HIV-1慢性感染者外周血中CD8+干细胞样记忆型T细胞变化以及对疾病进展的影响
宋冰冰 陆小凡 翁文佳 粟 斌 张 彤 高艳青*
(1.首都医科大学附属北京佑安医院皮肤性病科,北京 100069;2.北京市艾滋病研究北京市重点实验室,北京 100069)
·皮肤病性病诊疗与研究·
HIV-1慢性感染者外周血中CD8+干细胞样记忆型T细胞变化以及对疾病进展的影响
宋冰冰1陆小凡2翁文佳1粟 斌2张 彤2高艳青1*
(1.首都医科大学附属北京佑安医院皮肤性病科,北京 100069;2.北京市艾滋病研究北京市重点实验室,北京 100069)
目的观察人类免疫缺陷病毒-1(human immunodeficiency virus-1, HIV-1)慢性感染者外周血中CD8+干细胞样记忆型T细胞(CD8+stem memory T cells, CD8+TSCM)随治疗的动态变化,分析其与疾病进展的关系。方法观察25例经抗反转录病毒治疗(antiretroviral therapy,ART)的慢性HIV-1感染者(治疗方案为替诺福韦+依非韦伦+拉米夫定)以及36例健康对照者, 对样本外周血单个核细胞(peripheral blood mononuclear cells, PBMC)分别用CD3-APCCy7、CD4-FITC、CD8-PerCPCy5、CD45RA-PECy7、 CCR7-APC、CD27-PE、 CD95-Pacific Blue抗体和CD38-PE、HLA DR-APC抗体进行细胞表面染色并通过流式细胞仪检测CD8+TSCM细胞比例。比较HIV-1慢性感染者和健康者CD8+TSCM细胞比例的差异,并分析其与疾病进展指标(CD4+T细胞计数、HIV病毒载量以及免疫活化指标)的关系。结果HIV-1慢性感染组的CD8+TSCM细胞比例小于健康对照组;CD8+TSCM细胞比例与CD4+T细胞计数呈正相关,与HIV-1病毒载量以及免疫活化指标CD38+HLA-DR+CD8+T细胞呈负相关;随着ART治疗,CD8+TSCM细胞比例在144周后升至健康对照水平。结论CD8+TSCM在慢性HIV-1感染中发挥一定的保护作用,HIV-1相关特异性CD8+TSCM细胞可能是未来T细胞疫苗设计的靶点。
人类免疫缺陷病毒;干细胞样记忆性T细胞;疾病进展
干细胞样记忆型T细胞(stem memory T cells, TSCM )是一类具有干细胞特性的记忆性T淋巴细胞。在分化的同时又能够维持自我更新,占外周血循环T淋巴细胞的2%~4%[1-2]。
TSCM细胞具有很强的抗肿瘤功能[3]。有可能成为最有效的免疫细胞过继转移疗法(the adoptive cell transfer) 治疗肿瘤的细胞类型[4-6]。
TSCM细胞在人/猴获得性免疫缺陷病毒 (human/simian immunodeficiency virus, HIV/SIV) 感染中的作用也有相关报道[7-9]。首先,TSCM细胞表面表达CCR5和CXCR4,可以被HIV-1感染。同时,研究[10-11]显示与其他记忆性T细胞亚群相比,TSCM细胞中潜伏的HIV-1具有极高的稳定性。而且,在病毒血症无进展者(viremic non-progressors, VNP)人群中外周血中TSCM数量高于潜在进展者(putative progressor,PP),而且VNP病人中央记忆性T细胞(central memory T cells, TCM)和TSCM中HIV-1 DNA含量低于PP组,表明TCM/TSCM亚群被HIV-1感染较少可能与疾病进展缓慢相关[12]。本研究欲通过分析CD8+TSCM 与 CD4+T细胞计数、HIV-1病毒载量、免疫活化指标CD38+HLA-DR+CD8+T淋巴细胞的相关性,总结CD8+TSCM细胞与HIV-1疾病进程的关系。
1 对象与方法
1.1研究对象
选取首都医科大学附属北京佑安医院随访的HIV-1感染者25例, 发现HIV-1抗体确认阳性时间均大于12个月,均经替诺福韦、依非韦伦、拉米夫定抗病毒治疗;健康对照36例为首都医科大学附属北京佑安医院门诊的健康志愿者, HIV-1抗体确认阴性, 既往3个月未进行任何治疗。病人资料详见表1。所有临床标本的获取均通过北京佑安医院伦理委员会批准, 并有受试者书面同意。
1.2材料
EDTA抗凝管采血10 mL,4 h内用Ficoll(Pharmacia公司,瑞典)方法分离单个核细胞,送实验室-135 ℃冰箱冻存待用。荧光单克隆抗体 CD3-APCCy7、CD4-FITC、CD8-PerCPCy5、CD45RA-PECy7、CCR7-APC、CD27-PE、CD95-Pacific Blue、CD38-PE、HLA DR-APC抗体均购自美国BD公司;病毒载量检测试剂盒购自德国罗氏公司。
