利用酵母双杂交系统筛选与草莓镶脉病毒P6蛋白互作的森林草莓寄主因子
李帅,蒋西子,梁伟芳,陈思涵,张享享,左登攀,胡亚会,江彤
(安徽农业大学植物保护学院,合肥 230036)
植物保护
利用酵母双杂交系统筛选与草莓镶脉病毒P6蛋白互作的森林草莓寄主因子
李帅,蒋西子,梁伟芳,陈思涵,张享享,左登攀,胡亚会,江彤
(安徽农业大学植物保护学院,合肥 230036)
【目的】草莓镶脉病毒(Strawberry vein banding virus,SVBV)是侵染草莓的主要病毒,但其侵染草莓的机制尚不清楚。论文以SVBV的P6蛋白为诱饵筛选森林草莓(Fragaria vesca)cDNA文库的寄主因子,为解析SVBV侵染草莓的分子机制提供理论依据。【方法】SVBV接种森林草莓,提取出现明显症状叶片的总RNA,DnaseI处理后,用SMART法反转录合成ds cDNA,均一化处理cDNA并酶切纯化,将<400 bp的短片段去除,其余片段连接到pGAD-T7质粒载体上,构建森林草莓初级cDNA文库。同时将SVBV P6构建到酵母双杂交诱饵载体pGBK-T7上,再将pGBK-P6和pGBK-T7分别转化酵母菌株AH109,阳性酵母菌株接种SD/-Trp液体培养基,鉴定诱饵载体对酵母细胞的毒性。将转化pGBK-P6的酵母菌分别涂布SD/-Trp、SD/-Leu-Trp和SD/-His-Trp平板,测定菌落生长情况,分析P6蛋白对酵母报告基因的自激活活性。然后用森林草莓初级cDNA文库质粒转化含有诱饵载体pGBK-P6的AH109酵母菌株,共转化子依次涂布SD/-Leu-Trp、SD/-Leu-Trp-His和SD/-Trp-Leu-His-Ade/X-α-Gal平板,最终筛选蓝色且长势较好的阳性菌落,提取酵母质粒并测序,GenBank中初步比对候选基因,利用Uniprot在线网站的gene ontology(GO)通路注释互作蛋白因子,分析互作蛋白的生物学功能。【结果】3种cDNA文库平均库容超过2.0×106cfu,平均文库重组率为97%,文库插入片段平均扩增长度>1 kb,表明森林草莓cDNA文库符合试验标准。最终利用SD/-Trp-Leu-His-Ade/X-α-Gal培养基筛选得到230个酵母阳性克隆,经过序列相似性比对,除去重复序列、载体序列和移码序列,共筛得15个与SVBV P6互作的寄主因子。GO通路注释结果表明这些寄主因子参与了13种生物过程,包括泛素化、转录因子调节、防御反应、代谢过程、氧化还原和胞内氨基酸代谢等过程;这15个寄主因子的分子功能多样,包括乙酰转移酶活性、萜烯合酶活性、脱氢酶活性、金属离子结合活性、蛋白激酶活性和水解酶活性等。【结论】成功构建了森林草莓酵母cDNA文库,筛选出15个与SVBV P6互作的森林草莓寄主因子,为进一步探明SVBV与森林草莓互作的分子机理提供了理论依据。
草莓镶脉病毒;森林草莓;cDNA文库;酵母双杂交;筛选;寄主因子
Abstract:【Objective】Strawberry vein banding virus (SVBV) is a main virus infecting woodland strawberry (Fragaria vesca),but the SVBV infection mechanisms on woodland strawberry remains unknown. The objective of this study is to provide a theoretical basis for studying the SVBV infection mechanisms on woodland strawberry, SVBV P6 was used as a bait protein toscreen the host factors from the cDNA library of woodland strawberry. 【Method】 Woodland strawberries were inoculated with SVBV, and total RNA was extracted from the leaves showed obvious disease symptoms. The total RNA was treated with DnaseI and double-stranded cDNA was synthesized using SMART technology. cDNA was treated with homogenization and enzymatic digestion,and the short fragments with length less than 400 bp were removed. Then the other cDNA fragments were ligated to plasmid vector pGAD-T7 to construct the primary cDNA library of woodland strawberry. Simultaneously, SVBV P6 was ligated into the yeast two-hybrid bait vector pGBK-T7, and the plasmids of pGBK-P6 and pGBK-T7 were transformed into AH109, respectively. The positive yeast clones were grown in the SD/-Trp liquid medium for identifying the toxicity of pGBK-P6 on the yeast AH109. The yeast transformed with pGBK-P6 was grown on the plate of SD/-Trp, SD/-Leu-Trp and SD/-His-Trp medium, respectively, and then the growth situation of the yeast was tested and the self-activating effect of pGBK-P6 on the reporter gene of yeast was analyzed.Then the AH109 containing bait vector pGBK-P6 was transformed with the primary cDNA library of woodland strawberry, the co-transformed yeasts were coated on the plate of SD/-Leu-Trp, SD/-Leu-Trp-His and SD/-Trp-Leu-His-Ade/X-α-Gal medium in turn. Finally, the blue and well grown positive clones were selected. The plasmids of positive yeast clones were extracted and sequenced. The candidate genes were preliminarily compared in the GenBank, and the interacted protein factors were annotated and the protein’s biological functions were analyzed with gene ontology (GO) pathway of Uniprot online websites. 【Result】 Three libraries with the average capacity more than 2.0×106cfu were constructed, and the average library recombination rate was 97% and the average amplification sizes of inserts fragment of cDNA library were above 1 kb. It demonstrated that the cDNA library of woodland strawberry measured up to the experiment standard. The 230 positive clones were finally selected by using the SD/-Trp-Leu-His-Ade/X-α-Gal medium. After sequence similarity comparison, removing the repetitive sequences, the vector sequences and the frameshift sequences, the 15 host factors interacted with SVBV P6 were screened. GO pathway annotation showed that the 15 host factors were involved in 13 biological processes, including protein ubiquitination, regulation of transcription factor process, defense response, protein catabolic process, oxidation-reduction process and cellular amino acid metabolic process, etc.Moreover, molecular functions of the 15 host factors are mutiple, including acetyltransferase activity, terpene synthase activity,dehydrogenase activity, metalion binding activity, protease activity and hydrolase activity, etc.【Conclusion】The cDNA library of woodland strawberry was constructed successfully, and 15 host factors of woodland strawberry interacted with SVBV P6 were preliminarily screened. This work can provide a theoretical basis for further exploring the molecular interaction mechanism between SVBV and woodland strawberry.
