盐酸小檗碱预处理对青霉素钠诱发小鼠癫痫发作程度、发作潜伏期的影响及其机制
刘则烨,向雪瑞,范孟孟,向婷,房春云,江平
(河北医科大学,石家庄050017)
盐酸小檗碱预处理对青霉素钠诱发小鼠癫痫发作程度、发作潜伏期的影响及其机制
刘则烨,向雪瑞,范孟孟,向婷,房春云,江平
(河北医科大学,石家庄050017)
目的 观察盐酸小檗碱(BBH)预处理对青霉素钠诱发小鼠癫痫发作程度、发作潜伏期的影响,并探讨其机制。方法 雄性昆明种小鼠50只,适应性饲养3 d后随机分为生理盐水组、模型组、丙戊酸钠(VPA)组、BBH1组、BBH2组。VPA组腹腔注射VPA 40 mg/kg,BBH1组以BBH 25 mg/kg灌胃,BBH2组以BBH 50 mg/kg灌胃,生理盐水组和模型组分别以等量生理盐水和β-环糊精灌胃。各组每天给药1次,连续给药3 d。至末次给药次日,除生理盐水组外,其余四组均腹腔注射青霉素钠700万IU/kg诱发癫痫。记录各组小鼠从给药到出现癫痫症状的潜伏期。根据Racine分级标准评估癫痫发作分级,记录癫痫大发作(>Ⅲ级)发生情况。发作持续1 h后,颈椎脱臼处死动物并断头取脑,采用液相色谱-质谱联用法(LC-MS)检测海马组织中的谷氨酸(Glu)、γ-氨基丁酸(GABA)、五羟色胺(5-HT)。结果 模型组癫痫发作Ⅲ级2只、Ⅳ级7只、Ⅴ级1只,癫痫大发作8只;VPA组Ⅲ级6只、Ⅳ级3只、Ⅴ级1只,共4只达到大发作;BBH1组Ⅲ级3只、Ⅳ级6只、Ⅴ级1只,7只大发作;BBH2组Ⅱ级1只、Ⅲ级6只、Ⅳ级3只、Ⅴ级1只,癫痫大发作4只。BBH2组小鼠癫痫发作达到Ⅲ级以上的数量与模型组相比,P均<0.05。模型组、VPA组、BBH1组、BBH2组癫痫发作潜伏期分别为(142.4±68.4)、(237.5±132.6)、(246.9±59.8)、(260.5±52.4)s,BBH1组、BBH2组癫痫发作潜伏期较模型组延长(P均<0.05)。VPA组海马组织中GABA表达水平高于模型组(P<0.05)。模型组Glu/GABA高于生理盐水组(P<0.05);VPA组Glu/GABA低于模型组(P<0.05);BBH1组、BBH2组Glu/GABA低于模型组,但差异无统计学意义。BB1组Glu/5-HT低于模型组(P<0.05)。结论 BBH预处理有助于降低小鼠癫痫发作分级,延长癫痫发作潜伏期,其中50 mg/kg剂量效果优于25 mg/kg剂量;BBH的抗癫痫机制可能与调节海马组织内神经递质水平有关。
小檗碱;癫痫;神经递质;谷氨酸;γ-氨基丁酸;五羟色胺;动物实验
癫痫是慢性反复发作性短暂脑功能失调综合征,以脑神经元异常放电引起反复痫性发作为特征[1]。目前癫痫的发病机制仍不完全清楚[2,3],一般认为遗传、中枢神经系统感染、恶性肿瘤、脑血管疾病、先天及围生期异常、颅脑损伤、寄生虫病等都是癫痫的可能致病因素[4],免疫和细胞信号通路改变、神经递质改变、神经胶质细胞改变、神经元形态和功能异常、离子通道异常都可能参与癫痫的发病过程[5,6]。癫痫的临床治疗颇为棘手,一般首选药物治疗,目前临床常用的抗癫痫药物[如丙戊酸钠(VPA)等]各有不足。如VPA治疗过程中常会出现腹泻、消化不良、恶心、呕吐、胃肠道痉挛、月经周期改变等不良反应,还可出现血小板减少引起紫癜、出血时间延长、肝功能损伤及不可逆的听力损坏等严重不良后果。这些均在一定程度上限制了药物的应用。小檗碱又称黄连素,是一种异喹啉生物碱,用药后组织分布广泛[7]。研究[8]表明小檗碱对癫痫发作有抑制作用,但具体作用机制尚不明确,也未形成系统的抗癫痫剂型和疗法,未能在临床上广泛应用于癫痫的治疗。本研究观察了盐酸小檗碱(BBH)预处理对青霉素钠诱发小鼠癫痫发作程度、发作潜伏期的影响,并检测了小鼠海马组织中的谷氨酸(Glu)、γ-氨基丁酸(GABA)和五羟色胺(5-HT),以期探讨小檗碱的抗癫痫作用机制,现报告如下。
1 材料与方法