1.3方法
1.3.1 标本采集及外周血单个核细胞(peripheral blood mononuclear cells, PBMCs)的分离
用 10 mL EDTA抗凝负压真空采血管采集外周静脉血,采用Ficoll密度梯度离心法分离获得PBMCs。
1.3.2 CD4+T细胞计数检测
取新鲜全血50 μL,放入专用的CD4绝对计数管中, 加CD3-FITC/CD4-PE/CD8-Percp抗体,混匀后,室温避光孵育15 min,加入450 μL免洗溶血素,充分混匀,室温避光 15 min 后上机, 采用 MultiSET 软件自动计数系统检测CD4+T细胞绝对计数。
1.3.3 病毒载量检测
病毒载量依据检测试剂盒说明书进行检测, 检测下限为40拷贝/mL。
1.3.4 流式检测
取冻存的PBMC分别加入不同单色荧光抗体:CD3-APCCy7,CD4-FITC,CD8-PerCPCy5,CD45RA-PECy7,CCR7-APC,CD27-PE,CD95-Pacific Blue,CD38-PE, HLA DR-APC进行细胞表面染色,用FAC SC licar流式细胞仪(美国BD公司)检测T细胞和CD8+TSCM细胞比例。
1.4统计学方法

2 结果
2.12组人群外周血CD8+TSCM细胞比例的比较
本研究包括25名慢性HIV-1感染者和36名健康对照者。基本资料详见表1。所有研究对象均为男性。 HIV-1组CD4+T 淋巴细胞的比例明显低于健康对照组(P<0.000 1)。为确定慢性HIV-1 对CD8+TSCM细胞比例的影响, 笔者对健康对照组和HIV-1慢性感染组外周血中CD8+TSCM比例进行了比较。CD8+TSCM细胞定义为 CD3+CD8+CD45RA+CCR7+CD27+CD95+(图1A)。HIV-1慢性感染组的CD8+TSCM细胞的比例明显小于健康对照组 (P<0.000 1,图1B)。

表1 HIV-1感染者及健康志愿者资料Tab.1 Summary of HIV-1 patients andhealthy control subjects characteristics
***P<0.000 1vshealthy control;HIV-1: human immunodeficiency virus-1;NA: not applicable.
2.2CD8+TSCM细胞比例与疾病进展指标的相关性
HIV-1人群基线水平CD8+TSCM比例与HIV-1
RNA病毒载量呈负相关(r=-0.57,P=0.007,图2A),与CD4+T 细胞计数呈正相关 (r=0.42,P=0.04,图2B)。CD38+HLA-DR+CD8+T 细胞与CD8+TSCM细胞比例呈负相关 (r=-0.45,P=0.04,图2C)。
2.3随着ART治疗CD8+TSCM细胞比例的动态变化
与设想的一致,随着抗反转录病毒治疗(antiretrovial therapy, ART),CD4+T 细胞的计数逐渐升高 (P<0.000 1, 图3A);CD8+TSCM细胞比例在ART 治疗12周前呈下降趋势,随后上升,在治疗48周后又出现下降,随后逐步上升直至144周升至健康对照水平(P<0.01, 图3B);活化指标 CD38+HLA-DR+CD8+T在长期的ART治疗后明显下降(P<0.000 1, 图3C)。 总体, CD8+TSCM细胞随ART的动态变化与外周循环中CD4+T计数变化一致,与 CD38+HLA-DR+CD8+T 细胞变化水平大致相反。

图1 CD8+ TSCM细胞比例的比较Fig.1 Comparison of CD8+ TSCM cells proportion
A:flow cytometry gating strategy for CD8+TSCM cells;B:decreased proportion of CD8+TSCM cells in chronically HIV-1-infected patients;***P<0.000 1vshealthy controls;TSCM:stem memory T cells;HIV-1:human immunodeficiency virus-1;HC:healthy controls;CM:central memory;EM:effector memory;SCM:memory stem cells.
