由半刚性三羧酸配体构筑的一维和二维钴配位聚合物的合成、晶体结构及磁性质
顾文君 顾金忠
(1西南大学动物科技学院,重庆400715)(2兰州大学化学化工学院,兰州730000)
顾文君1顾金忠*,2
(1西南大学动物科技学院,重庆400715)
(2兰州大学化学化工学院,兰州730000)
采用水热方法,用半刚性三羧酸配体(H3cpta)和菲咯啉(phen)或2,2′-联吡啶(2,2′-bipy)与CoCl2·6H2O反应,合成了一个一维链状配位聚合物[Co(μ2-Hcpta)(phen)(H2O)]n(1)和一个二维层状配位聚合物[Co3(μ5-cpta)2(2,2′-bipy)2]n(2),并对其结构和磁性质进行了研究。结构分析结果表明2个配合物均属于单斜晶系,P21/c空间群。配合物1具有一维链状结构,而且这些一维链状结构通过C-H…O氢键作用进一步形成了二维超分子网络。而配合物2具有由三核钴单元构筑的二维层状结构。研究表明,配合物1和2中相邻钴离子间存在反铁磁相互作用。
配位聚合物;氢键;三羧酸配体;磁性
0 Introduction
The design and construction of the coordination polymershasattractedgreatattentionfortheir potential applications,architectures,and topologies[1-8]. Many factors such as the coordination geometry of the central atom,the structural characteristics of the ligand,the solvent system,and the counterions canplaythekeyroleintheconstructionofthe coordination networks[9-12].The selection of the special ligands is very important in the construction of these coordination polymers.
A lot of aromatic polycarboxylic acids has been extensively applied as multifunctional building blocks in the construction of metal-organic networks because of their abundant coordination modes to metal ions, allowing different type of structural topologies and because of their ability to act as H-bond acceptors and donors,depending on the number of deprotonated carboxylicgroupstoassemblesupramolecular structures[13-16].Amongthem,semi-rigidV-shaped ligands enable the formation of uncommon frameworks or even novel topologies and interesting properties becauseoftheirflexibilityandconformational diversity[17-20].
In order to extend our research in this field,we chose a new semi-rigid polycarboxylate ligand,2-(2-carboxyphenoxy)terephthalic acid(H3cpta)to construct novelcoordinationpolymers.TheH3cptaligand possesses the following features:(1)two rigid benzene rings of H3cpta ligand are connected by a rotatable -O-group,whichallowstheligandwithsubtle conformationaladaptation;(2)sevenpotential coordination sites(six carboxylate O and one ether O) of H3cpta ligand,which can provide more varied coordination patterns in the construction of fascinating coordinationframeworks,especiallywithhigh dimensionalities.However,the metal-organic networks constructed from the H3cpta ligand have not been reported.
Taking into account these factors,we herein report the syntheses,crystal structures,and magnetic propertiesoftwoCocoordinationpolymers constructed from H3cpta.
1 Experimental
1.1 Reagents and physical measurements
All chemicals and solvents were of AR grade and used without further purification.The content of carbon,hydrogen and nitrogen were determined using an Elementar Vario EL elemental analyzer.IR spectra wererecordedusingKBrpelletsandaBruker EQUINOX55spectrometer.Thermogravimetric analysis(TGA)data were collected on a LINSEIS STA PT1600 thermal analyzer with a heating rate of 10℃· min-1.Powder X-ray diffraction patterns(PXRD)were determined with a Rigaku-Dmax 2400 diffractometer using Cu Kα radiation(λ=0.154 060 nm)and 2θ ranging from 5°to 45°,in which the X-ray tube was operated at 40 kV and 40 mA.Magnetic susceptibility data were collected in the 2~300 K temperature range with a Quantum Design SQUID Magnetometer MPMS XL-7 with a field of 0.1 T.A correction was made for the diamagnetic contribution prior to data analysis.