1.1 实验动物与主要材料 雄性清洁级昆明种小鼠50只,体质量18~20 g,由河北省实验中心提供。小鼠饲养于普通环境,自由摄食饮水,经适应性饲养3 d后,按体质量随机分为生理盐水组、模型组、VPA组、BBH1组和BBH2组。注射用青霉素钠,BBH,生理盐水,API 4000 Q-Trap质谱仪,LC-30A液相色谱系统,Analysis1.6软件,BS124S电子天平。
1.2 小檗碱预处理及癫痫诱导
1.2.1 药物剂量选择及给药方式 在预实验中,将注射10、20、40 mg/kg VPA的小鼠用于制作癫痫模型,观察各组癫痫发作潜伏期并最终确定VPA给药浓度为40 mg/kg(此组平均潜伏期最短);VPA组参考临床及实验室给药方式,决定选择腹腔注射给药。预实验中采用丙二醇、二甲亚砜溶解BBH,但因BBH在二者内溶解性低或溶剂毒性过大等原因放弃,最终选择用25%β-环糊精作为小檗碱溶剂。预实验发现50 mg/kg的BBH为实验对象可耐受最大剂量,并显示出一定效果,故选择25、50 mg/kg的BBH用于实验;BBH腹腔注射后实验对象死亡率过高,故BBH组采用灌胃给药。在安全性及控制癫痫发作方面,各组具有可比性。
1.2.2 药物预处理及癫痫诱导 VPA组腹腔注射VPA 40 mg/kg,BBH1组以BBH 25 mg/kg灌胃,BBH2组以BBH 50 mg/kg灌胃,生理盐水组和模型组分别以等量生理盐水和β-环糊精灌胃。各组每天给药1次,连续给药3 d。至末次给药次日,除生理盐水组外,其余四组均腹腔注射青霉素钠700万IU/kg诱导癫痫。
1.3 癫痫发作分级评价及潜伏期观察 根据Racine分级标准[5]评价癫痫发作的分级:无抽搐发作为0级;耳、面部抽搐为Ⅰ级;节律性点头、无站立为Ⅱ级;肌阵挛伴双前肢立起为Ⅲ级;肌阵挛伴有双后肢立起或跳跃,或阵发性全身强直发作为Ⅳ级;频繁的强直阵挛发作,失去体位控制,或持续30 min以上的强制阵挛发作为Ⅴ级。癫痫发作>Ⅲ级记为大发作。记录各组小鼠从给药到出现癫痫症状的潜伏期。
1.4 海马组织中神经递质含量测定 癫痫发作持续1 h后,颈椎脱臼处死动物并立即断头取脑,在冰上完整取出双侧海马,称重后剪碎,以10倍体积的甲醇/水在冰水浴下间断匀浆,离心(5 000 r/min×5 min,4 ℃)后取上清与等体积甲醇充分混合,离心(12 000 r/min×10 min,4 ℃)后取上清液备用。采用液相色谱-质谱联用法(LC-MS)检测海马组织中的Glu、GABA及5-HT。
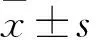
2 结果
2.1 癫痫发作级别 模型组癫痫发作均在Ⅲ级以上,其中Ⅲ级2只、Ⅳ级7只、Ⅴ级1只,癫痫大发作8只;VPA组Ⅲ级6只、Ⅳ级3只、Ⅴ级1只,共4只达到大发作;BBH1组Ⅲ级3只、Ⅳ级6只、Ⅴ级1只,7只达到大发作;BBH2组Ⅱ级1只、Ⅲ级6只、Ⅳ级3只、Ⅴ级1只,癫痫大发作4只。BBH2组小鼠癫痫发作达到Ⅲ级以上的数量与模型组相比,P均<0.05。
2.2 癫痫发作潜伏期 模型组、VPA组、BBH1组、BBH2组癫痫发作潜伏期分别为(142.4±68.4)、(237.5±132.6)、(246.9±59.8)、(260.5±52.4)s,BBH1组、BBH2组癫痫发作潜伏期较模型组延长(P均<0.05)。
2.3 海马组织中Glu、GABA、5-HT表达比较 VPA组海马组织中GABA表达水平高于模型组(P<0.05)。模型组Glu/GABA高于生理盐水组(P<0.05);VPA组Glu/GABA低于模型组(P<0.05);BBH1组、BBH2组Glu/GABA低于模型组,但差异无统计学意义。BB1组Glu/5-HT低于模型组(P<0.05)。详见表1。

表1 各组小鼠海马组织中Glu、GABA、5-HT表达水平比较
注:与生理盐水组相比,*P<0.05;与模型组相比,#P<0.05。
