状,即使在15V·s-1下,石墨烯电极的体积比容量仍为4F·cm-3。这表明该电极具有快速的电流响应速率,同时电解液在电极表面发生了快速扩散。另外,在体积能量密度4.43mWh·cm-3下,其体积功率密度为42.74kW·cm-3。
Sun等[85]采用微喷出打印技术打印出平面超级电容器微电极。石墨烯墨水浓度高达20mg·mL-1,并且墨水中未添加黏结剂和分散剂。打印过程采用微喷出打印技术制备出逐层堆叠结构的氧化石墨烯薄膜电极。该结构表现出高机械强度、高电导率以及良好的电解液侧向渗透性。经测量,4层结构电极材料的电导率高达(114.0±17.7)S·cm-1。电化学测试结果表明,电容器表现出典型的双电层电容特性,在0.06A·cm-3电流密度下,超级电容器的体积能量密度和体积功率密度分别为7mWh·cm-3和30mV·cm-3。为验证该电极材料应用于柔性电子器件的可能性,将石墨烯墨水打印在PET薄膜上并进行电化学性能测试(图5),其电压-电流曲线并未因电极的弯曲而发生变化。充放电循环过程中,初始容量为38.4F·cm-3,经300次循环后容量下降到约20.3F·cm-3,并且在随后的10000次循环过程中逐渐保持稳定。

图5 分层结构微型超级电容器电极电化学性能测试结果[85](a)平直和弯曲状态下电容器的CV曲线;(b)电容器体积比容量与充放电循环次数的关系Fig.5 Electrochemical performance of planar micro-supercapacitors electrodes[85](a)CVs at flat or bent states;(b)dependence of volumetric capacitance on charge-discharge cycling numbers
Zhu等[43]采用高浓度氧化石墨烯作为3D打印墨水,并将3D打印石墨烯用作超级电容器电极材料。为提高电极材料导电性,墨水中还添加适量石墨烯纳米片。与未添加石墨烯纳米片3D打印石墨烯相比,添加适量石墨烯纳米片可以有效降低电极内阻,同时电极材料仍然具有较高比表面积。3D打印石墨烯电极容量性能十分优异,当电流密度从0.5A·g-1增加到10A·g-1时,石墨烯电极容量保留率达到90%。如果考虑电极厚度,该性能甚至更优异,因为3D打印石墨烯电极厚度为1mm,而其他大多数电极厚度都不到100μm。另外,该电极性能极其稳定,经过10000次充放电循环后容量保持率高达95.5%,高于其他研究报告中的性能(见表4)。