1.2 Synthesis of[Co(μ2-Hcpta)(phen)(H2O)]n(1)
A mixture of CoCl2·6H2O(0.071 g,0.30 mmol), H3cpta(0.060 g,0.2 mmol),phen(0.060 g,0.3 mmol), NaOH(0.016 g,0.40 mmol),and H2O(10 mL)was stirred at room temperature for 15 min,and then sealed in a 25 mL Teflon-lined stainless steel vessel, and heated at 160℃for 3 days,followed by cooling to room temperature at a rate of 10℃·h-1.Orange block-shaped crystals of 1 were isolated manually,and washed with distilled water.Yield:63%(based on H3cpta).Anal.Calcd.for C27H18CoN2O8(%):C 58.18,H 3.26,N 5.03;Found(%):C 58.46,H 3.24,N 5.01.IR (KBr,cm-1):3 438w,3 050w,1 702w,1 625m,1 594s, 1 559s,1 528w,1 493w,1 452w,1 400s,1 355s, 1 299w,1 243w,1 151w,1 100w,1 048w,956w,850m, 809w,787w,762w,727m,691w,665w,640w,594w, 537w.
1.3 Synthesis of[Co3(μ5-cpta)2(2,2′-bipy)2]n(2)
The preparation of 2 was similar to that of 1 except 2,2′-bipy(0.047 g,0.30 mmol)was used instead of phen and different amount of NaOH(0.024 g,0.60 mmol)was used.Pink block-shaped crystals of 2 were isolated manually,washed with distilled water. Yield:55%(based on H3cpta).Anal.Calcd.for C50H30Co3N4O14:C 55.22,H 2.78,N 5.15;Found(%):C 55.02,H 2.81,N 5.11.IR(KBr,cm-1):1 590s,1 554m, 1 529w,1 477w,1 452m,1 396s,1 345s,1 237m, 1 156w,1 094w,1 059w,1 018w,956w,879w,870w, 834w,798m,767s,737w,706w,661w,589w,537m.The compounds are insoluble in water and common organicsolvents,such as methanol,ethanol,acetone and DMF.
1.4 Structure determinations
The data of two single crystals with dimensions of 0.26 mm×0.24 mm×0.21 mm for 1 and 0.27 mm×0.22 mm×0.21 mm for 2,respectively,were collected at 293(2)K on a Bruker SMART APEXⅡCCD diffractometer with Mo Kα radiation(λ=0.071 073 nm).The structures were solved by direct methods and refined by full matrix least-square on F2using the SHELXTL-97 program[21].All non-hydrogen atoms were refined anisotropically.Allthehydrogenatomswere positioned geometrically and refined using a riding model.A summary of the crystallography data and structure refinements for 1 and 2 is given in Table 1. The selected bond lengths and angles for compounds 1 and 2 are listed in Table 2.Hydrogen bond parameters of compound 1 are given in Table 3.