3 讨论
癫痫是大脑神经元突发性异常放电,导致短暂大脑功能障碍的一种慢性疾病。由于异常放电的起始部位、累及范围和传递方式的不同,癫痫发作的临床表现复杂多样[9~11]。癫痫作为一种常见病和多发病,其治疗方案的研究不断进展,尤其是脑保护药物的研究进展迅速。在癫痫动物模型制备中,青霉素是较早被公认的致痫物,它可以破坏大脑皮层兴奋与抑制的平衡关系,削弱抑制性神经递质的作用,进而引起癫痫发作[12,13]。本实验中模型组小鼠癫痫发作均达Ⅲ级及以上,成功复制了急性小鼠癫痫模型。
在本实验中,模型组癫痫发作Ⅲ级2只、Ⅳ级7只、Ⅴ级1只,癫痫大发作8只;VPA组Ⅲ级6只、Ⅳ级3只、Ⅴ级1只,共4只达到大发作;BBH1组Ⅲ级3只、Ⅳ级6只、Ⅴ级1只,7只大发作;BBH2组Ⅱ级1只、Ⅲ级6只、Ⅳ级3只、Ⅴ级1只,癫痫大发作4只。25、50 mg/kg的BBH不同程度地降低了小鼠癫痫发作级数,其中BBH2组癫痫发作级别明显低于模型组;作为阳性对照,VPA组癫痫发作级别亦降低,效果与BBH2组接近。BBH1组、BBH2组癫痫发作潜伏期较模型组延长,表明小檗碱在一定剂量条件下具有延缓癫痫发作、减轻发作严重程度的效果。
有研究认为引起癫痫发作的神经元反复阵发性过度放电与脑内兴奋性和抑制性神经递质的比例失衡有关,其中Glu、GABA、5-HT尤其受到关注,本实验也选择这三种神经递质进行检测。生理状态下,Glu是中枢系统重要的兴奋性神经递质,而GABA和5-HT则是中枢神经系统重要的抑制性神经递质。它们与相应受体进行作用会影响神经元的内外离子流动平衡进而影响神经元兴奋性,三者水平失衡与癫痫发作有密切关系。GABA的受体分为A、B、C三型,其中A型属于配体门控的氯离子通道,其兴奋可以产生抑制性突触后电位而抑制癫痫发作[14],故GABA减少在癫痫发作中可能有重要作用[15]。同理,5-HT的中枢抑制作用也有可能抑制癫痫发作,虽然可见其水平与BBH剂量有关,但无统计学差异,可能需要通过增大样本量进行验证。Glu的受体包括离子型与代谢型受体,Glu大量蓄积并触发离子型受体会使突触兴奋性过度增加而诱发癫痫[16]。总之,中枢神经系统内Glu的增加和GABA的减少均有可能增大癫痫发作的风险。姚君茹等[17]认为Glu等神经递质早期胞内大量合成并释放可能是癫痫发作的重要诱因。
研究[18,19]提示小檗碱具有调节脑内神经递质含量、影响神经元兴奋性的作用,而Lin等[20]则提出小檗碱具有增加脑内抑制性神经递质GABA表达的作用。本实验结果显示,模型组Glu/GABA高于生理盐水组,说明癫痫状态下Glu/GABA平衡被打乱。VPA组海马组织中GABA水平高于模型组,Glu/GABA低于模型组,提示VPA的抗癫痫作用可能与调节Glu/GABA有关。BBH1组Glu/5-HT低于模型组,推测小檗碱可抑制兴奋性递质与海马区受体结合,开放钙通道、减少钙内流,进而减轻细胞器损伤,减少细胞膜不稳定的发生机会,最终达到降低神经元兴奋性、提高癫痫发生阈值的目的[21]。我们还发现,BBH1组、BBH2组Glu/GABA低于模型组,但差异无统计学意义,这可能与纳入样本量较小有关。我们推测小檗碱有助于降低兴奋性递质与抑制性递质的比值,可能对减少癫痫发生频率、减轻发作严重程度有重要意义。就本实验而言,可认为BBH 50 mg/kg对延长癫痫发作潜伏期和降低发作级别更有效,具体更加精确的剂量有待进一步研究。至于小檗碱通过何种方式影响Glu与GABA的代谢,仍是今后进一步研究的方向。
[1] Ngugi AK, Bottomley C, Fegan G, et al. Premature mortality in active convulsive epilepsy in rural Kenya: causes and associated factors[J]. Neurology, 2014,82(7):582-589.