Key words:Strawberry vein banding virus; woodland strawberry; cDNA library; yeast two-hybrid; screen; host factors
0 引言
【研究意义】草莓镶脉病毒(Strawberry vein banding virus,SVBV)是侵染草莓的主要病毒之一,在美洲、欧洲、澳大利亚和日本等多个国家和地区广泛分布[1-2],中国河南、河北、吉林和浙江等省也均有报道,给草莓生产造成严重损失[3]。自然条件下,SVBV主要借助蚜虫以半持久性方式传染[4]。SVBV侵染森林草莓(Fragaria vesca)表现出沿着叶脉黄化、小叶扭曲等症状[5];侵染栽培草莓可造成植株生长衰弱,匍匐茎数量减少,果实偏小,产量和品质大幅度降低[6]。实验室前期研究发现SVBV编码的P6蛋白是一个多功能蛋白,既是病毒 RNA沉默抑制子,又是症状决定子,在病毒侵染寄主和致病过程中起到重要的作用。明确与SVBV P6蛋白互作的寄主因子,对探明病毒致病机理、解析寄主如何抵御病毒侵染均具有重要意义。【前人研究进展】SVBV为花椰菜花叶病毒科(Caulimovidae)花椰菜花叶病毒属(Caulimovirus)的重要成员[7],是一种大小为45—50 nm的环状ds DNA病毒。PETRZIK等[8]于20世纪90年代完成了第一个SVBV美国分离物全基因组序列测定,SVBV核苷酸序列全长7 876 nt,基因组结构与同属的花椰菜花叶病毒(Cauliflower mosaic virus,CaMV)的结构相似,包含7个开放阅读框(open reading frame,ORF)[9]。有关SVBV各个ORF的功能研究目前在全世界范围内均未见报道,只能根据CaMV相应ORF功能进行推测。在线预测SVBV保守功能域,ORF I可能编码移动蛋白(movement protein,MP);ORF II编码一个与蚜虫传染有关的蛋白;ORF III可能编码一个非序列专化性的 DNA结合蛋白;ORF IV编码病毒的外壳蛋白(coat protein,CP);ORF V编码逆转录酶蛋白;ORF VI编码一个多功能蛋白;ORF VII编码蛋白的功能尚不明确[10-11]。CaMV ORF VI编码的P6蛋白功能多样,相关研究较深入。CaMV P6蛋白是一个反式激活因子,能够与翻译起始因子eIF3以及60S核糖体的L18和L24蛋白亚基互作,调控35S RNA下游蛋白的翻译[12];P6蛋白是病毒内含体的主要组分,也是一个 RNA沉默抑制子,决定症状类型和症状严重度以及寄主范围[13];P6蛋白还能调控寄主植物多个基因mRNA的表达量[14]。而关于SVBV P6蛋白的功能研究至今尚未见报道。近年来,酵母双杂交系统(yeast two-hybrid system,Y2H)广泛应用于病毒蛋白与寄主因子互作研究,Y2H应用于病毒核酸复制及病毒基因表达调控、病毒介体传播的分子机制、病毒致病机制等方面的研究已见报道[15]。构建感病寄主酵母cDNA文库,再用诱饵载体筛选出与病毒蛋白互作的寄主因子,可为进一步研究互作蛋白的功能提供理论依据。何乙坤等[16]利用 Y2H技术筛选出苹果褪绿叶斑病毒(Apple chlorotic leaf spot virus,ACLSV)CP可以与光系统 II(PSII)的装配因子蛋白互作,推测ACLSV影响植物光系统的稳定性及叶绿素的形成,导致苹果树产生褪绿症状;楼望淮等[17]筛选到与菊花 B病毒(Chrysanthemum virus B,CVB)CP蛋白互作的E3泛素连接酶ARIADNE-like蛋白和ATP结合蛋白,推测CP可能参与泛素-蛋白酶体降解途径(UPP),并在病毒的侵染过程中起关键作用;赵艺泽等[18]构建了异沙叶蝉(Psammotettix alienus)cDNA文库,筛选得到9个与小麦矮缩病毒(Wheat dwarf virus,WDV)CP蛋白互作的寄主因子,这些寄主因子参与介体体内多条重要的代谢通路,可能与病毒突破昆虫介体中肠屏障,进入血淋巴循环有重要关系,为进一步深入研究病毒与昆虫介体的相互作用打下了基础;肖冬来等[19]利用水稻酵母文库筛选出了与水稻黑条矮缩病毒(Rice black-streaked dwarf virus,RBSDV)P6蛋白互作的水稻代谢途径关键酶 tAPX、IM和 A1EP,推测 P6可能参与水稻的相关代谢途径。