CCDC:1507502,1;1507503,2.
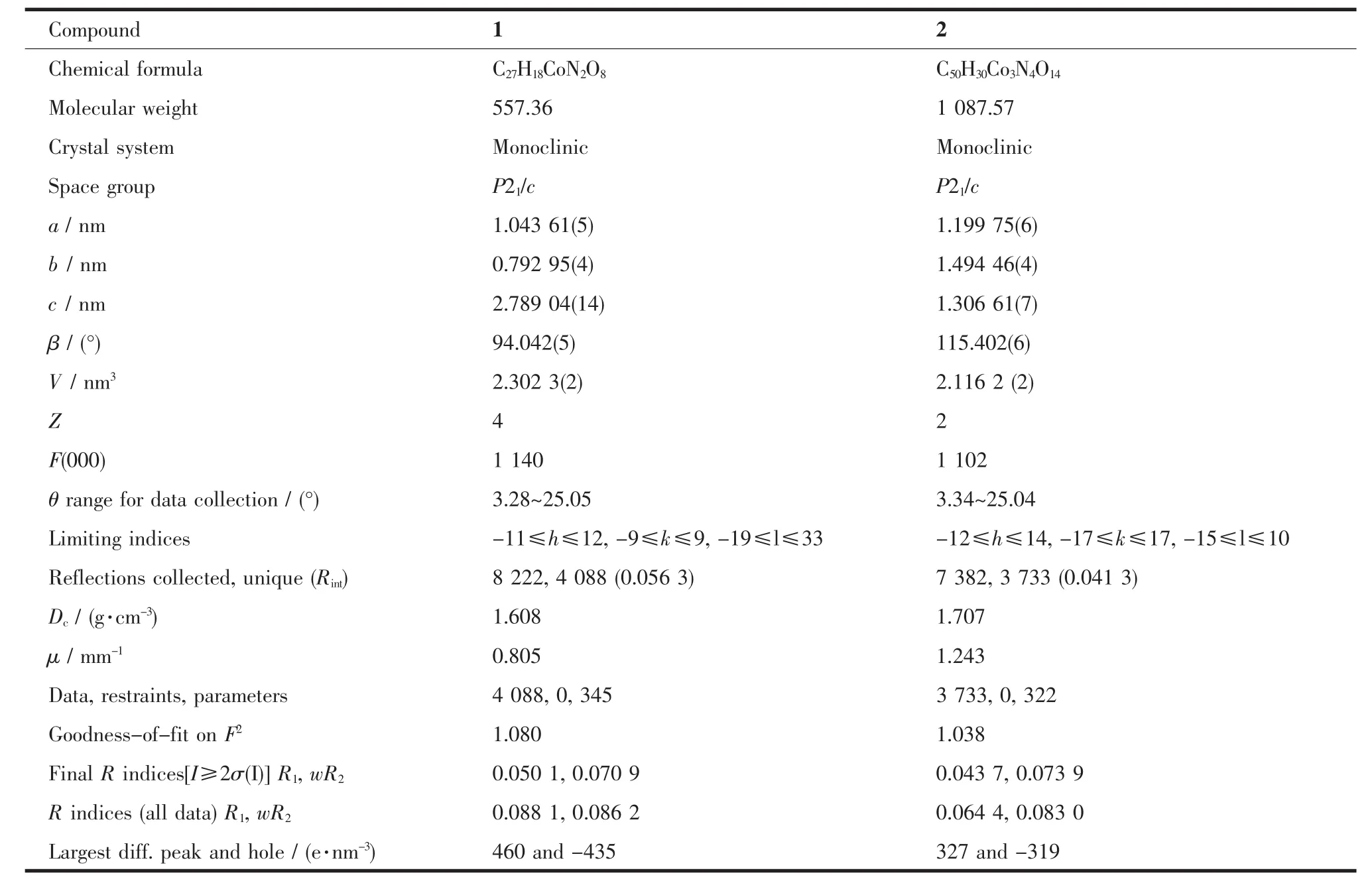
Table 1Crystal data for compounds 1 and 2

Table 2Selected bond distances(nm)and bond angles(°)for compounds 1 and 2

Continued Table 2

Table 3Hydrogen bond lengths(nm)and angles(°)of compound 1
2 Results and discussion
2.1 Description of the structure
2.1.1 [Co(μ2-Hcpta)(phen)(H2O)]n(1)
The X-ray crystallography analyses reveal that compound 1 has a 1D chain structure.Its asymmetric unit contains one crystallographically unique Coatom,one μ2-Hcpta2-block,one phen moiety,and one coordination water molecule.As depicted in Fig.1,the six-coordinatedCo1atomdisplaysadistorted octahedral{CoN2O4}geometry filled by four O atoms from two different μ2-Hcpta2-blocks and one coordinated water molecule and two N atoms from one phen ligand.The lengths of the Co-O bonds range from 0.203 8(2)to 0.212 7(3)nm,whereas the Co-N distances vary from 0.214 8(3)to 0.218 7(3)nm;these bonding parameters are comparable to those found in other reported Cocompounds[10,22-24].In 1,the Hcpta2-ligand acts as a μ2-linker(Scheme 1,modeⅠ),in which two deprotonated carboxylate groups show theη1:η0monodentateandη1:η1bidentatemodes, respectively.The dihedral angle between two phenyl rings in the Hcpta2-is 81.03°.The angle of C-Oetheric-C is 119.16°.The carboxylate groups of Hcpta2-ligands bridge alternately neighboring Coatoms in a syn-anti coordination fashion to form a 1D chain with the Co…Co separation of 0.505 5(3)nm(Fig.2).The present structure shows 1D zigzag metal organic chain wherein the 2-connected Co1 nodes are interconnected by the μ2-Hcpta2-linkers(Fig.3).These chains can betopologically classified as a uninodal 2-connected net with the 2C1 topology.The adjacent chains are connected together by O-H…O hydrogen bonds(Table 3),forming a 2D supramolecular sheet(Fig.4).