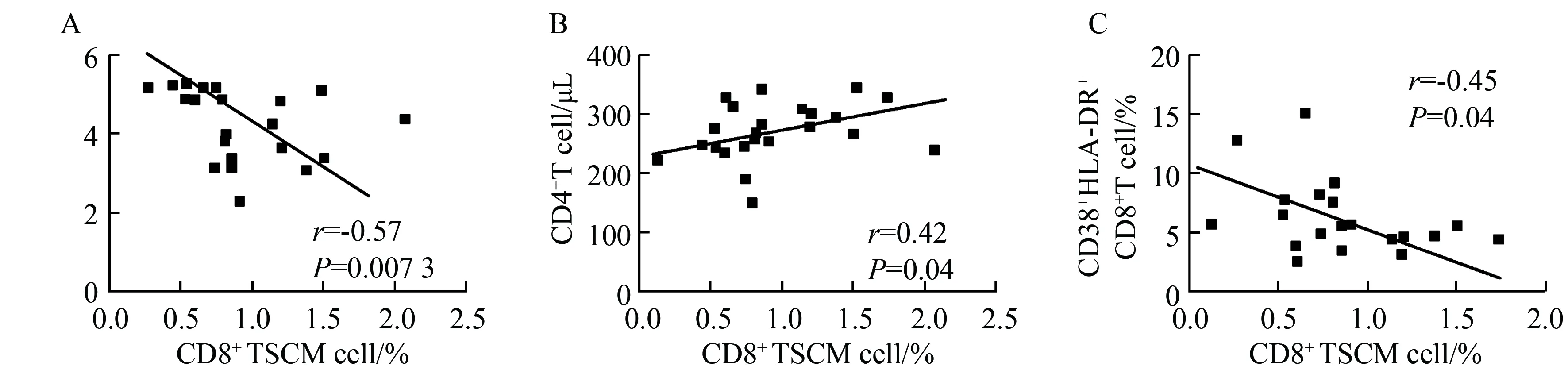
图2 CD8+TSCM细胞比例与疾病进展指标的相关性Fig.2 CD8+TSCM cell proportion were correlated with disease progression markers
A: correlation between the baseline level of CD8+TSCM cell proportion and plasma viral blad;B:CD4+T cell counts;C: T cell immune activation;TSCM:stem memory T cells;pVL:plasma viral load.
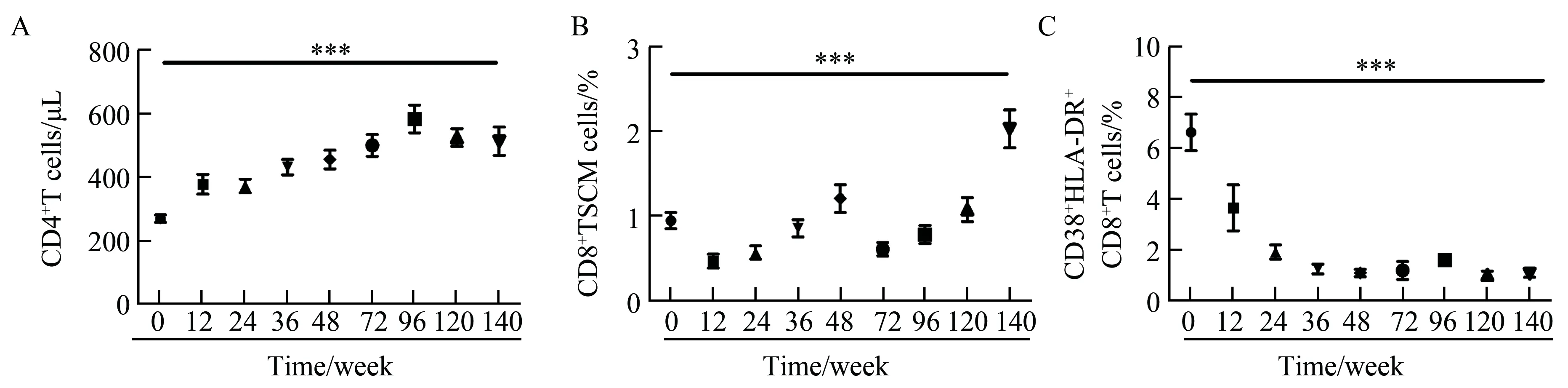
图3 HIV-1慢性感染组CD8+TSCM细胞随着ART治疗的变化Fig.3 Response of CD8+TSCM cells to ART in chronic HIV-1 infected patients
A: changes in the CD8+T cell count;B: CD8+TSCM cell count;C: level of T cell immune activation;***P<0.000 1;ART:antiretroviral therapy;HIV-1:human immunodeficiency virus-1.