【本研究切入点】研究病毒致病因子与寄主因子的互作关系,对探明病毒致病机理、解析寄主如何抵御病毒侵染十分重要,这也是当前植物病毒学研究的热点问题。目前运用酵母双杂交体系研究病毒蛋白与寄主因子互作的方法已经很成熟,笔者实验室已证明SVBV ORF VI基因编码的P6蛋白是一个致病因子,能够抑制GFP局部沉默和系统沉默,加重寄主的症状表型,与病毒的致病性密切相关。而关于SVBV 致病因子P6蛋白与寄主因子互作机理尚未见报道。因此,发现与P6蛋白互作的寄主因子是探明 SVBV致病机理的关键。【拟解决的关键问题】构建森林草莓叶片酵母cDNA文库,以SVBV P6为诱饵蛋白,筛选出与P6互作的寄主因子。再进一步研究寄主因子的生物学功能,为探明SVBV致病特征,明晰病毒与寄主的互作机理提供依据。
1 材料与方法
试验于 2015—2016年在安徽农业大学植物保护学院植物病毒实验室完成。
1.1 试验材料
森林草莓种植于温室大棚,感病草莓cDNA文库由宝生物工程(大连)有限公司构建;SVBV-T simple-P6质粒和SVBV侵染性克隆pBIN-1.25SVBVUS由笔者实验室保存,酵母菌株AH109,pGAD-T7和pGBK-T7等质粒购自Clontech公司;酵母质粒小提试剂盒、大肠杆菌DH5α感受态细胞购自北京康为世纪公司;Primer STAR GXL DNA聚合酶、pMD18-T simple载体、T4-DNA连接酶、Nde I、Sal I限制性内切酶和琼脂糖凝胶回收试剂盒等购自宝生物工程(大连)有限公司;引物合成与序列测序由上海生工生物股份有限公司完成。
1.2 试验方法
1.2.1 森林草莓的接种及 cDNA文库的构建 利用SVBV全长基因组侵染性克隆pBIN-1.25SVBV-US接种森林草莓,8周后系统叶表现出沿叶脉黄化症状,SDS法提取草莓显症叶片总RNA,具体步骤详见说明书,使用1%琼脂糖凝胶电泳检测RNA的完整性,将RNA样本送宝生物工程(大连)有限公司构建森林草莓cDNA文库。
1.2.2 P6扩增和诱饵载体 pGBK-P6的构建 设计SVBV P6特异性引物 P6-Nde I-F/P6-Sal I-R,以SVBV-P6-T simple质粒为模板扩增P6,胶回收纯化P6基因片段,连接pMD18-T simple载体,转化DH5α感受态细胞,涂布于氨苄青霉素抗性LB平板,菌落PCR筛选阳性克隆,提取质粒命名为 pMD-P6。用Nde I和Sal I酶双酶切重组质粒pMD-P6,将P6插入pGBK-T7,筛选阳性克隆,命名为pGBK-P6。
1.2.3 诱饵载体 pGBK-P6毒性及自激活检测 醋酸锂法制备酵母菌AH109感受态细胞,分别转化诱饵载体pGBK-P6和空载体pGBK-T7。涂布SD/-Trp培养基,30℃倒置培养3—5 d,挑取单菌落并筛选阳性克隆,鉴定诱饵载体pGBK-P6是否成功转化至AH109。
含有pGBK-P6和空载体pGBK-T7的酵母菌分别接种于 50 mL SD/-Trp(20 μg·mL-1)液体培养基中,30℃ 250 r/min 振荡培养24 h,检测菌液的OD600,鉴定诱饵载体对细胞的毒性;将含有pGBK-P6重组质粒的酵母菌株AH109分别涂布SD/-Trp、SD/-Trp-His和SD/-Trp-Ade平板,30℃倒置培养3—5 d,观察平板菌落生长情况,分析pGBK-P6对酵母细胞的自激活活性。
1.2.4 cDNA文库质粒转化携带 pGBK-P6的酵母菌醋酸锂法制备含有pGBK-P6的酵母菌AH109感受态细胞,再将插入森林草莓cDNA片段的pGAD-T7文库质粒转化到 AH109感受态细胞,转化产物涂布于SD/-Trp-Leu固体培养板,30℃倒置培养3—5 d;无菌水收集 SD/-Trp-Leu上的酵母菌落,再涂布于SD/-His-Leu-Trp固体培养基,30℃倒置培养 3—5 d后;挑取SD/-His-Leu-Trp固体培养基上长势良好的酵母单菌落,转移到SD/-Trp-Leu-His-Ade/X-α-Gal固体培养基,30℃倒置培养。
1.2.5 cDNA文库阳性克隆的鉴定 挑取 SD/-Trp-Leu-His-Ade/X-α-Gal上显蓝色的酵母单菌落,接种于5.0 mL SD/-Leu(20 μg·mL-1)液体培养基,30℃,250 r/min振荡扩繁1—2 d后,提取酵母质粒。
