托吡酯腹腔注射后癫痫大鼠海马组织损伤变化及其机制探讨
李香丹,尹明姬,杨洋,林贞花,咸哲民,李今子
(1延边大学附属医院,吉林延吉133002;2延边大学)
托吡酯腹腔注射后癫痫大鼠海马组织损伤变化及其机制探讨
李香丹1,尹明姬1,杨洋2,林贞花2,咸哲民1,李今子1
(1延边大学附属医院,吉林延吉133002;2延边大学)
目的 观察托吡酯(TPM)腹腔注射后癫痫大鼠海马组织损伤改变,并探讨其可能机制。方法 将40只大鼠依据随机数字表法分为正常组、模型组、TPM低剂量组、TPM高剂量组。模型组、TPM低剂量组、TPM高剂量组采用腹腔注射氯化锂180 mg/kg、匹罗卡品30 mg/kg制作癫痫模型。模型组、TPM低剂量组、TPM高剂量组大鼠分别于癫痫发作后5 h经腹腔注射生理盐水10 mL/(kg·d)、TPM 40 mg/(kg·d)、TPM 80 mg/(kg·d)。各组连续用药4周后处死大鼠取海马组织,HE染色观察大鼠海马组织病理变化;应用基因芯片技术筛选各组差异表达的miRNA,并用实时荧光定量PCR法进行验证;采用TUNEL法检测海马组织凋亡细胞,并计算凋亡细胞数;分别采用Western blotting法、免疫荧光法和免疫组化法检测各组大鼠海马组织中的Caspase-8。结果 与正常组相比,模型组海马组织损伤明显,TPM低剂量组和TPM高剂量组海马组织损伤较模型组减轻,且TPM低剂量组损伤最轻。基因芯片检测发现模型组出现12个表达上调的miRNA和14个表达下调的miRNA,经实时荧光定量PCR验证,只有miR-146a在各组表达的差异有统计学意义。模型组、TPM高剂量组、TPM低剂量组、正常组海马组织中miR-146a相对表达量依次降低(P均<0.05)。细胞凋亡检测发现,与正常组相比,模型组大鼠CA1区、CA3区和DG区凋亡细胞细胞数高于正常组(P均<0.05),而TPM高剂量组和TPM低剂量组凋亡细胞数低于模型组(P均<0.05)。Western blotting结果显示,模型组Caspase-8蛋白相对表达量高于正常组,模型组、TPM高剂量组、TPM低剂量组Caspase-8蛋白相对表达量依次降低(P均<0.01)。免疫荧光结果显示,与正常组相比,模型组大鼠CA1区、CA3区和DG区Caspase-8阳性细胞数高于正常组(P均<0.05),TPM高剂量组和TPM低剂量组Caspase-8阳性细胞数低于模型组(P均<0.05)。免疫组化结果显示,模型组IOD值高于正常组,模型组、TPM高剂量组、TPM低剂量组IOD值依次降低(P均<0.05)。结论 TPM腹腔注射可减轻癫痫大鼠海马组织病理改变,减少海马组织细胞凋亡,下调海马组织中miR-146a和Caspase-8的表达,其中40 mg/(kg·d)的TPM较80 mg/(kg·d)效果更加明显。TPM的抗癫痫作用机制可能与调节miRNA表达、减少细胞凋亡、下调Caspase-8表达有关。
癫痫;托吡酯;海马;微小RNA;微小RNA146a;细胞凋亡;含半胱氨酸的天冬氨酸蛋白水解酶8