3D打印三维石墨烯电极表现出来的良好性能,一方面,得益于电极中石墨烯纳米片的加入,另一方面,得益于三维石墨烯中的大孔径结构,这有利于提高离子的传输速率。需要强调的是,由于打印过程中由管嘴造成的剪切应力使得墨水中的石墨烯沿着喷出方向排列,从而在电极中产生有序的大孔径,有助于厚电极结构中的质量传输,并有效降低传输过程中的内阻。随后,3D打印石墨烯被组装成准固态对称超级电容器,其功率密度和能量密度的测试结果也非常优秀。超级电容器在能量密度0.26Wh·kg-1下,具有最大功率密度4079.9W·kg-1;而在功率密度76.36W·kg-1下,具有最大能量密度0.43Wh·kg-1(能量密度和功率密度计算基于整个设备质量)。
随后,Liu等[100]采用电化学离子插层法对3D打印石墨烯进行改性,并将其应用到超级电容器上。电化学离子插层法分为两步,包括锂离子插层和高氯酸根离子插层。随后高氯酸根离子插层化合物发生分解,从而在石墨烯表面功能化含氧基团。电化学离子插层法是一种易实现且环境友好的石墨烯功能化方法。被用作超级电容器电极材料的功能化石墨烯能有效提高超级电容器的电化学性能。测试结果表明,在0.5A·g-1电流密度下,石墨烯电极的比容量达到124.7F·g-1,是相同电流密度下未经功能化石墨烯电极的两倍。当电流密度达到10A·g-1时,石墨烯电极的比容量仍能达到101.7F·g-1。另外,该电极在200mV·s-1扫速下,经10000次充放电循环后,其容量保持率高达97%。功能化3D打印石墨烯电极(3DG-N-Ox)与原始3D打印石墨烯电极(3DG)电化学结果对比如表5所示[100]。石墨烯电极电化学性能的显著提高与石墨烯表面功能化后比表面积增大以及表面存在的含氧官能团有关。该研究为3D打印石墨烯功能化提供一种简单易行的方法,同时也是提高超级电容器电化学性能的一种有效方法。
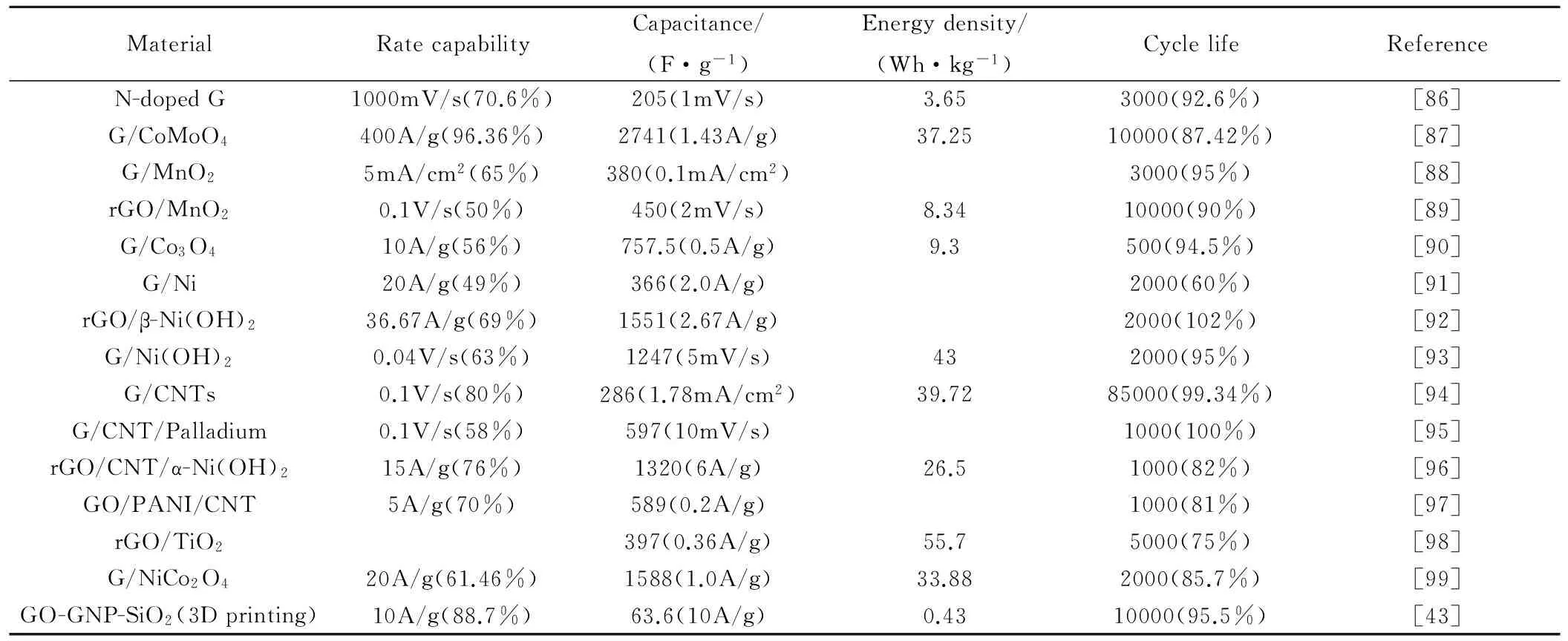
表4 超级电容器三维石墨烯电极电容性能Table 4 Capacitive performance of three dimensional graphene electrodes for supercapacitors
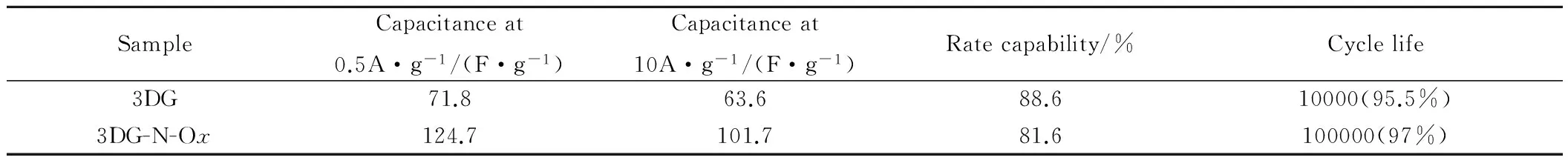
表5 3D打印石墨烯与功能化3D打印石墨烯电极电化学性能[100]Table 5 Electrochemical performance of 3DG and 3DG-N-Ox[100]
Note:rate capability was from 0.5A·g-1to 10A·g-1.