1.2.6 阳性克隆的序列分析与生物信息学分析 通用引物(T7-Promoter:5′-CTATTCGATGATGAAGAT ACCCCACCAAACCC-3′;3′ AD:5′-GTGAACTTGCG GGGTTTTTCAGTATCTACGATT-3′)PCR 扩增酵母质粒中的插入片段,选择插入片段>400 bp的酵母质粒,转化大肠杆菌DH5α,送上海生工生物公司测序,利用GenBank网站(http://blast.ncbi.nlm.nih.gov/Blast.Cgi)分析插入片段所属基因,再用Uniprot在线网站GO通路注释(http://www.uniprot.org/)该基因编码的蛋白因子。
2 结果
2.1 cDNA文库的质量检测
提取感病森林草莓总RNA,琼脂糖凝胶电泳检测发现,5.8S、18S和28S条带清晰,完整性好(图1)。NanoDrop检测 RNA OD260/OD280=1.97,OD260/OD230=2.19,RNA浓度为2 086.4 ng·μL-1,说明RNA质量较好,可以用于文库构建。森林草莓 cDNA文库报告结果显示,3个读码框初级文库库容分别为 1.5×106、2.5×106和2.0×106cfu,文库重组率为97%,均一化处理ds cDNA并酶切纯化,显示合成的ds cDNA呈弥散状分布,其片段大小分布范围为0.5—3.0 kb(图2)。文库质粒转入大肠杆菌DH5α,随机挑取16个克隆,PCR扩增插入片段,平均长度>1 kb(图3)。
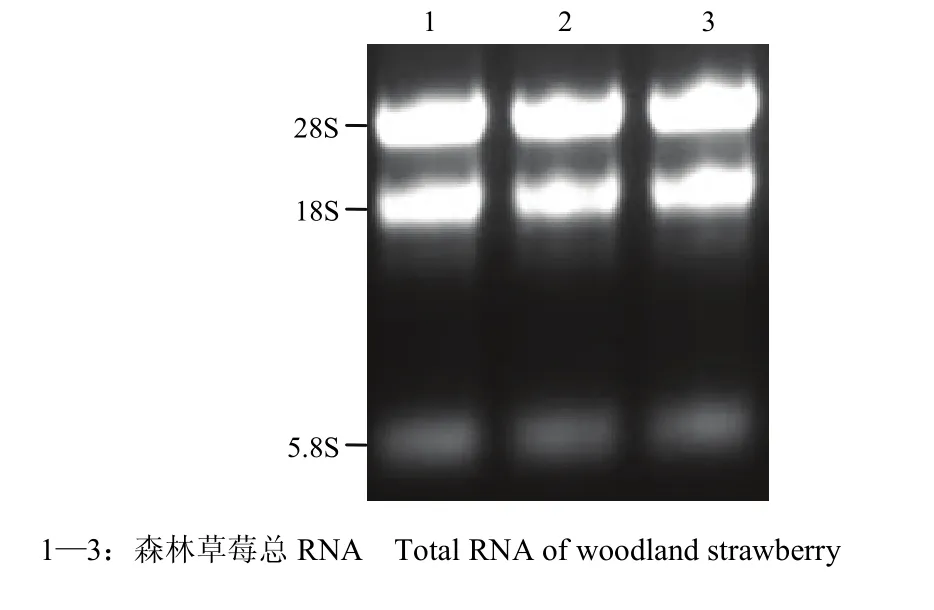
图1 SVBV侵染的森林草莓总RNA琼脂糖凝胶电泳Fig. 1 Agarose gel electrophoresis of RNA of woodland strawberry infected with SVBV
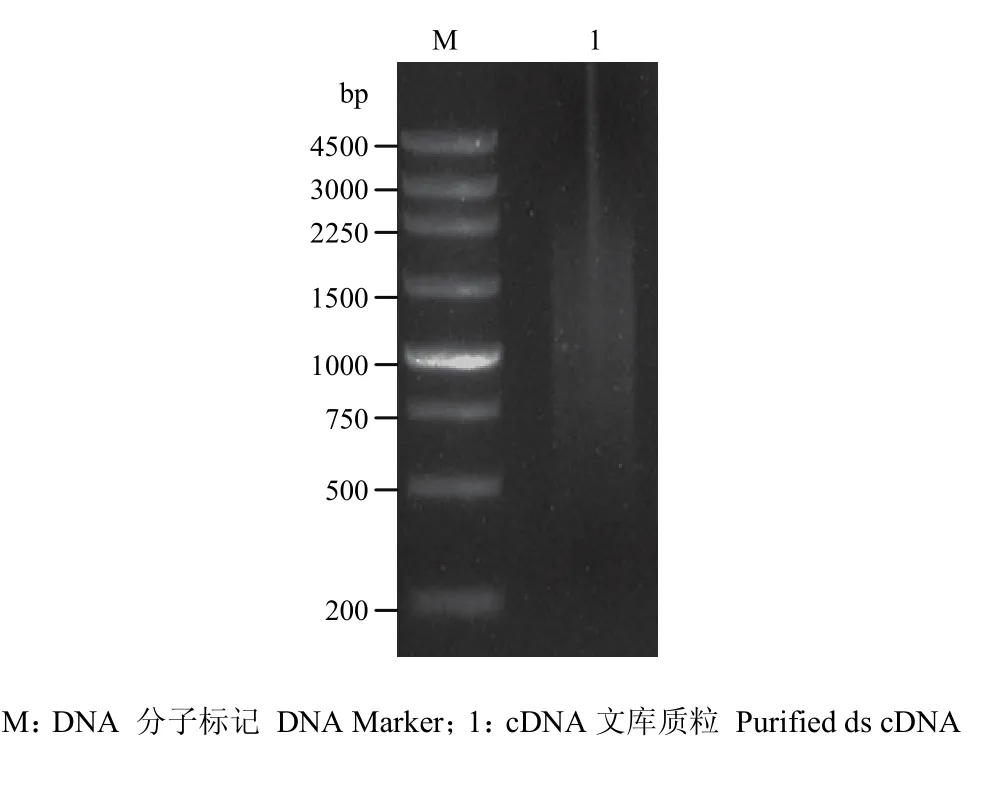
图2 森林草莓ds cDNA纯化后的电泳图Fig. 2 Electrophoresis of purified ds cDNA of woodland strawberry
2.2 诱饵载体pGBK-P6的构建及转化
以 SVBV-P6-simple模板,用引物 P6-NdeI-F、P6-SalI-R进行PCR扩增,可扩增出1条约为1 600 bp的特异性条带,片段大小与预期结果相符。将此特异性条带克隆并测序,证明插入pMD19-T simple的P6没有发生变异。再将P6克隆到酵母载体pGBK-T7上,PCR筛选pGBK-P6阳性克隆,Nde I和Sal I双酶切验证pGBK-P6质粒,片段大小和理论值完全相符(图4),第2次测序进一步验证P6基因序列未发生突变,说明插入的P6读码框正确。pGBK-P6质粒转化酵母菌株AH109,提取酵母质粒,PCR扩增得到大小约1 600 bp的特异性条带(图5),证明pGBK-P6质粒已成功转化到酵母菌株AH109。
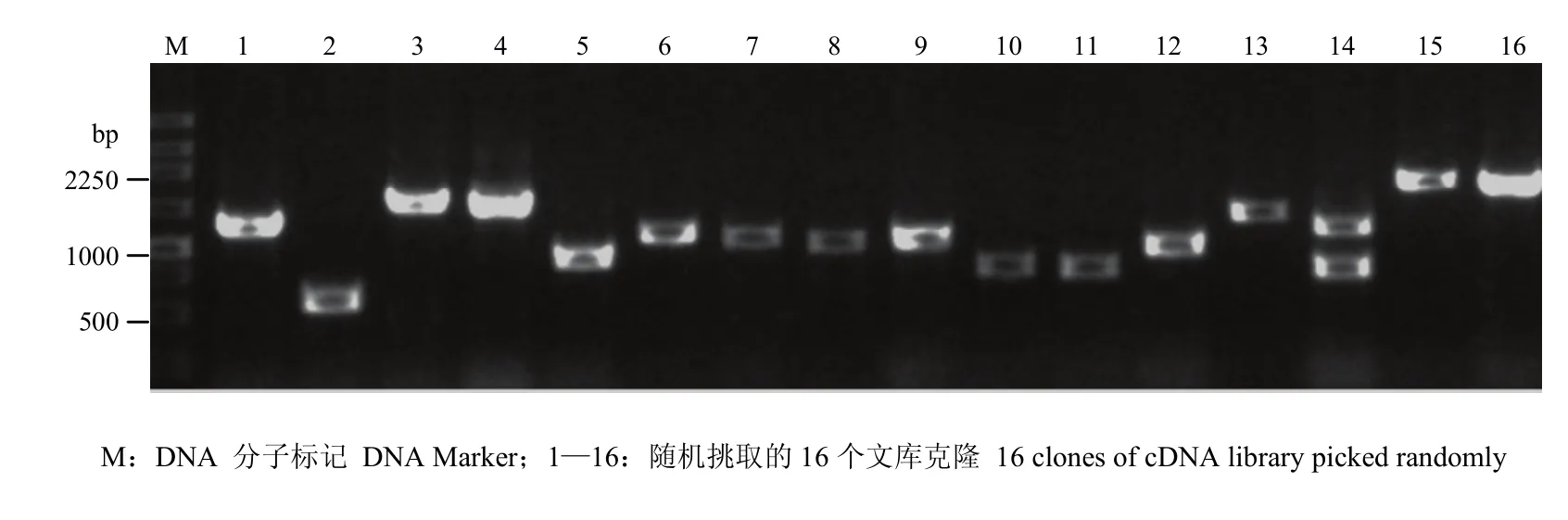
图 3 cDNA文库插入片段的PCR鉴定Fig. 3 PCR identification of inserts in the cDNA library
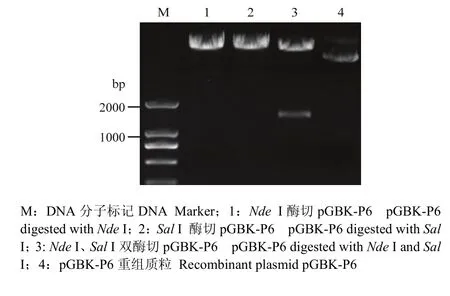
图4 pGBK-P6重组质粒酶切电泳图谱Fig. 4 Electrophoresis patterns of recombinant plasmid pGBK-P6 digested with restriction endonuclease enzymes
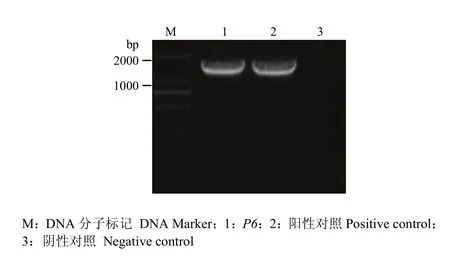
图5 重组质粒pGBK-P6转化酵母菌阳性克隆的PCR鉴定Fig. 5 Positive clone verification of recombinant plasmid pGBK-P6 expression in yeast by PCR
2.3 诱饵载体的自激活及毒性检测