Scheme 1Coordination modes of Hcpta2-/cpta3-ligands in compounds 1 and 2

Fig.1Drawing of the asymmetric unit of compound 1 with 30%probability thermal ellipsoids

Fig.2View of a 1D metal-organic chain parallel to the bc plane

Fig.4Perspective of a 2D supramolecular network along the bc plane in 1
2.1.2 [Co3(μ5-cpta)2(2,2′-bipy)2]n(2)
Theasymmetricunitof2consistsoftwo crystallographically distinct Coatoms(Co1 with full occupancy;Co2 is located on a 2-fold rotation axis), one μ5-cpta3-block,and one 2,2′-bipy ligand.As shown in Fig.5,the Co1 atom is six-coordinated and adopts a distorted octahedral{CoN2O4}geometry completed by four carboxylate O from three distinct μ5-cpta3-blocks and two N atoms from one 2,2′-bipy ligand.The six-coordinated Co2 center is located on a 2-fold rotation axis and is surrounded by six O atoms from six different cpta3-blocks,thus adopting a distorted octahedral{CoO6}geometry.TheCo-O distances range from 0.201 7(2)to 0.223 9(2)nm, whereas the Co-N distances vary from 0.210 6(3)to 0.214 5(3)nm;these bonding parameters are comparable to those observed in other Cocompounds[10,22-24]. In 2,the cpta3-block acts as a μ5-spacer(Scheme 1, modeⅡ),in which the carboxylate groups exhibit the μ2-η1:η1and μ2-η0:η2bidentate and μ2-η1:η2tridentate modes.In the cpta3-,the dihedral angle between the two phenyl rings is 87.37°.The angle C-Oetheric-C is 119.73°.Three adjacent Coions are bridged by means of six carboxylate groups from the four different cpta3-blocks,givingrise to a centrosymmetric tricobaltsubunit(Fig.6).In this Co3unit,the Co…Co distance of 0.322 3(3)nm is close to those reported for other carboxylate-bridged trinuclear Cocompounds[25-27].The adjacent Co3subunits are further interlinked by the cpta3-blocks into a 2D metalorganic network(Fig.7).It has the shortest distance of 1.107 0(3)nm between the neighboring tricobaltsubunits.This structure features an intricate 2D metalorganic layer,which from the topological point of view,is assembled from the 3-connected Co1 and 4-connected Co2 nodes,as well as the 5-connected μ5-cpta3-nodes(Fig.8).Its topological analysis discloses a trinodal 3,4,5-connected underlying net with a very rare 3,4,5L45 topology and point symbol of(43)2(44.86)2(45.6).The(43),(44.86),and(45.6)notations correspond to the Co1,μ5-cpta3-,and Co2 nodes,respectively.
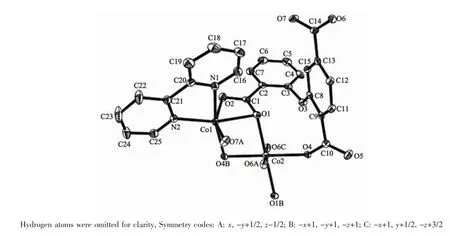
Fig.5Drawing of the asymmetric unit of compound 2 with 30%probability thermal ellipsoids
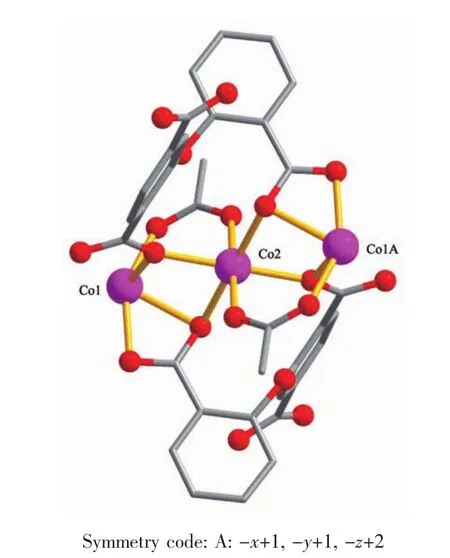
Fig.6Tricobaltsubunit in compound 2

Fig.72D metal-organic framework along the a axis in compound 2

Fig.8Topological representation of a 2D metal-organic network in 2 displaying a trinodal 3,4,5-connected underlying layer with the 3,4,5L45 topology and point symbol of(43)2(44.86)2(45.6)
2.2 TGA analysis and PXRD results
To determine the thermal stability of compounds 1 and 2,their thermal behaviors were investigated undernitrogenatmospherebythermogravimetric analysis(TGA).As shown in Fig.9,the TGA curve of 1 reveals that one coordinated aqua ligand is released between 148~210℃(Obsd.3.5%;Calcd.3.2%),and the dehydrated solid begins to decompose at 308℃. The TGA curve of 2 indicates that the compound is stable up to 367℃,and then decompose upon further heating.The patterns for the as-synthesized bulk material closely match the simulated ones from the single-crystal structure analysis,which is indicative of the pure solid-state phase(Fig.10 and 11).