3D打印石墨烯除在超级电容器上有着广泛应用外,Fu等[44]应用3D打印技术打印出带有活性物质的锂离子电池正极(LiFePO4,LFP)/还原氧化石墨烯、负极(Li4Ti5O12,LTO)/还原氧化石墨烯、隔膜以及固态电解液。对半电池及全电池进行电化学性能测试,如图6所示,在10mA·g-1电流密度下,3D打印磷酸铁锂正极材料充放电比容量约为168mAh·g-1和164mAh·g-1(图6(a)),该比容量明显优于表6中其他锂离子电池正极材料。同时,第10次和第20次充放电循环对应的比容量已接近磷酸铁锂理论比容量170mAh·g-1。这表明石墨烯/磷酸铁锂正极材料具有优异的容量性能。同样,在10mA·g-1电流密度下,3D打印钛酸锂负极材料充放电比容量约为184mAh·g-1和185mAh·g-1(图6(c))。这一数值略高于钛酸锂理论比容量(175mAh·g-1),这可能源于还原氧化石墨烯对比容量的贡献。倍率性能测试结果表明,与正极材料相比,负极材料的容量衰减更大。这可能与电极材料电导率有关。正负极材料电导率分别为31.6S·cm-1和6.1S·cm-1,负极材料电导率明显小于正极材料电导率,较低的电导率会导致更大的容量衰减。这一问题可通过向负极材料中添加高导电物质来提高材料电导率。另外,在50mA·g-1电流密度下,全电池初始充放电比容量分别为117mAh·g-1和91mAh·g-1。结果表明,以3D打印石墨烯为电极材料的锂离子电池展现出优异的电化学性能,这为锂离子电池一体化打印提供了支持。
3 结束语
三维石墨烯具有多孔结构、超大的比表面积以及超高的电导率,使其在储能领域得到广泛应用。3D打印技术有望实现三维石墨烯结构精确可控及规模化制备。然而,石墨烯3D打印作为一种新兴的三维石墨烯制备工艺,还有一些问题有待进一步研究。石墨烯墨水的黏度及其可打印性是制约石墨烯3D打印发展的重要因素。通常,氧化石墨烯墨水因黏度不足无法实现有效打印,须添加聚合物作为黏结剂以提高墨水黏度,但引入聚合物会降低三维石墨烯的电导率。将3D打印与纳米尺度3D打印技术或冷冻铸造技术相结合可实现无黏结剂石墨烯墨水3D打印。但是该类打印工艺条件苛刻,难以实现三维石墨烯规模化制备。最新研制成功的高浓度氧化石墨烯3D打印技术被视为最具应用潜力的制备方法。高浓度氧化石墨烯3D打印无需黏结剂,同时可有效提高三维石墨烯电导率。打印得到的三维石墨烯具有高电导率、轻质以及高度可压缩性等优点。这些都为实现三维石墨烯结构设计和规模化制备提供有力支持。然而,目前采用高浓度氧化石墨烯墨水打印得到的三维石墨烯的比表面积还有待进一步提高。如何实现工艺简单、浓度可控的石墨烯3D打印成为目前亟待解决的问题。研制3D打印氧化石墨烯同步还原技术对简化石墨烯3D打印制备工艺、拓展石墨烯3D打印的规模化应用具有重要意义。3D打印石墨烯已被用作超级电容器和锂离子电池的电极材料,其容量性能均得到显著提高。除超级电容器和锂离子电池外,3D打印石墨烯还有望应用于锂硫电池、燃料电池、太阳能电池等众多储能元件中。石墨烯储能元件一体化打印成型是3D打印石墨烯在储能领域应用的发展方向。

图6 3D打印石墨烯电极电化学性能[44](a)LFP/rGO半电池充放电曲线;(b)不同电流密度下LFP/rGO半电池的倍率性能曲线;(c)10mA·g-1电流密度下LTO/rGO半电池充放电曲线;(d)不同电流密度下LTO/rGO半电池的倍率性能曲线;(e)3D打印全电池循环稳定性;(f)3D打印全电池充放电曲线Fig.6 Electrochemical performance of 3D-printed graphene electrodes[44](a)charge and discharge curves of the LFP/rGO half-cell;(b)rate curves of the LFP/rGO half-cell at various currents;(c)charge and discharge curves of the LTO/rGO half-cell at a current of 10mA·g-1;(d)rate curves of the LTO/rGO half-cell at various currents;(e)cycling stability of the 3D-printed full cell;(f)charge and discharge curves of the 3D-printed full cell

MaterialCyclingperformanceRateperformanceCurrentdensity/(mA·g-1)Initialcapacity/(mAh·g-1)CycleRemaincapacity/(mAh·g-1)Capacityretention/%Currentdensity/(mA·g-1)Capacity/(mAh·g-1)ReferenceG/TiO20.5C31410019762.720C124.0[101]G/SiO210001200100109090.920000500.0[102]rGO/SnO2-Fe2O32001556100830531000550.0[103]G/LFP/CNT0.2C168.9100≈165.5≈9820C115.8[104]G/NiO/C20010405075472.51600580.0[105]G/CNT/C-LVP20C147.5200012282.720C122[106]G/TiO2500500500082[107]G/CoFe2O45003009668000146[108]G/MnO280151560111373.41200440[109]G/MoS210067730670993200≈400[110]G/NiCo2O420013876098571600761[111]GA100256610095437800587[112]C/Si/rGO0.5C19933501913965C830[113]G(3Dprinting)1016820170[44]
[1] NOVOSELOV K S,GEIM A K,MOROZOV S V,et al.Electric field effect in atomically thin carbon films[J].Science,2004,306(5696):666-669.
[2] GEIM A K,NOVOSELOV K S.The rise of graphene[J].Nature Materials,2007,6(3):183-191.
[3] SINGH V,JOUNG D,ZHAI L,et al.Graphene based materials: past,present and future[J].Progress in Materials Science,2011,56:1178-1271.
[4] CHAE H K,SIBERIO-PÉREZ D Y,KIM J,et al.A route to high surface area,porosity and inclusion of large molecules in crystals[J].Nature,2004,427(6974):523-527.