pGBK-P6和pGBK-T7质粒分别转化AH109感受态细胞,2组转化菌在SD/-Trp培养基上均生长良好,菌落无明显差异(图6);2组转化菌在SD/-Trp(20 μg·mL-1)液体培养基培养 24 h 后,菌液 OD600均>0.8,说明 pGBK-P6载体对酵母细胞没有毒性。将含有pGBK-P6的酵母菌分别涂布于SD/-Trp、SD/-Trp-His和SD/-Trp-Ade培养基,发现酵母菌能在SD/-Trp培养基上生长,而不能在 SD/-Trp-His和 SD/-Trp-Ade培养基上生长,说明pGBK-P6不能激活报告基因His及Ade的表达,没有自激活活性(图7),可进行下游筛库试验。
2.4 筛选森林草莓cDNA文库中与P6互作的寄主因子
将森林草莓cDNA文库质粒转化到含有pGBK-P6诱饵载体的酵母菌AH109感受态细胞中,转化产物经SD/-Trp-Leu、SD/-His-Leu-Trp和 SD/-Trp-Leu-His-Ade/X-α-Gal固体培养基顺次筛选后,最终在SD/-Trp-Leu-His-Ade/X-α-Gal培养基筛选到230个生长状况良好且显蓝色的菌落(图8)。
2.5 寄主因子的扩增及测序分析
扩繁230个显蓝色菌落的菌株,分别提取质粒,转化大肠杆菌 DH5α,PCR筛选阳性菌株,其中 180个菌株呈阳性,可扩增出约1 kb的特异性片段。测序并进行序列比对,除去重复序列、载体序列和移码序列,最终获得174个有效序列,共对应15个寄主基因,这15个基因编码的寄主因子可能与P6蛋白互作(表1)。这15个寄主因子筛选丰度差异较大,筛选频率最高的是CHY型锌指蛋白(CHY zinc finger protein,ZFP),其次是低温诱导的半胱氨酸蛋白酶(lowtemperature-induced cysteine proteinase,LTICP),然后是3S,6E-橙花叔醇合酶1(3S,6E-nerolidol synthase 1,36NS),这3个寄主因子的筛选频数达118次,占筛选总数174个有效序列的67.8%。

图6 转化pGBK-P6和pGBK-T7质粒的酵母菌在SD/-Trp培养基的生长情况Fig. 6 The growth situation of yeast containing plasmids pGBK-P6 and pGBK-T7 in SD/-Trp medium
利用Uniprot在线网站的GO通路注释这15个森林草莓寄主因子(图9),发现这15个寄主因子参与了13种生物过程,包括泛素化、转录因子调节、防御反应、代谢过程、氧化还原、胞内氨基酸代谢、蛋白水解、生物合成、胞内信号转导、蛋白质磷酸化、诱导系统抗性、光系统II修复和细胞分裂等过程;其分子功能有12种,包括乙酰转移酶活性、萜烯合酶活性、脱氢酶活性、金属离子结合活性、蛋白激酶活性、水解酶活性、氧化还原酶活性、磷酸酶活性、DNA结合活性、ATP结合活性、蛋白自身结合和肽链内切酶抑制剂活性。
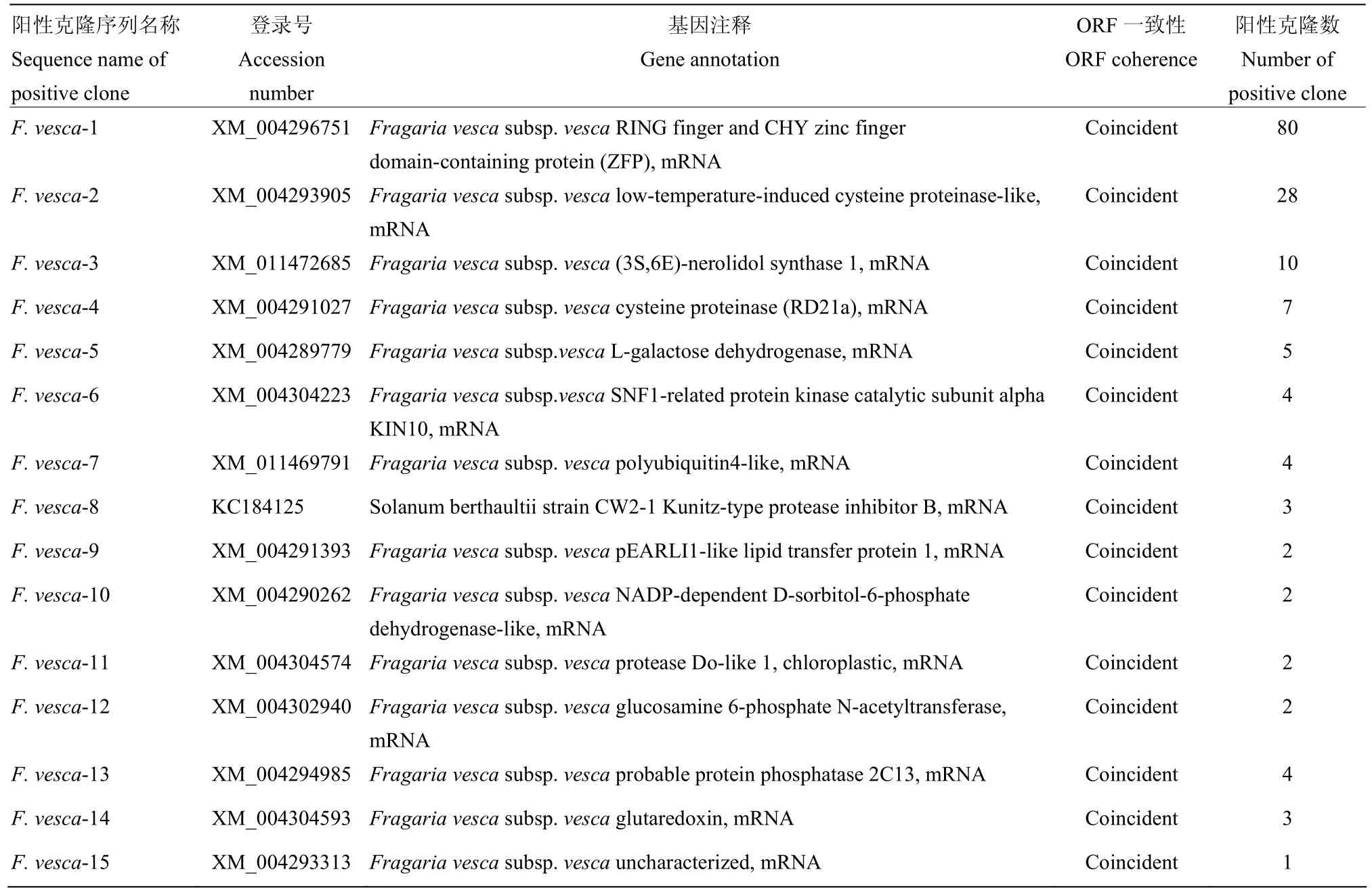
表1 15个与SVBV P6互作的森林草莓寄主因子Table 1 Fifteen host factors of woodland strawberry interacting with SVBV P6
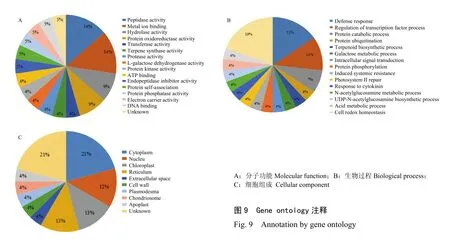
图9 Gene ontology注释Fig. 9 Annotation by gene ontology
3 讨论
目前,世界范围内已经鉴定出了30多种植物病毒沉默抑制子,其功能主要为增加病毒的积累、协助病毒的胞间运动及促进病毒的长距离运输等[20]。而SVBV P6蛋白作为沉默抑制子的主要功能及作用机理尚不清楚。本试验构建了高质量的森林草莓 cDNA文库,以SVBV P6为诱饵蛋白,从感染SVBV的森林草莓酵母cDNA文库中筛选与P6互作的寄主因子,为进一步探究SVBV的致病机理,解析病毒与寄主的分子互作机制打下了基础。
本研究共筛得15个寄主因子,序列比对发现15个寄主因子中,ZFP筛选丰度最高,筛选比率达到46%,说明ZFP与P6密切互作,具有较高的研究参考价值。锌指蛋白ZFP是一类结合锌离子折叠成手指状结构域的蛋白,广泛分布于动植物和微生物[21]。主要由半胱氨酸(Csy)和/或组氨酸(His)组成,与锌离子结合形成“指”状四面体结构,锌离子在稳定锌指蛋白结构和发挥调控功能方面具有关键作用[22]。锌指蛋白可以与靶分子DNA、RNA、DNA-RNA的序列特异性结合[23],在转录和翻译水平上调控基因的表达、细胞分化以及胚胎发育等[24]。