癫痫是一种慢性神经系统疾病,其中耐药的难治性癫痫占到了1/3[1]。miRNA能通过碱基互补结合靶基因3′非编码区,抑制其转录和(或)增加其降解[2]。我们前期研究发现miRNA广泛参与癫痫的发生发展,可调节癫痫诱发神经元死亡的过程[3]。托吡酯(TPM)是一种由氨基磺酸酯取代单糖的新型广谱抗癫痫药物,生物利用度高,具有多重抗癫痫机制[4]。目前研究表明TPM不仅有助于治疗难治性癫痫,也可辅助或单药治疗非难治性癫痫[5]。TPM治疗癫痫的具体机制仍不十分明确。本研究采用氯化锂、匹罗卡品制作癫痫大鼠模型并给予TPM治疗,观察TPM处理后癫痫大鼠海马组织中miRNA、Caspase-8表达改变,初步探讨TPM治疗癫痫的可能机制。
1 材料与方法
1.1 实验动物与主要材料 清洁级雄性SD大鼠40只,体质量(220±20)g,由延边大学医学院实验教学中心动物部提供,分笼饲养在温度18~25 ℃、相对湿度50%~60%、人工昼/夜循环照明(7:00至19:00)环境中。大鼠可自由饮食。氯化锂,匹罗卡品,水合氯醛,TRIzol试剂,miRNAs和U6的引物,RNA纯化试剂盒,RT-qPCR试剂盒,TUNEL 染色试剂盒,鼠抗Caspase-8抗体,辣根过氧化物酶标记山羊抗鼠IgG,BCA蛋白定量试剂盒,DAB显色液。
1.2 癫痫模型制作及TPM用法 将大鼠依据随机数字表法分为正常组、模型组、TPM低剂量组、TPM高剂量组。模型组、TPM低剂量组、TPM高剂量组采用腹腔注射氯化锂180 mg/kg、匹罗卡品30 mg/kg诱发大鼠癫痫发作。依据Racine标准[6],达到Ⅳ~Ⅴ级癫痫发作标准即为造模成功。未出现癫痫发作的大鼠追加10 mg/kg的匹罗卡品,每间隔30 min给药1次,直到出现癫痫发作,每只大鼠匹罗卡品用量最多不超过60 mg/kg。正常组给予等量生理盐水。模型组、TPM低剂量组、TPM高剂量组大鼠分别于癫痫发作后5 h经腹腔注射生理盐水10 mL/(kg·d)、TPM 40 mg/(kg·d)、TPM 80 mg/(kg·d),连续用药4周。
1.3 海马组织病理观察 在饲养第28天时(用药4周后)将各组大鼠以10%水合氯醛3 mL/kg腹腔注射麻醉,断头取脑,迅速在含有生理盐水的冰面上钝性分离出双侧海马组织,左脑海马立即进行4%多聚甲醛固定,制备海马组织石蜡标本,以5 μm厚连续切片,HE染色观察各组大鼠组织学改变。右脑海马组织冻存于-80 ℃液氮中用于RNA提取。
1.4 海马组织中差异表达miRNA筛选 取各组大鼠右脑海马组织解冻,溶于适量TRIzol试剂中提取总RNA。选取各组RNA标本,依据操作说明加多聚A尾、标记、杂交、洗涤、染色后,用miRNA3.0仪器进行检测,图片输入Expression Console软件进行分析,获得原始数据,经背景校正及筛选后,采用BMA进行对数转化,使用Genesrping软件进行数据分析。候选miRNAs变化倍数用Java Treeview软件进行可视化处理。随后采用实时荧光定量基因验证差异表达的miRNA。将纯化的RNA进行逆转录成cDNA,以U6作为内参,所用到的PCR引物序列详见表1。PCR反应条件为94 ℃ 15 min、94 ℃ 30 s、60 ℃ 30 s、72 ℃ 30 s,共循环40次,最后72 ℃延伸8 min。每组样品设3个复孔。以2-ΔΔCt表示目的基因相对表达量。
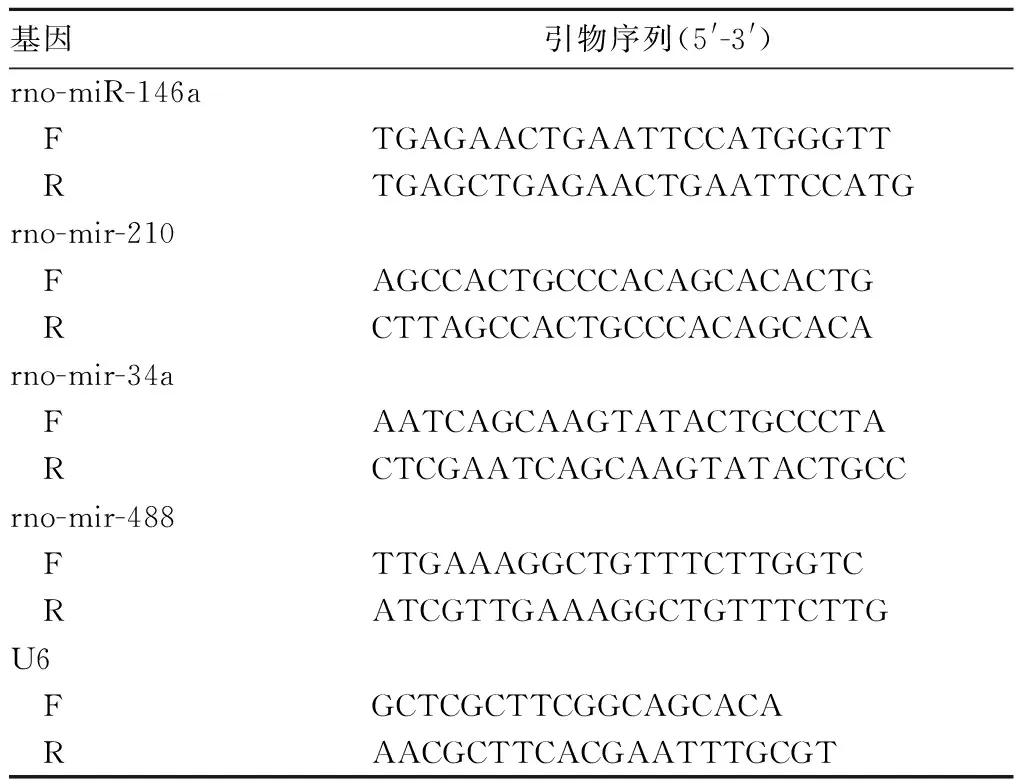