3 讨论
笔者研究发现与健康对照组相比,HIV-1感染组外周血中CD8+TSCM细胞比例明显降低,而且该比例与CD4+T细胞计数呈正相关,与HIV-1病毒载量以及免疫活化指标CD38+HLA-DR+CD8+T细胞呈负相关。这一现象提示高比例的CD8+TSCM亚群,可能降低免疫活化水平,该亚群可能与功能性免疫系统相关。本课题组的结果也表明CD8+TSCM细胞可能利于免疫系统对HIV-1病毒的抑制,同时有助于免疫功能的维护和较低水平的T细胞活化。而且随着抗反转录病毒治疗CD8+TSCM细胞比例逐渐接近健康个体,在一定程度上反映了治疗对免疫功能恢复的效果。
既往研究[13-14]已经明确CD8+T细胞可以直接杀伤靶细胞抑制病毒复制维持CD4+T细胞数量。本研究中发现CD8+TSCM比例与HIV-1病毒载量呈负相关,与CD4+T细胞计数呈正相关,这也提示该细胞亚群利于疾病的预后。
HIV-1感染与系统性免疫激活有关,这种激活导致免疫衰竭,增加非艾滋病相关疾病的发生,最终发展到艾滋病[15]。而本研究表明CD8+TSCM百分数与免疫活化指标CD38+HLA-DR+CD8+T细胞百分数呈负相关,也提示CD8+TSCM对慢性HIV-1感染起保护作用。但是该细胞亚群对免疫活化影响的机制仍未研究清楚。
综上所述,本研究表明CD8+TSCM细胞与HIV疾病进程具有相关性,CD8+TSCM在慢性HIV-1感染中发挥一定的保护作用,HIV-1相关特异性CD8+TSCM细胞可能是未来T细胞疫苗设计的靶点。
[1] Farber D L, Yudanin N A, Restifo N P. Human memory T cells: generation, compartmentalization and homeostasis[J].Nat Rev Immunol,2014,14(1):24-35.
[2] Lugli E, Dominguez M H, Gattinoni L,et al. Superior T memory stem cell persistence supports long-lived T cell memory[J]. J Clin Invest,2013,123(2):594-599.
[3] Dudley M E, Wunderlich J R, Shelton T E, et al. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients[J]. J Immunother,2003,26: 332-342.
[4] Stroncek D F, Berger C, Cheever M A, et al. New directions in cellular therapy of cancer: a summary of the summit on cellular therapy for cancer [J]. J Transl Med,2012,10: 48.
[5] Zhou J, Shen X, Huang J, et al. Telomere length of transferred lymphocytes correlates withinvivopersistence and tumor regression in melanoma patients receiving cell transfer therapy[J]. J Immunol, 2005,175(10): 7046-7052.
[6] Louis C U, Savoldo B, Dotti G, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuro- blastoma[J]. Blood,2011,118(23): 6050-6056.
[7] Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell-like properties[J]. Nat Med,2011,17(10):1290-1297.