锌指蛋白ZFP在调节植物生长发育和抵御病原物侵染过程中均发挥重要的作用。将棉花锌指蛋白基因GhZFP1转入烟草,GhZFP1能够上调烟草抗病相关蛋白(resistance related protein)GZIRD21A和GZIPR5的表达,提高烟草对立枯丝核菌(Rhizoctonia solani)侵染的抗性[25]。稻瘟病菌(Magnaporthe oryzae)侵染水稻,水稻锌指蛋白 OsSYP71能提高病程相关基因PR-1b的表达,同时增强过氧化物酶的活性,清除寄主体内的过氧化氢(H2O2),抵御病菌的侵染[26]。烟草花叶病毒(Tobacco mosaic virus,TMV)侵染普通烟(Nicotiana tabacum)时,寄主WRKY转录因子锌指蛋白 TIZZ的表达量提高,诱导寄主产生过敏性坏死反应(hypersensitive response,HR),抵御病毒的进一步侵染[27]。黄瓜花叶病毒(Cucumber mosaic virus,CMV)侵染普通烟,锌指蛋白Tsip1能够与CMV的复制复合体在液泡膜上互作,影响病毒的胞间运输,Tsip1还能与CMV 1a和CMV 2a形成复合体,干扰病毒的复制和转移[28]。SVBV的复制的机理与CaMV极为相似,P6作为一个反式激活因子,可在细胞质中调控35S RNA翻译出病毒其余蛋白[29]。因此,推测锌指蛋白ZFP和SVBV致病因子P6蛋白的互作,能够干扰P6蛋白与35S RNA的结合能力,影响病毒的复制过程,抵御病毒的侵染。
半胱氨酸蛋白酶(cysteine protease)作为一类重要的蛋白酶家族,广泛参与植物的各种生理过程[30]。研究发现,半胱氨酸蛋白酶在各种衰老的植物器官中大量表达,可降解光合作用必需酶 Rubisco(1,5-二磷酸核酮糖脱羧/加氧酶)大亚基,抑制光合作用,促进植物衰老[31]。当植物受到病原菌侵染时,半胱氨酸蛋白酶mRNA含量会显著增加,参与响应寄主的过敏性坏死反应,诱导植物细胞的程序性死亡(PCD),阻止病原菌在寄主的进一步扩散[32]。因此,笔者推测受SVBV侵染后,P6蛋白与半胱氨酸蛋白酶RD21a结合,上调RD21a的表达,抑制叶片光合作用,使植物提前衰老,进一步表现出沿着叶脉黄化、小叶扭曲等感病症状。
植物SNF1激酶(SNF1-related protein kinase)是动物AMP激活蛋白激酶的同源物,为体内代谢的全局调控因子,在消耗ATP的胁迫反应中起重要作用[33]。SUNTER等[34]将番茄金色花叶病毒(Tomato golden mosaic virus,TGMV)AC2和甜菜曲顶病毒(Beet curly top virus,BCTV)的C2分别转入烟草,发现转基因植株对TGMV、BCTV和TMV的感病性增加,进一步利用酵母双杂交技术验证蛋白互作,发现 TGMV AC2和 BCTV的 C2蛋白与拟南芥(Arabidopsis thaliana)的蔗糖非发酵激酶SNF1互作,并在体外和体内抑制SNF1激酶的活性,表达反义SNF1的拟南芥对双生病毒的感病性增强,而过量表达正义 SNF1的拟南芥对双生病毒表现出抗性。表达 AC2/C2的拟南芥对双生病毒的感病性增强,但在拟南芥中表达缺失与SNF1激酶互作结构域的AC2/C2则不能增强对双生病毒的感病性[35]。这些结果表明植物代谢通路的调节可能是植物应对病毒侵染的一种重要防卫反应。由此推测SVBV P6蛋白可能与寄主SNF1激酶互作,抑制SNF1的活性,调节寄主代谢通路,影响草莓的正常生理代谢,导致草莓表现出生长衰弱、匍匐茎数量减少等不良生长状况。
4 结论
成功构建了高质量的森林草莓cDNA文库,筛选得到了15个与SVBV P6蛋白互作的寄主因子,这些蛋白因子生物功能多样,可能参与SVBV侵染寄主过程中的多条重要通路,在协助寄主抵御病毒侵染,减轻发病症状等方面发挥重要作用,为进一步深入研究SVBV与寄主的相互作用打下了理论基础。
[1]RATTI C, PISI A, AUTONELL C R, BABINI A, VICCHI V. First report of Strawberry vein banding virus on strawberry in Italy. Plant Disease, 2009, 93(6): 675.