[2] Pittau F, Fahoum F, Zelmann R, et al. Negative BOLD response to interictal epileptic discharges in focal epilepsy[J]. Brain Topogr, 2013,26(4):627-640.
[3] Khaing M, Lim KS, Tan CT. Focal epilepsy recruiting a generalised network of juvenile myoclonic epilepsy: a case report[J]. Epileptic Disord, 2014,16(3):370.
[4] Hildebrand MS, Dahl HH, Damiano JA, et al. Recent advances in the molecular genetics of epilepsy[J]. J Med Genet, 2013,50(5):271-279.
[5] 刘明刚,朱权,孙美珍,等.血脑屏障通透性在外伤性癫痫大鼠中的作用机制研究[J].中西医结合心脑血管病杂志,2013,11(10):1244-1247.
[6] Mishra V, Shuai B, Kodali M, et al. Resveratrol treatment after status epilepticus restrains neurodegeneration and abnormal neurogenesis with suppression of oxidative stress and inflammation[J]. Sci Rep, 2015,5:17807.
[7] Tan XS, Ma JY, Feng R, et al. Tissue distribution of berberine and its metabolites after oral administration in rats[J]. PLoS One, 2013,8(10):e77969.
[8] Bhutada P, Mundhada Y, Bansod K, et al. Anticonvulsant activity of berberine, an isoquinoline alkaloid in mice[J]. Epil Behav, 2010,18(3):207.
[9] 蔡秀侠,宋昌鹏,牛艳花.卡马西平联合丙戊酸钠治疗早期癫痫的效果观察[J].中国实用神经疾病杂志,2015,5(7):101-102.
[10] 曹利,戴艳萍,李军,等.神经内科癫痫病临床诊断及治疗效果探讨[J].中外医疗,2015(8):114-115.
[11] 苏艳,赵世刚,杨蕴天,等.癫痫病因及发病机制[J].脑与神经疾病杂志,2016,24(4):262-264.
[12] 黄小平,欧阳国,丁煌,等.黄芪甲苷与三七有效成分配伍对小鼠脑缺血再灌注后神经细胞凋亡和内质网应激的影响[J].中草药,2015,46(15):2257-2264.
[13] 刘春,宋成云,王旭,等.不同剂量青霉素致SD大鼠癫痫的实验研究[J].交通医学,2011,25(2):126-128.
[14] Pavlov I, Walker MC. Tonic GABA(A) receptor-mediated signalling in temporal lobe epilepsy[J]. Neuropharmacology, 2013,69(6):55-61.
[15] 肖文,李臣鸿.GABA紧张性抑制电流及其对癫痫的功能性研究[J].科教导刊(下旬),2016(12):50-51.
[16] Cho CH. New mechanism for glutamate hypothesis in epilepsy[J]. Front Cellu Neurosci, 2013,7(7):127.
[17] 姚君茹,潘三强,吕来清,等.慢性癫痫模型大鼠脑谷氨酶神经元的变化[J].解剖科学进展,2004,10(1):26-29.
[18] Peng WH, Lo KL, Lee YH, et al. Berberine produces antidepressant-like effects in the forced swim test and in the tail suspension test in mice[J]. Life Sciences, 2007,81(11):933-938.