[5] BOLOTINA K I,SIKESB K J,JIANG Z,et al.Ultrahigh electron mobility in suspended graphene[J].Solid State Communications,2008,146:351-355.
[6] WANG H B,MAIYALAGAN T,WANG X.Review on recent progress in nitrogen-doped graphene: synthesis,characterization,and its potential applications[J].ACS Catalysis,2012,2(5):781-794.
[7] CHOI W,LAHIRI I,SEELABOYINA R,et al.Synthesis of graphene and its applications: a review[J].Critical Reviews in Solid State and Materials Sciences,2010,35:52-71.
[8] BROWNSON D A C,KAMPOURIS D K,BANKS C E.An overview of graphene in energy production and storage applications[J].Journal of Power Sources,2011,196:4873-4885.
[9] HUANG Y,LIANG J J,CHEN Y S.An overview of the applications of graphene-based materials in supercapacitors[J].Small,2012,8(12):1805-1834.
[10] DAI L M.Functionalization of graphene for efficient energy conversion and storage[J].Accounts of Chemical Research,2012,46(1):31-42.
[11] SUN Y Q,WU Q,SHI G Q.Graphene based new energy materials[J].Energy & Environmental Science,2011(4):1113-1132.
[12] XU C H,XU B H,GU Y,et al.Graphene-based electrodes for electrochemical energy storage[J].Energy & Environmental Science,2013(5):1388-1414.
[13] STOLLER M D,PARK S J,ZHU Y W,et al.Graphene-based ultracapacitors[J].Nano Letters,2008,8(10):3498-3502.
[14] LI N,CHEN Z P,RWN W C,et al.Flexible graphene-based lithium ion batteries with ultrafast charge and discharge rates[J].Proceedings of the National Academy of Science of the United State of American,2012,109(43):17360-17365.
[15] XIA X H,CHAO D L,ZHANG Y Q,et al.Three-dimensional graphene and their integrated electrodes[J].Nano Today,2014,9(6):785-807.
[16] CHEN Z P,REN W C,GAO L B,et al.Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition[J].Nature Materials,2011,10(6):424-428.
[17] YOON J C,LEE J S,KIM S I,et al.Three-dimensional graphene nano-networks with high quality and mass production capabilityviaprecursor-assisted chemical vapor deposition[J].Science Reports,2013,3(19):1788-1795.
[18] HE Y M,CHEN W J,LI X D,et al.Freestanding three-dimensional graphene/MnO2composite networks as ultralight and flexible supercapacitor electrodes[J].ACS Nano,2013,7(1):174-182.
[19] LEE J S,KIM S I,YOON J C,et al.Chemical vapor deposition of mesoporous graphene nanoballs for supercapacitor[J].ACS Nano,2013,7(7):6047-6055.
[20] SHI J L,TANG C,PENG H J,et al.3D mesoporous graphene: CVD self-assembly on porous oxide templates and applications in high-stable Li-S batteries[J].Small,2015,11(39):5243-5252.
[21] ZHAO B,HUANG S Y,WANG T.Hollow SnO2&Co3O4core-shell spheres encapsulated in three-dimensional graphene foams for high performance supercapacitors and lithium-ion batteries[J].Journal of Power Sources,2015,298:83-91.
[22] BURRESS J W,GADIPELLI S,FORD J.Graphene oxide framework materials: theoretical predictions and experimental results[J].Angewandte Chemie,2010,122(47):8902-8904.
[23] WORSLEY M A,PAUZAUSKIE P J,OLSON T Y,et al.Synthesis of graphene aerogel with high electrical conductivity[J].Journal of the American Chemical Society,2010,132(40):14067-14069.
[24] CHOI B G,YANG M,HONG W H,et al.3D macroporous graphene frameworks for supercapacitors with high energy and power densities[J].ACS Nano,2012,6(5):4020-4028.
[25] ZHOU G M,PAEK E,HWANG G S,et al.Long-life Li/polysulphide batteries with high sulphur loading enabled by lightweight three-dimensional nitrogen/sulphur-codoped graphene sponge[J].Nature Communications,2015,6:7760-7770.
[26] SHI Q W,HOU C Y,WANG H Z,et al.Rapid formation of superelastic 3D reduced graphene oxide networks with simultaneous removal of HI utilizing NIR irradiation[J].Journal of Materials Chemistry A,2015,3(18):9882-9889.
[27] SUN H Y,XU Z,GAO C,et al.Multifunctional,ultra-flyweight,synergistically assembled carbon aerogels[J].Advanced Materials,2013,25(18):2554-2560.
[28] CHEN W F,LI S R,CHEN C H,et al.Self-assembly and embedding of nanoparticles byinsitureduced graphene for preparation of a 3D graphene/nanoparticle aerogel[J].Advanced Materials,2011,23(47):5679-5683.
[29] ZHU C,HAN Y-J T,DUOSS E B,et al.Highly compressible 3D periodic graphene aerogel microlattices[J].Nature Communication,2015,6:6962-6969.