表1 目的基因与内参基因引物序列
1.5 海马组织中凋亡细胞检测 采用TUNEL法检测海马组织凋亡细胞。切片脱蜡后按照TUNEL染色试剂盒说明书进行染色,中性树胶封片。400倍光镜下,每份脑组织随机选取3个切片,每个海马切片的不同区域随机选取5个区域,计数TUNEL染色阳性细胞数,计算平均数。
1.6 海马组织中Caspase-8检测 ①Western blotting法:提取各组海马组织总蛋白,BCA法蛋白定量,SDS-PAGE电源分离目的条带,每孔加入20 μL的样品进行电泳,完成后用孔径0.45 μm的PVDF膜进行湿转;用山羊血清常温封闭30 min,加入鼠抗人Caspase-8一抗(或β-actin一抗,1∶1 000)4 ℃孵育过夜,次日常温下TBST洗膜3次,加入辣根过氧化物酶标记的羊抗鼠二抗孵育1 h;ECL化学发光法检测,柯达胶片暗室显影,采用Quantity one软件对反应条带进行分析,蛋白相对表达量用Caspase-8条带灰度与内参的比值表示。②免疫荧光法:海马组织石蜡切片经脱蜡、梯度乙醇脱水后,进行抗原修复,PBST漂洗5 min×3次;2% BSA 37 ℃湿盒内封闭30 min,用Caspase-8一抗4 ℃染色过夜,在标本片上滴加1∶20稀释的荧光标记抗体,放在湿盒中37 ℃孵育60 min,PBS洗5 min×3次;加DAPI(1∶1 000)复染细胞核,PBS漂洗缓冲,甘油封片,在荧光显微镜下观察,各组切片随机选5个视野计算阳性细胞数。③免疫组化法:海马组织切片常规脱蜡、水化、抗原修复,用3% H2O2去离子水孵育10 min,PBS冲洗;正常山羊血清封闭液孵育2 h,加入鼠抗鼠Caspase-8一抗(1 μg/mL)4 ℃孵育过夜,加辣根过氧化物酶标记山羊抗兔IgG(1∶200)室温孵育1 h,其间使用PBS充分洗涤;最后切片入DAB显色液中显色5~10 min,贴片,常规脱水透明封片。采用Olympus高清晰彩色图像处理系统观察和采集图像,每只大鼠各取切片3张,随机选择5个高倍镜视野,以Image Pro Plus6.0软件进行图像分析,测定并记录每张图片的积分光密度(IOD)值。
2 结果
2.1 各组大鼠海马组织病理改变 正常组海马神经元呈条带状分布,排列规则,细胞核大,细胞质少,无神经元坏死表现,CA1和CA3区见大量致密锥体细胞,排列规则,边缘清晰。模型组海马结构明显受损,CA1区、CA3区及齿状回(DG)区可见神经元细胞肿胀、增大,多数出现坏死,细胞形态改变,界限不清。TPM高剂量组和TPM低剂量组海马结构损伤较模型组减轻,CA1区细胞形态较好、无明显变性,CA3区见少量核固缩现象、大部分胞核清晰,DG区颗粒细胞排列尚齐、偶有核固缩和变性,以TPM低剂量组海马组织损伤最轻。见图1。
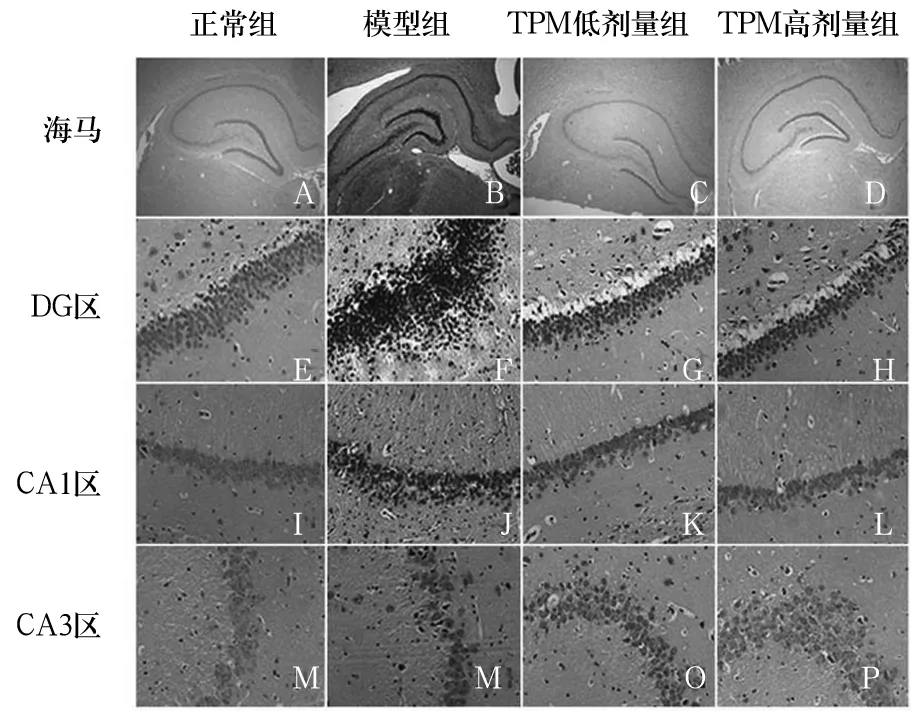
图1 各组大鼠海马组织结构变化