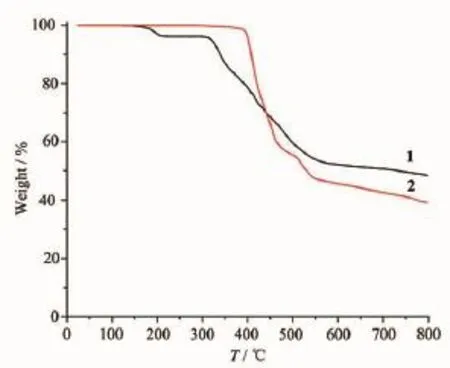
Fig.9TGA curves of compounds 1 and 2
2.3 Magnetic properties
Variable-temperaturemagneticsusceptibility studieswerecarriedoutonpowdersamplesof compounds 1 and 2 in the 2~300 K temperature range.For1,asshowninFig.12,theroom temperature values of χMT(3.21 cm3·mol-1·K)is larger than the value(1.83 cm3·mol-1·K)expected for one magnetically isolated high-spin Coions(S=3/2,g= 2.0).This is a common phenomenon for Coions due to their strong spin-orbital coupling interactions[10,22-24]. When the temperature is lowered,theχMT values decrease slowly until about 116 K,then decrease quickly to 1.23 cm3·mol-1·K at 2.0 K.Between 88and 300 K,the magnetic susceptibilities can be fitted to the Curie-Weiss law with C=3.55 cm3·mol-1·K and θ=-28.4 K.These results indicate an antiferromagnetic interaction between the adjacent Cocenters in compound 1.We tried to fit the magnetic data of 1 using the following expression for a 1D Cochain[28]:

Fig.10PXRD patterns of compound 1 at room temperature
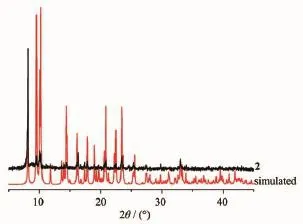
Fig.11PXRD patterns of compound 2 at room temperature

Fig.12Temperature dependence of χMT(О)and 1/χM(□) vs T for compound 1

Using this rough model,the susceptibilities above 65 K were simulated,leading to J=-9.87 cm-1,g=2.43. The negative J parameters indicate that an weak antiferromagnetic exchange coupling exists between the adjacent Cocenters in 1,which is agreement with negative θ value.According to the structure of 1, there is one magnetic exchange pathway within the chain through one syn-anti carboxylate bridges,which couldberesponsiblefortheobserved antiferromagnetic exchange.