[30] 李小丽,马剑雄,李萍,等.3D打印技术及应用趋势[J].自动化仪表,2014,35(1):1-5.
LI X L,MA J X,LI P,et al.3D printing technology and its application trend[J].Process Automation Instrumentation,2014,35(1):1-5.
[31] REDDY B V,REDDY N V,GHOSH A.Fused deposition modelling using direct extrusion[J].Virtual Phys Prototyping,2007,2(1):51-60.
[32] DUL S,FAMBRI L,PEGORETTI A.Fused deposition modelling with ABS-graphene nanocomposites[J].Composites Part A:Applied Science & Manufacturing,2016,85:181-191.
[33] ZEIN I,HUTMACHER D W,TAN K C,et al.Fused deposition modeling of novel scaffold architectures for tissue engineering applications[J].Biomaterials,2002,23(4):1169-1185.
[34] MASOOD S H,SONG W Q.Development of new metal/polymer materials for rapid tooling using fused deposition modelling[J].Materials & Design,2004,25:587-594.
[35] WEI X J,LI D,JIANG W,et al.3D printable graphene composite[J].Scientific Reports,2015,5:11181.
[36] HWANG S,REYES E I,MOON K,et al.Thermo-mechanical characterization of metal/polymer composite filaments and printing parameter study for fused deposition modeling in the 3D printing process[J].Journal of Electronic Materials,2015,44(3):771-777.
[37] DAVER F,BAZE E,SHANKS R A,et al.Conductive polyolefin-rubber nanocomposites with carbon nanotubes[J].Composites: Part A,2016,80:13-20.
[38] SUNPREET S,RUPINDER S.Effect of process parameters on micro hardness of Al-Al2O3composite prepared using an alternative reinforced pattern in fused deposition modelling assisted investment casting[J].Robotics and Computer-Integrated Manufacturing,2016,37:162-169.
[39] JAKUS A E,SECOR E B,RUTZ A L,et al.Three-dimensional printing of high-content graphene scaffolds for electronic and biomedical applications[J].ACS Nano,2015,9(4):4636-4648.
[41] KIM J H,CHANG W S,KIM D,et al.3D printing of reduced graphene oxide nanowires[J].Advanced Materials,2015,27(1):157-161.
[42] ZHANG Q Q,ZHANG F,MEDARAMETLA S P,et al.3D printing of graphene aerogels[J].Small,2016,12(13):1-7.
[43] ZHU C,LIU T Y,QIAN F,et al.Supercapacitors based on three-dimensional hierarchical graphene aerogels with periodic macropores[J].Nano Letter,2016,16(6):3448-3456.
[44] FU K,WANG Y B,YAN C Y,et al.Graphene oxide-based electrode inks for 3D-printed lithium-ion batteries[J].Advanced Materials,2016,28(13):2587-2594.
[45] PIERIN G,GROTTA C,COLOMBO P,et al.Direct ink writing of micrometric SiOC ceramic structures using a preceramic polymer[J].Journal of the European Ceramic Society,2016,36(7):1589-1594.
[46] LARSON C M,CHOI J J,GALLARDO P A,et al.Direct ink writing of silicon carbide for microwave optics[J].Advanced Engineering Materials,2015,18:39-45.
[47] ZOU F,ZHAO N,FU X L.Enhanced osteogenic differentiation and biomineralization in mouse mesenchymal stromal cells on a β-TCP robocast scaffold modified with collagen nanofibers[J].RCS Advances,2016,6(28):23588-23598.
[48] FEILDEN E,BLANCA G-T E,GIULIANI F,et al.Robocasting of structural ceramic parts with hydrogel inks[J].Journal of the European Ceramic Society,2016,36:2525-2533.
[49] EQTESADI S,MOTEALLEH A,PAJARES A,et al.Improving mechanical properties of 13-93 bioactive glass robocast scaffold by poly (lactic acid) and poly (ε-caprolactone) melt in filtration[J].Journal of Non-Crystalline Solids,2016,432:111-119.
[50] EQTESADI S,MOTEALLEH A,PAJARES A,et al.Effect of milling media on processing and performance of 13-93 bioactive glass scaffolds fabricated by robocasting[J].Ceramics International,2015,41:1379-1389.
[51] DANACI S,PROTASOVA L,LEFEVERE J,et al.Efficient CO2methanation over Ni/Al2O3coated structured catalysts[J].Catalysis Today,2016,273:234-243.
[52] URRIOS A,PARRA-CABRERA C,BHATTACHARJEE N,et al.3D-printing of transparent bio-microfluidic devices in PEG-DA[J].Lab on A Chip,2016,16(12):2287-2294.
[53] ALESSANDRI K,FEYEUX M,GURCHENKOV B,et al.A 3D printed microfluidic device for production of functionalized hydrogel microcapsules for culture and differentiation of human neuronal stem cells[J].Lab on A Chip,2016,16(9):1593-1604.
[54] PETIT C,MAIRE E,MEILLE S,et al.CoCrMo cellular structures made by electron beam melting studied by local tomography and finite element modelling[J].Materials Characterization,2016,116:48-54.