2.2 各组大鼠海马组织中miRNA表达改变 相比于正常组,模型组共发现12个表达上调的miRNA(rno-miR-146a、rno-miR-183、rno-miR-204、rno-miR-210、rno-miR-339、rno-miR-34、rno-miR-351、rno-miR-429、rno-miR-455、rno-miR-466b-1、rno-miR-503、rno-miR-532)和14个表达下调的miRNA(rno-miR-101a、rno-miR-382、rno-miR-451、rno-miR-380、rno-miR-488、rno-miR-499、rno-miR-338、rno-miR-551b、rno-miR-383、rno-miR-592、rno-miR-598-3p、rno-miR-374、rno-miR-9、rno-miR-33)。与模型组比较,TPM低剂量组和TPM高剂量组miRNAs表达谱改变不同。其中关于rno-miR-146a、rno-mir-210、rno-mir-34和rno-mir-488在癫痫中的作用研究甚少。采用实时荧光定量PCR进一步验证芯片结果发现,只有miR-146a在各组表达的差异有统计学意义。模型组、TPM高剂量组、TPM低剂量组、正常组海马组织中miR-146a相对表达量依次降低(P均<0.05)。见表2。

表2 各组海马组织中rno-miR-146a、rno-mir-210、rno-mir-34a、rno-mir-488相对表达量比较
2.3 各组海马组织中凋亡细胞数比较 与正常组相比,模型组大鼠CA1区、CA3区和DG区凋亡细胞数高于正常组(P均<0.05),而TPM高剂量组和TPM低剂量组凋亡细胞数低于模型组(P均<0.05)。见表3。
表3 各组大鼠海马组织中凋亡细胞数比较(个
2.4 各组大鼠海马组织中Caspase-8表达比较 Western blotting结果显示,正常组、模型组、TPM低剂量组和TPM高剂量组海马组织中Caspase-8蛋白相对表达量分别为1.03±0.26、4.33±0.37、1.96±0.19、3.05±0.22,其中模型组Caspase-8蛋白相对表达量高于正常组,模型组、TPM高剂量组、TPM低剂量组Caspase-8蛋白相对表达量依次降低(P均<0.01)。免疫荧光结果显示,与正常组相比,模型组大鼠CA1区、CA3区和DG区Caspase-8阳性细胞数高于正常组(P均<0.05),TPM高剂量组和TPM低剂量组Caspase-8阳性细胞数低于模型组(P均<0.05)。免疫组化结果显示,模型组IOD值高于正常组,模型组、TPM高剂量组、TPM低剂量组IOD值依次降低(P均<0.05)。详见表4、5。
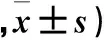
表4 免疫荧光法测各组大鼠海马组织中Caspase-8阳性细胞数比较(个
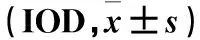
表5 免疫组化法测各组大鼠海马组织中Caspase-8表达比较
3 讨论