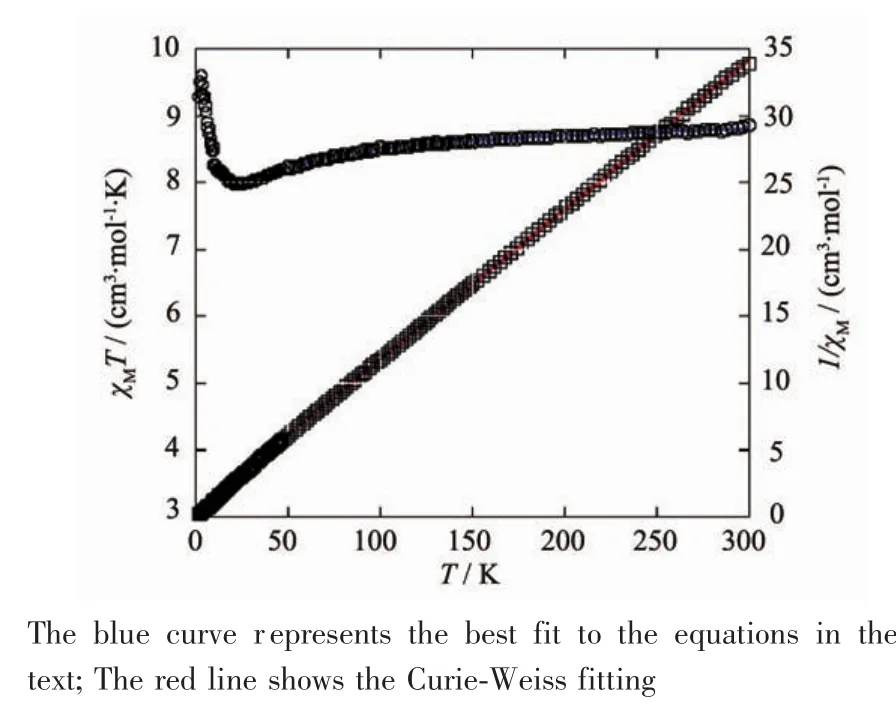
Fig.13Temperature dependence of χMT(О)and 1/χM(□) vs T for compound 2
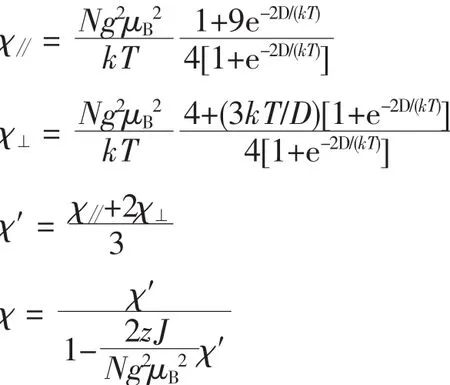
Where N is Avogadro′s number,μBis Bohr magneton,k is Boltzmann′s constant,and g is Lande factor.The best fit in the range of 50~300 K was obtained with values of g=2.37,D=96.3 cm-1,and zJ= 0.78 cm-1with the agreement factor R(R=∑[(χMT)obs-(χMT)calc]2/∑(χMT)2)of 5.76×10-5.The main magnetic exchange pathway is the exchange between adjacent Coions within the Co3cluster to the μ2-Ocarboxylbridge with the superexchange angle Co-Ocarboxyl-Co of 99.05(3)°.
3 Conclusions
In summary,two new coordination polymers, namely[Co(μ2-Hcpta)(phen)(H2O)]n(1)and[Co3(μ5-cpta)2(2,2′-bipy)2]n(2)have beensynthesized under hydrothermal conditions.The compounds feature the 1Dchainand2Dsheetstructures,respectively.Magneticstudiesfortwocompoundsshowan antiferromagnetic coupling between the adjacent Cocenters.