[55] LIU Y J,LI S J,HOU W T,et al.Electron beam melted beta-type Ti-24Nb-4Zr-8Sn porous structures with high strength-to-modulus ratio[J].Journal of Materials Science & Technology,2016,32:505-508.
[56] BAUDANA G,BIAMINO S,KLÖDEN B,et al.Electron beam melting of Ti-48Al-2Nb-0.7Cr-0.3Si: feasibility investigation[J].Intermetallics,2016,73:43-49.
[57] PENG H,LIU C,GUO H B,et al.Fabrication of WCp/NiBSi metal matrix composite by electron beam melting[J].Materials Science and Engineering:A,2016,666:320-323.
[58] RAGHAVAN N,DEHOFF R,PANNALA S,et al.Modeling of heat-transfer and the influence of process parameters on tailoring the grain morphology of IN718 in electron beam additive manufacturing[J].Acta Materialia,2016,112:303-314.
[59] ALMANGOUR B,GRZESIAK D,YANG J M.Rapid fabrication of bulk-form TiB2/316L stainless steel nanocomposites with novel reinforcement architecture and improved performance by selective laser melting[J].Journal of Alloys and Compounds,2016,680:480-493.
[60] VAITHILINGAM J,PRINA E,GOODRIDGE R D,et al.Surfa-ce chemistry of Ti6Al4V components fabricated using selective laser melting for biomedical applications[J].Materials Science and Engineering:C,2016,67:294-303.
[61] REN L,MEMARZADEH K,ZHANG S Y,et al.A novel coping metal material CoCrCu alloy fabricated by selective laser melting with antimicrobial and antibiofilm properties[J].Materials Science and Engineering:C,2016,67:461-467.
[62] SUN Z J,TAN X P,TOR S B,et al.Selective laser melting of stainless steel 316L with low porosity and high build rates[J].Materials & Design,2016,104:197-204.
[63] ABOULKHAIR N T,MASKERY I,TUCK C,et al.Improving the fatigue behaviour of a selectively laser melted aluminium alloy: influence of heat treatment and surface quality[J].Materials & Design,2016,104:174-182.
[64] KANG N,CODDET P,LIAO H L,et al.Applied surface science wear behavior and microstructure of hypereutectic Al-Si alloys prepared by selective laser melting[J].Applied Surface Science,2016,378:142-149.
[65] POPOVICH A,SU V,POLOZOV,et al.Microstructure and mechanical properties of additive manufactured copper alloy[J].Materials Letters,2016,79:38-41.
[66] SUN H,HE S W,WU P,et al.A novel MgO-CaO-SiO2system for fabricating bone scaffolds with improved overall performance[J].Materials,2016,9(4):1-12.
[67] XIE F X,HE X M,YU J H.Fabrication and characterization of porous Ti-4Mo alloy for biomedical applications[J].J Porous Mater,2016,23:783-790.
[68] SHISHKOVSKY I,YADROITSEV I,MOROZOV Y,et al.Laser-assisted synthesis in Cu-Al-Ni system and some of its properties[J].Journal of Alloys and Compounds,2016,658:875-879.
[69] VOLYANSKI I,SHISHKOVSKYI I V,YADROITSEV I,et al.Layer-by-layer laser synthesis of Cu-Al-Ni intermetallic compounds and shape memory effect[J].Inorganic Materials,2016,52(6):566-572.
[70] LIU K,SUN H J,SHI Y S,et al.Research on selective laser sintering of Kaolin-epoxy resin ceramic powders combined with cold isostatic pressing and sintering[J].Ceramics International,2016,42:10711-10718.
[71] GIRARDIN E,BARUCCA G,MENGUCCI P,et al.Biomedical Co-Cr-Mo components produced by direct metal laser sintering[J].Materials Today Proceedings,2016,3(3):889-897.
[72] CHANG K,GU D D.Direct metal laser sintering synthesis of carbon nanotube reinforced Ti matrix composites: densification,distribution characteristics and properties[J].Journal of Material Research,2016,31(2):281-291.
[73] CABRINI M,LORENZI S,PASTORE T,et al.Effect of heat treatment on corrosion resistance of DMLS AlSi10Mg alloy[J].Electrochimica Acta,2016,206:346-355.
[74] MENGUCCI P,BARUCCA G,GATTO A,et al.Effects of thermal treatments on microstructure and mechanical properties of a Co-Cr-Mo-W biomedical alloy produced by laser sintering[J].Journal of the Mechanical Behavior of Biomedical Materials,2016,60:106-117.
[75] CABRINI M,LORENZI S,PASTORE T,et al.Evaluation of corrosion resistance of Al-10Si-Mg alloy obtained by means of direct metal laser sintering[J].Journal of Materials Processing Technology,2016,231:326-335.
[76] VANDERESSE N,KY I,QUEVEDO G F,et al.Image analysis characterization of periodic porous materials produced by additive manufacturing[J].Materials & Design,2016,92:767-778.
[77] IDELL Y,LEVINE L E,ALLEN A J,et al.Unexpected δ-phase formation in additive-manufactured Ni-based super alloy[J].JOM,2016,68(3):950-959.