[2]HONETSLEGROVA J, MRAZ I, SPAK J. Detection and isolation of Strawberry vein banding virus in the Czech Republic. Acta Horticulturae,1995, 385: 29-32.
[3]肖敏, 张志宏. 草莓镶脉病毒研究进展. 辽宁农业科学, 2005(4):36-38.XIAO M, ZHANG Z H. Research advance in Strawberry vein banding virus. Liaoning Agricultural Sciences, 2005(4): 36-38. (in Chinese)
[4]MORRIS T J, MULLIN R H, SCHLEGEL D E, COLE A, ALOSI M C. Isolation of a Caulimovirus from strawberry tissue infected with Strawberry vein banding virus. Phytopathology, 1980, 70(2): 156-160.
[5]PETRZIK K, MRAZ I, DULIC-MARKOVIC I. Quarantine Strawberry vein banding virus firstly detected in Slovakia and Serbia.Acta Virologica, 1998, 42(2): 87-89.
[6]FRAZIER N W. Detection of graft-transmissible diseases in strawberry by a modified leaf grafting technique. Plant Disease Reporter, 1974, 58: 203-207.
[7]洪健, 李德葆, 周雪平. 植物病毒分类图谱. 北京: 科学出版社,2001: 12.HONG J, LI D B, ZHOU X P. Classification Atlas of Plant Viruses.Beijing: Science Press, 2001: 12. (in Chinese)
[8]PETRZIK K, BENES V, MRAZ I, HONETSLEGROVA F J,ANSORGE W, SPAK J. Strawberry vein banding virus-definitive member of the genus Caulimovirus. Virus Genes, 1998, 16(3):303-305.
[9]PAPPU H R, DRUFFEL K L. Use of conserved genomic regions and degenerate primers in a PCR-based assay for the detection of members of the genus Caulimovirus. Journal of Virological Methods, 2009,157(1): 102-104.
[10]KAREL P, VLADIMIR B, IVAN M, HONETSLEGROVA- FRANOVA J,ANSORGE W, SPAK J. Strawberry vein banding virus-definitive member of the genus Caulimovirus. Virus Genes, 1998, 16(3):303-305.
[11]LEH V, YOT P, KELLER M. The Cauliflower mosaic virus translational transactivator interacts with the 60S ribosomal subunit protein L18 of Arabidopsis thaliana. Virology, 2000, 266(1): 1-7.
[12]ANDREW J L, JANET L, JUSTIN H, HAMILTON A J,SADANANDOM A, MILNER J J. Cauliflower mosaic virus protein P6 is a suppressor of RNA silencing. Journal of General Virology,2007, 88(12): 3439-3444.
[13]GAIL A B, JERRY D J, STEPHEN H H. Cauliflower mosaic virus gene VI produces a symptomatic phenotype in transgenic tobacco plants. Proceeding of the National Academy Sciences of the United States of America, 1988, 85(3): 733-737.
[14]CHIARA G, CECCHINI E, MARIA E G,SIMON N C, JOEL J M.Altered patterns of gene expression in Arabidopsis elicited by Cauliflower mosaic virus (CaMV) infection and by a CaMV gene VI transgene. Molecular Plant, 1999, 12(5): 377-384.