[19] Ikhyun K, Hyunsook C, Kunseong S, et al. Effects of berberine on 6-hydroxydopamine-induced neurotoxicity in PC12 cells and a rat model of Parkinson's disease[J]. Neuroscience Letters, 2010,486(1):29.
[20] Lin TY, Lin YW, Lu CW, et al. Berberine inhibits the release of glutamate in nerve terminals from rat cerebral cortex[J]. PLoS One, 2013,8(6):e67215.
[21] Sivam SP, Hudson PM, Tilson HA, et al. GABA and dopamine interaction in the basal ganglia: dopaminergic supersensitivity following chronic elevation of brain gamma-aminobutyric acid levels[J]. Brain Res, 1987,412(1):29.
Effects of berberine pretreatment on degree and incubation period of epileptic seizure in mice with penicillin-induced epilepsy
LIUZeye,XIANGXuerui,FANMengmeng,XIANGTing,FANGChunyun,JIANGPing
(HebeiMedicalUniversity,Shijiazhuang050017,China)
Objective To observe the effects of berberine (BBH) pretreatment on the degree and the incubation period of epileptic seizure induced by penicillin in mice and to discuss the mechanism. Methods After adaptive feeding of 3 days, fifty male Kunming mice were randomly divided into five groups including the saline group, model group, VPA group, BBH 1 group, and BBH 2 group. The mice in the VPA group were given intraperitoneal injection of 40 mg/kg VPA , mice in the BBH 1 group were given 25 mg/kg BBH by gavage and BBH 2 group with 50 mg/kg BBH in the same way. The saline and model groups were given the same volume of saline or 25% β-cyclodextrin, and all the groups were administered once a day for three days. Up to the next day of the last time, except for the saline group, all the other four groups were given an intraperitoneal injection of 7×106IU/kg penicillin to induce epileptic seizure. We recorded the incubation period for each group of mice from administration to the onset of epilepsy. We evaluated the onset classification byRacine criterion and recorded the seizure attack (more than III-grade). Animals showing seizure attack for 1 h were killed by dislocation of cervical vertebra. Brain tissues were taken out and processed for further estimation, we used LC-MS to test the content of glutamic acid (Glu), γ-aminobutyric acid (GABA) and serotonin (5-HT). Results As for the seizures, there were 2 cases of class Ⅲ, 7 cases of class Ⅳ, 1 case of class V, and 8 cases of grand mal epilepsy, respectively, in the model group; in the VPA group, they were 6, 3, 1, and 4; in the BBH 1 group, they were 3, 6, 1, and 7; in the BBH 2 group, there was i case of class II, 6 cases of class Ⅲ, 3 cases of class Ⅳ, 1 case of class V, and 4 cases of grand mal epilepsy. The number of mice with seizures more than class Ⅲ in the BBH 2 group was significantly different as compared with that of the model group (allP<0.05). The incubation period of the model group, VPA group, BBH 1 and BBH 2 groups were (142.4±68.4),(237.5±132.6),(246.9±59.8), and (260.5±52.4)s . The incubation period of the BBH 1 and BBH 2 groups was longer than that of the model group (allP<0.05). The level of GABA in the VPA group was higher than that of the model group (P<0.05). The Glu/GABA of the model group was higher than that of the saline group (P<0.05). The Glu/GABA of the VPA group was lower than that of the model group (P<0.05). The levels of Glu/GABA in the BBH1 group and the BBH2 group were lower than that of the model group, but the difference was not statistically significant. The Glu/5-HT in the BBH1 group was lower than that of the model group (P<0.05). Conclusions The BBH pretreatment helps to reduce the scale of epileptic seizures in mice and prolong the incubation period. Among them, the overcome of 50 mg/kg dose is better than 25 mg/kg. The anti-epileptic mechanism of BBH may be associated with regulating neurotransmitter levels in the hippocampus.
berberine; epilepsy; neurotransmitter; glutamic acid; γ-aminobutyric acid; serotonin; animal experiment
河北医科大学大学生创新计划项目(USIP2016045)。
刘则烨(1995-),男,本科,主要研究方向为神经药理学。E-mail:1482080766@qq.com
江平(1973-),女,博士,副教授,主要研究方向为神经药理学。E-mail:jp_sjz2002@163.com
10.3969/j.issn.1002-266X.2017.27.005
R741.05;R965.1
A
1002-266X(2017)27-0019-04
2017-03-15)