[1]Sun Y J,Zhou H C.Sci.Technol.Adv.Mater.,2015,16: 054202
[2]Li J R,Sculley J,Zhou H C.Chem.Rev.,2012,112:869-932
[3]Cui Y,Yue Y,Qian G,et al.Chem.Rev.,2012,112:1126-1162
[4]Kuppler R J,Timmons D J,Fang Q R,et al.Coord.Chem. Rev.,2009,253:3042-3066
[5]Zheng X D,Lu T B.CrystEngComm,2010,12:324-336
[6]Jiang X,Liu C M,Kou H Z.Inorg.Chem.,2016,55:5880 -5885
[7]Zhang W J,Yuan D Q,Liu D H,et al.Chem.Mater.,2012, 24:18-25
[8]Zhang H,Yang J,Liu Y Y,et al.Cryst.Growth Des.,2016, 16:3244-3255
[9]Karmakar A,Desai A V,Ghosh S K,Coord.Chem.Rev., 2016,07:313-341
[10]Gu J Z,Gao Z Q,Tang Y.Cryst.Growth Des.,2012,12: 3312-3323
[11]Gao Q,Xie Y B,Li J R,et al.Cryst.Growth Des.,2012,12: 281-288
[12]Lee J,Kang Y J,Cho N S,et al.Cryst.Growth Des., 2016,16:996-1004
[13]Li L,Cao X Y,Huang R D.Chin.J.Chem.,2016,34:143-156
[14]Lu W G,Su C Y,Lu T B,et al.J.Am.Chem.Soc.,2006, 128:34-35
[15]Wang H H,Yang H Y,Shu C H,et al.Cryst.Growth Des., 2016,16:5394-5402
[16]Cui L T,Niu Y F,Han J,et al.J.Solid State Chem., 2015,227:155-164
[17]Wang H L,Zhang D P,Sun D F,et al.CrystEngComm, 2010,12:1096-1102
[18]Chen X,Wang Y Y,Liu B,et al.Dalton Trans.,2013,42: 7092-7100
[19]Hu J S,Huang X H,Pan C L,et al.Cryst.Growth Des., 2015,15:2272-2281
[20]Zhao H W,Li B.Chin.J.Struct.Chem.,2012,31:61-66
[21]Sheldrick G M.SHELXTL-97,University of Göttingen, Germany,1997.
[22]Tang L,Fu F,Wang J J,et al.Polyhedron,2015,88:116-124
[23]GU Jin-Zhong(顾金忠),GAO Zhu-Qing(高竹青),DOU Wei (窦伟),et al.Chinese J.Inorg.Chem.(无机化学学报), 2009,25(5):920-923
[24]QIAO Yu(乔宇),WEI Bing(尉兵),WANG Lu-Yao(王璐瑶), et al.Chinese J.Inorg.Chem.(无机化学学报),2016,32(7): 1261-1266
[25]Marshall S R,Rheingold A L,Dawe L N,et al.Inorg. Chem.,2002,41:3599-3601
[26]Su Z,Fan J,Chen M,et al.Cryst.Growth Des.,2011,11: 1159-1169
[27]Niu C Y,Zheng X F,Wan X S,et al.Cryst.Growth Des., 2011,11:2874-2888
[28]Kahn O.Molecular Magnetism.New York:VCH Publishers, 1993:258
Syntheses,Crystal Structures and Magnetic Properties of 1D and 2D CobaltCoordination Polymers Constructed from Semir-igid Tricarboxylic Acid
GU Wen-Jun1GU Jin-Zhong*,2
(1College of Animal Science and Technology,Southwest University,Chongqing 400715,China)
(2College of Chemistry and Chemical Engineering,Lanzhou University,Lanzhou 730000,China)
Two 1D and 2D cobaltcoordination polymers,namely[Co(μ2-Hcpta)(phen)(H2O)]n(1)and[Co3(μ5-cpta)2(2,2′-bipy)2]n(2),have been constructed hydrothermally using H3cpta(H3cpta=2-(2-carboxyphenoxy)terephthalic acid),phen(phen=phenanthroline)or 2,2′-bipy(2,2′-bipy=2,2′-bipyridine),and cobalt chloride.Single-crystal X-ray diffraction analyses revealed that the two compounds crystallize in the monoclinic system,space groupIn compound 1,the carboxylate groups of Hcpta2-ligands bridge alternately neighboring metal ions to form a chain.Adjacent chains are assembled to a 2D supramolecular network through C-H…O hydrogen bond.Compound 2 shows a 2D sheet based on Co3units.Magnetic studies for compounds 1 and 2 demonstrate an antiferromagnetic coupling between the adjacent Cocenters.CCDC:1507502,1;1507503,2.
coordination polymer;hydrogen bonding;tricarboxylic acid;magnetic properties
O614.81+2
A
1001-4861(2017)02-0227-10
10.11862/CJIC.2017.035
2016-09-30。收修改稿日期:2016-12-02。
*通信联系人。E-mail:gujzh@lzu.edu.cn;会员登记号:S06N5892M1004。