[8] Lugli E, Gattinoni L, Roberto A,et al. Identification, isolation andinvitroexpansion of human and nonhuman primate T stem cell memory cells[J]. Nat Protoc,2013,8(1):33-42.
[9] Cartwright E K, McGary C S, Cervasi B,et al. Divergent CD4+T memory stem cell dynamics in pathogenic and nonpathogenic simian immunodeficiency virus infections[J]. J Immunol,2014,192(10):4666-4673.
[10] Buzon M J, Sun H, Li C, et al. HIV-1 persistence in CD4+T cells with stem cell-like properties[J]. Nat Med,2014,20(2):139-142.
[11] Jaafoura S, de Goer de Herve M G, Hernandez-Vargas E A, et al. Progressive contraction of the latent HIV reservoir around a core of less-differentiated CD4+memory T Cells[J]. Nat Commun,2014,5:5407.
[12] Klatt N R, Bosinger S E, Peck M, et al. Limited HIV infection of central memory and stem cell memory CD4+T cells is associated with lack of progression in viremic individuals[J].PLoS Pathog, 2014,10(8):e1004345.
[13] Walker B D, Chakrabarti S, Moss B, et al. HIV-specific cytotoxic T lymphocytes in seropositive individuals [J].Nature,1987,328(6128):345-348.
[14] Betts M R, Nason M C, West S M, et al. HIV non-progressors preferentially maintain highly functional HIV-specific CD8+T cells [J]. Blood,2006,107(12):4781-4789.
[15] Giorgi J V, Hultin L E, McKeating J A, et al. Shorter survival in advanced human immunodeficiency virus type 1 in- fection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage[J]. J Infect Dis,1999,179(4):859 -870.
DynamicchangesofCD8+stemmemoryTcellsandtheireffectsondiseaseprogressioninchronicHIV-1infection
Song Bingbing1, Lu Xiaofan2, Weng Wenjia1, Su Bin2, Zhang Tong2, Gao Yanqing1*
(1.DepartmentofDermatology,BeijingYouanHospital,CapitalMedicalUniversity,Beijing100069,China; 2.BeijingKeyLaboratoryofAIDSResearch,Beijing100069,China)
ObjectiveTo study the dynamics of CD8+stem memory T cells (TSCM) and the impact of CD8+TSCM cells on disease progression of human immunodeficiency virus-1 (HIV-1) infection.MethodsTwenty-five cases with chronic HIV-1 infection receiving antiretroviral therapy (ART) with tenofovir plus efavirenz + lamivudine and 36 healthy controls were enrolled and observed. Peripheral blood mononuclear cells were stained with using CD3-APCCy7, CD4-FITC, CD8-PerCPCy5, CD45RA-PECy7, CCR7-APC, CD27-PE, CD95-Pacific Blue, CD38-PE, HLA DR-APC monoclonal antibodies, then CD8+TSCM cell percentage were determined by flow cytometry.To compare the difference of CD8+TSCM cell percentage between HIV-1 chronic infected persons and healthy subjects, and to analyze the relationship between CD8+TSCM cell percentage and disease progression index (CD4+T cell count, HIV-1 viral load and immune activation index).ResultsChronic HIV-1 infection resulted in a decrease of the CD8+TSCM cell proportion in HIV-1 patients. CD8+TSCM cells positively correlated with CD4+T cell counts and negatively correlated with plasma viral load and CD8+T cell activation. Prolonged ART successfully recovered the CD8+TSCM cells, and the dynamic change of CD8+TSCM cells was in parallel with CD4+T cell restoration and a decrease in the level of T cell immune activation.ConclusionIn summary, this report identifies CD8+TSCM as a correlate of protection from disease progression. HIV-1-specific CD8+TSCM can presumably directly contribute to the design of T cell-based vaccines.
human immunodeficiency virus; stem memory T cells; disease progression
北京市科技计划(D141100000314005)。This study was supported by Science and Technology Plan of Beijing (D141100000314005).
*Corresponding author, E-mail:gyqing2001bj@sina.com
时间:2017-10-14 16∶19
http://kns.cnki.net/kcms/detail/11.3662.R.20171014.1619.028.html
10.3969/j.issn.1006-7795.2017.05.004]
R512
2017-05-09)
编辑 孙超渊