[15]吴建国, 蔡丽君, 胡梅群, 谢荔岩, 林奇英, 吴祖建, 谢联辉. 水稻瘤矮病毒 P3、P7、P8、Pn9、Pn10、Pnl1、Pnl2的酵母双杂交载体的构建及自激活效应检测. 热带作物学报, 2009, 30(9):1364-1368.WU J G, CAI L J, HU M Q, XIE L Y, LIN Q Y, WU Z J, XIE L H.Construction of yeast two-hybrid vectors of P3, P7, P8, Pn9, Pn10,Pn11 and Pn12 of Rice gall dwarf virus and identification of their self-activation. Chinese Journal of Tropical Crops, 2009, 30(9):1364-1368. (in Chinese)
[16]何乙坤, 钟敏, 胡同乐, 王树桐, 段豪, 丁丽, 王亚南, 曹克强. 利用酵母双杂交筛选与苹果褪绿叶斑病毒CP互作的寄主因子. 中国农业科学, 2014, 47(24): 4821-4829.HE Y K, ZHONG M, HU T L, WANG S T, DUAN H, DING L,WANG Y N, CAO K Q. Screening of the host factors interacting with CP of Apple chlorotic leaf spot virus by yeast two-hybrid system.Scientia Agricultura Sinica, 2014, 47(24): 4821-4829. (in Chinese)
[17]楼望淮, 蒋甲福, 陈素梅, 房伟民, 陈发棣, 管志勇, 廖园. 菊花 B病毒外壳蛋白互作蛋白的筛选. 南京农业大学学报, 2013, 36(4):43-48.LOU W H, JIANG J F, CHEN S M, FANG W M, CHEN F D, GUAN Z Y, LIAO Y. Screening of proteins interacting with the coat protein of Chrysanthemum virus B. Journal of Nanjing Agricultural University, 2013, 36(4): 43-48. (in Chinese)
[18]赵艺泽, 刘艳, 王锡锋. 利用酵母双杂交系统筛选介体异沙叶蝉中与小麦矮缩病毒外壳蛋白互作的蛋白质. 中国农业科学, 2015,48(12): 2354-2363.ZHAO Y Z, LIU Y, WANG X F. Screening of putative proteins in vector Psammotettix alienus L. that are interacted with coat protein of Wheat dwarf virus by a split-ubiquitin yeast membrane system.Scientia Agricultura Sinica, 2015, 48(12): 2354-2363. (in Chinese)
[19]肖冬来, 邓慧颖, 谢荔岩, 吴祖建, 谢联辉. 酵母双杂交系统筛选与水稻黑条矮缩病毒 P6互作的水稻蛋白. 热带作物学报, 2010,31(3): 435-438.XIAO D L, DENG H Y, XIE L Y, WU Z J, XIE L H. Screening of rice proteins interacting with P6 of Rice black-streaked dwarf virusfrom rice cDNA library by yeast two hybrid system. Chinese Journal of Tropical Crops, 2010, 31(3): 435-438. (in Chinese)
[20]蒋琳, 魏春红, 李毅. 病毒基因沉默抑制子及其作用机制. 中国科学: 生命科学, 2012, 42(1): 16-28.JIANG L, WEI C H, LI Y. Viral suppressor of RNA silencing. Scientia Sinica Vitae, 2012, 42(1): 16-28. (in Chinese)
[21]FRANKEI A D, PABO C O. Fingering too many proteins. Cell, 1998,53(6): 675.
[22]HOOVERS J M, MANNENS M, JOHN R, BLIEK J, VERONICA V H, PORTEOUS D J, LESCHOT N J, WESTERETVELD A, LITTLE P F. High-resolution localization of 69 potential human zinc finger protein genes: a number are clustered. Genomics, 1992, 12(2):254-263.
[23]ESPINOSA J M, PORTAL D, LOBO G S, PEREIRA C A, ALONSO G D, GOMEZ E B, LAN G H, POMAR R, FLAWIA M M, TORRES H N. Trypanosoma cruzi poly-zinc finger protein: a novel DNA/RNA-binding CCHC-zinc finger protein. Molecular and Biochemical Parasitology, 2003, 131(1): 35-44.
[24]JAUCH R, BOURENKOV G P, CHUNG H R, URLAUB H, REIDT U, JACKLE H, WAHL M C. The zinc finger associated domain of the Drosophila transcription factor grauzone is a novel zinc-coordinating protein-protein interaction modules. Structure, 2003, 11(11):1393-1402.
[25]GUO Y H, YU Y P, WANG D, WU C A, YANG G D, HUANG J G,ZHENG C C. GhZFP1, A novel CCCH-type zinc finger protein from cotton, enhances salt stress tolerance and fungal disease resistance in transgenic tobacco by interacting with GZIRD21A and GZIPR5. New Phytologist, 2009, 183(1): 62-75.
[26]BAO Y M, SUN S J, LI M, LI L, CAO W L, LUO J, TANG H J,HUANG J, WANG Z F, WANG J F. Overexpression of the Qc-SNARE gene OsSYP71 enhances tolerance to oxidative stress and resistance to rice blast in rice (Oryza sativa). Gene, 2012, 504(2):238-244.
[27]YODA H, OGAWA M, YAMAGUCHI Y, KOIZUMI N, KUSANO T,SANO H. Identification of early-responsive genes associated with the hypersensitive response to Tobacco mosaic virus and characterization of a WRKY-type transcription factor in tobacco plants. Molecular Genetics and Genomics, 2002, 267(2): 154-161.
[28]HUH S U, KIM M J, HAM B K, PAEK K H. A zinc finger protein Tsip1 controls Cucumber mosaic virus infection by interacting with the replication complex on vacuolar membranes of the tobacco plant.New Phytologist, 2011, 191(3): 746-762.
[29]MURIEL H, MARINA B, ANGÈLE G, PIERRE Y, MARIO K.Cauliflower mosaic virus: still in the news. Molecular Plant Pathology,2002, 3(6): 419-429.
[30]李思滨, 刘英, 祖元刚. 半胱氨酸蛋白酶在植物细胞程序性死亡中的作用. 植物生理学通讯, 2008, 44(2): 345-349.LI S B, LIU Y, ZU Y G. Role of cysteine proteinase in programmed cell death of plant. Plant Physiology Communications, 2008, 44(2):345-349. (in Chinese)
[31]WANG W, ZHANG L, GUO N, ZHANG X, ZHANG C, SUN G, XIE J. Functional properties of a cysteine proteinase from pineapple fruit with improved resistance to fungal pathogens in Arabidopsis thaliana.Molecules, 2014, 19: 2374-2389.
[32]HARRAK H, AZELMAT S, BAKER E N, TABAEIZADEH Z.Isolation and characterization of a gene encoding a drought-induced cysteine protease in tomato (Lycopersicon esculentum). Genome, 2001,44: 368-374.
[33]NIGEL G H, GRAHAME D H. SNF1-related protein kinases: global regulators of carbon metabolism in plants. Plant Molecular Biology,1998, 37: 735-748.
[34]SUNTER G, SUNTER J L, BISARO D M. Plants expressing Tomato golden mosaic virus AL2 or Beet curly top virus L2 transgenes show enhanced susceptibility to infection by DNA and RNA viruses.Virology, 2001, 285: 59-70.
[35]HAO L H, WANG H, SUNTER G, BISARO D M. Geminivirus AL2 and L2 proteins interact with and inactivate SNF1 kinase. The Plant Cell, 2003, 15(4): 1034-1048.
(责任编辑 岳梅)
Screening of the Host Factors of Woodland Strawberry Interacting with P6 of Strawberry vein banding virus by Yeast Two-Hybrid System
LI Shuai, JIANG XiZi, LIANG WeiFang, CHEN SiHan, ZHANG XiangXiang,ZUO DengPan, HU YaHui, JIANG Tong
(School of Plant Protection, Anhui Agricultural University, Hefei 230036)
2017-03-20;接受日期:2017-05-09
国家公益性行业(农业)科研专项(201303028)、国家自然科学基金(31671999,31371915)
联系方式:李帅,E-mail:18356086590@163.com。通信作者江彤,E-mail:jiangtong4650@sina.com