[78] MCLACHLAN D S,CHITEME C,PARK C,et al.AC and DC percolative conductivity of single wall carbon nanotube polymer composites[J].Journal of Polymer Science: Part B:Polymer Physics,2005,43(22):3273-3287.
[79] ZHANG Q Q,XU X,LI H,et al.Mechanically robust honeycomb graphene aerogel multifunctional polymer composites[J].Carbon,2015,93:659-670.
[80] JALILI R,ABOUTALEBI S H,ESRAFILZADEH D,et al.Scalable one-step wet-spinning of graphene fibers and yarns from liquid crystalline dispersions of graphene oxide: towards multifunctional textiles[J].Advanced Functional Materials,2013,23(43):5345-5354.
[81] NAFICY S,JALILI R,ABOUTALEBI S H,et al.Graphene oxide dispersions: tuning rheology to enable fabrication[J].Material Horizons,2014,1(3):326-331.
[82] SUN K,WEI T S,AHN B Y,et al.3D printing of interdigitated Li-ion microbattery architectures[J].Advanced Materials,2013,25(33):4539-4543.
[83] ZHAO C,WANG C,GORKIN R,et al.Three dimensional (3D) printed electrodes for interdigitated supercapacitors[J].Electrochemistry Communications,2014,41:20-23.
[84] NATHAN-WALLESER T,LAZAR I-M,FABRITIUS M,et al.3D micro-extrusion of graphene-based active electrodes: towards high-rate AC line filtering performance electrochemical capacitors[J].Advanced Functional Materials,2014,24:4706-4716.
[85] SUN G Z,AN J,CHUA C K,et al.Layer-by-layer printing of laminated graphene-based interdigitated microelectrodes for flexible planar micro-supercapacitors[J].Electrochemistry Communications,2015,51:33-36.
[86] CHANG Y Z,HAN G Y,YUAN J P,et al.Using hydroxylamine as a reducer to prepare N-doped graphene hydrogels used in high-performance energy storage[J].Journal of Power Sources,2013,238:492-500.
[87] YU X Z,LU B A,XU Z.Super long-life supercapacitors based on the construction of nanohoneycomb-like strongly coupled CoMoO4-3D graphene hybrid electrodes[J].Advanced Materials,2014,26(7):1044-1051.
[88] YU G H,HU L B,LIU N,et al.Supporting information for enhancing the supercapacitor performance of graphene/MnO2-nanostructured electrodes by conductive wrapping[J].Nano Letters,2011,11(10):4438-4442.
[89] GE J,YAO H B,HU W,et al.Facile dip coating processed graphene/MnO2nanostructured sponges as high performance supercapacitor electrodes[J].Nano Energy,2013,2(4):505-513.
[90] YUAN J J,ZHU J W,BI H P,et al.Graphene-based 3D composite hydrogel by anchoring Co3O4nanoparticles with enhanced electrochemical properties[J].Phys Chem Chem Phys,2013,15(31):12940-12945.
[91] YE S B,FENG J C,WU P Y.Deposition of three-dimensional graphene aerogel on nickel foam as a binder-free supercapacitor electrode[J].ACS Applied Materials & Interfaces,2013,5(15):7122-7129.
[92] WANG Y,GAI S L,NIU N,et al.Fabrication and electrochemical performance of 3D hierarchical β-Ni(OH)2hollow microspheres wrapped in reduced graphene oxide[J].Electrochemistry Communications,2007,9(10):2606-2610.
[93] XU Y X,HUANG X Q,LIN Z Y,et al.One-step strategy to graphene/Ni(OH)2composite hydrogels as advanced three-dimensional supercapacitor electrode materials[J].Nano Research,2013,6(1):65-76.
[94] WANG W,GUO S R,PENCHEV M,et al.Three dimensional few layer graphene and carbon nanotube foam architectures for high fidelity supercapacitors[J].Nano Energy,2013,2(2):294-303.
[95] SRIDHAR V,KIM H J,JUNG J H,et al.Defect-engineered three-dimensional graphene-nanotube-palladium nanostructures with ultrahigh capacitance[J].ACS Nano,2012,6(12):10562-10570.
[96] CHEN X A,CHEN X H,ZHANG F Q,et al.One-pot hydrothermal synthesis of reduced graphene oxide/carbon nanotube/α-Ni(OH)2composites for high performance electrochemical supercapacitor[J].Journal of Power Sources,2013,243:555-561.
[97] NING G Q,LI T Y,YAN J,et al.Three-dimensional hybrid materials of fish scale-like polyaniline nanosheet arrays on graphene oxide and carbon nanotube for high-performance ultracapacitors[J].Carbon,2013,54:241-248.
[98] DING Y B,BAI W,SUN J H,et al.Cellulose tailored anatase TiO2nanospindles in three-dimensional graphene composites for high-performance supercapacitors[J].ACS Applied Materials Interfaces,2016,8(19):12165-12175.
[99] YU M,CHEN J P,LIU J H,et al.Mesoporous NiCo2O4nanoneedles grown on 3D graphene-nickel foam for supercapacitor and methanol electro-oxidation[J].Electrochim Acta,2015,151:99-108.
[100] LIU T Y,ZHUC,KOUT Y,et al.Ion intercalation induced capacitance improvement for graphene -based supercapacitor electrodes[J].Chem Nano Mat,2016,2:635-641.
[101] YU S X,YANG L W,TIAN Y,et al.Mesoporous anatase TiO2submicrospheres embedded in self-assembled three-dimensional reduced graphene oxide networks for enhanced lithium storage[J].Journal of Materials Chemistry A,2013,1(41):12750-12758.
[102] WU L L,YANG J,TANG J J,et al.Three-dimensional graphene nanosheets loaded with Si nanoparticles byinsitureduction of SiO2for lithium ion batteries[J].Electrochimica Acta,2016,190:628-635.
[103] ZHAO B,XU Y T,HUANG S Y,et al.3D RGO frameworks wrapped hollow spherical SnO2-Fe2O3mesoporousnano-shells: fabrication,characterization and lithium storage properties[J].Electrochimica Acta,2016,202:186-196.
[104] LEI X L,ZHANG H Y,CHEN Y M,et al.A three-dimensional LiFePO4/carbon nanotubes/graphene composite as a cathode material for lithium-ion batteries with superior high-rate performance[J].Journal of Alloys and Compounds,2015,626:280-286.
[105] WANG X W,ZHANG L,ZHANG Z H,et al.Growth of 3D hierarchical porous NiO@carbon nanoflakes on graphene sheets for high-performance lithium-ion batteries[J].Phys Chem Chem Phys,2016,18:3893-3899.
[106] CUI K,LI Y K.Monoclinic Li3V2(PO4)3/C nanocrystals co-modified with graphene nanosheets and carbon nanotubes as a three-dimensional-network cathode material for rechargeable lithium-ion batteries[J].RSC Advances,2016,6(10):8431-8439.
[107] LI F,JIANG J Z,WANG X,et al.Assembly of TiO2/graphene with macroporous 3D network framework as an advanced anode material for Li-ion batteries[J].RSC Advances,2016,6(4):3335-3340.
[108] WANG B B,WANG G,LV Z Y,et al.Insitusynthesis of hierarchical CoFe2O4nanoclusters/graphene aerogels and their high performance for lithium-ion batteries[J].Phys Chem Chem Phys,2015,17:27109-27117.
[109] ZHOU Y,LIU Q,LIU D B,et al.Carbon-coated MoO2dispersed in three-dimensional graphene aerogel for lithium-ion battery[J].Electrochimica Acta,2015,174(1):8-14.
[110] OUYANG B,WANG Y,ZHANG Z,et al.MoS2anchored free-standing three dimensional vertical graphene foam based binder-free electrodes for enhanced lithium-ion storage[J].Electrochimica Acta,2016,194:151-160.
[111] ZHANG C F,YU J S.Morphology-tuned synthesis of NiCo2O4-coated 3D graphene architectures used as binder-free electrodes for lithium-ion batteries[J].Chemistry-A European Journal,2016,22(13):4422-4430.
[112] SHAN H,XIONG D B,LI X F,et al.Tailored lithium storage performance of graphene aerogel anodes with controlled surface defects for lithium-ion batteries[J].Applied Surface Science,2016,364:651-659.
[113] CHANG J B,HUANG X K,ZHOU G H,et al.Three-dimensional carbon-coated Si/rGO nanostructures anchored by nickel foam with carbon nanotubes for Li-ion battery applications[J].Nano Energy,2015,15:679-687.
Research Progress on 3D Printed Graphene Materials Synthesis Technology and Its Application in Energy Storage Field
WANG Nan,YAN Shao-jiu,PENG Si-kan,CHEN Xiang,DAI Sheng-long
(Research Center of Graphene Applications,AECC Beijing Institute of Aeronautical Materials,Beijing 100095,China)
Graphene is an ideal material for energy storage application as its excellent mechanical and physical properties. 3D printed graphene materials will be widely applied in energy storage field for its precisely controllable structure and it is easy to realize large-scale preparation. In this paper, the progress of 3D printed graphene materials synthesis technology and its application in energy storage field were reviewed. The viscosity and printability of graphene ink are key factors for realizing graphene 3D printing. Scalable preparation of graphene ink with facile process, controllable concentration and additive free will be the research focus of graphene 3D printing technologies in the future. The integrated printing of graphene energy storage devices such as graphene supercapacitor, lithium-sulfur battery and lithium ion battery is the development direction in this area.
3D printing;graphene;energy storage;supercapacitor;lithium ion battery
10.11868/j.issn.1001-4381.2016.001102
O613.71
A
1001-4381(2017)12-0112-14
2016-09-14;
2017-05-02
燕绍九(1980-),男,高级工程师,博士,主要从事磁性材料及石墨烯应用方面的研究工作,联系地址:北京市81信箱72分箱(100095),E-mail:shaojiuyan@126.com
(本文责编:王 晶)