尽管目前已有许多抗癫痫药物(AEDs)大大增加了癫痫治疗的有效性,但癫痫病死率仍居高不下[7]。耐药型癫痫大部分都是颞叶癫痫(TLE)。本研究采用氯化锂、匹罗卡品诱导大鼠癫痫模型,与我们研究组先前采用戊四氮所致癫痫大鼠的行为类似[8]。与正常组比较,模型组海马组织出现核固缩、碎裂和溶解以及细胞坏死,同时出现大量凋亡细胞,这与我们先前所观察的癫痫大鼠模型表现一致[6]。TPM作为一种新型广谱抗癫痫药物,其作用机制可能包括:①选择性阻断钠离子通道,从而限制癫痫持续、重复发作;②提高γ-氨基丁酸活性,增强抑制性神经递质作用;③拮抗谷氨酸活性,阻断其介导的神经兴奋作用;④阻断T型钙通道,阻断癫痫持续、反复发作[9]。但是TPM发挥其作用的具体机制仍不明确,且不排除其他作用途径。本研究结果表明,TPM对癫痫大鼠有效。为进一步探索TPM治疗癫痫的机制,我们观察了各组大鼠海马组织中miRNA、Caspase-8的表达变化。
多项研究[10]报道miRNA在人类颞叶海马或癫痫模型中差异表达。有学者[11]首次报道miRNA可能参与神经精神疾病的发生。研究[12]发现,癫痫大鼠miR-181a表达上调,miR-181a能通过降低抗凋亡因子Bcl-2表达水平从而促进神经元细胞凋亡,对大鼠认知功能造成损伤。研究者[13]抑制癫痫大鼠海马组织中miR-210表达后,发现海马神经元形态改变减轻,细胞凋亡减少。我们在模型组发现了12个表达上调的miRNA和14个表达下调的miRNA,随后用实时荧光定量PCR进行验证,结果显示只有miR-146a在各组间表达有统计学差异。有学者[14]在大鼠TLE模型中发现miR-146a的表达量相比正常大鼠明显增高,并证明miR-146a可靶向作用于补体因子H,下调miR-146a能明显降低癫痫发作敏感性,提示miR-146a在癫痫发生发展有重要作用。本研究同样发现miR-146a在癫痫大鼠海马组织中表达升高,并且在TPM干预后miR-146a表达下降,TPM低剂量作用时下降更明显,于是推测TPM治疗癫痫的机制可能与调控miR-146a表达有关。
神经元凋亡参与了癫痫大鼠海马神经元的损伤过程[15],而Caspases在细胞凋亡过程中发挥重要作用。于是我们检测了各组大鼠海马组织中的Caspase-8,结果显示,TPM治疗后,大鼠海马CA1区、CA3区及DG区Caspase-8表达均减少,其中CA3区最为明显,且低剂量TPM较高剂量TPM作用明显;初步提示TPM的抗癫痫作用与抑制神经元凋亡有关。目前有关TPM治疗癫痫的剂量学者们仍存在争议,早期推荐其治疗剂量是400 mg/d,但考虑到不良反应,患者实际使用剂量往往更低。一个大型回顾性研究提出TPM治疗癫痫的使用剂量在175~200 mg/d[16]。在动物模型实验中,有研究者认为使用100 mg/(kg·d)剂量能达到控制癫痫的效果[17],也有学者发现用85 mg/(kg·d)和80 mg/(kg·d)[18]的TPM能有效抑制大鼠癫痫发作的频率和强度。本研究发现低剂量[40 mg/(kg·d)]TPM处理癫痫大鼠在海马组织形态学改善和抑制神经元凋亡方面效果均较高剂量[80 mg/(kg·d)]TPM要好,而且,低剂量TPM引起miRNA表达谱改变也更为明显。
结合上述研究结果,我们认为,TPM腹腔注射有助于减轻癫痫大鼠海马组织病理改变,抑制神经元凋亡,并下调海马组织中miR-146a和Caspase-8的表达,其中40 mg/(kg·d)的TPM较80 mg/(kg·d)效果更加明显。我们推测TPM的抗癫痫作用机制与调节miRNA表达、减少神经元细胞凋亡有关。
[1] Behr C, Goltzene MA, Kosmalski G, et al. Epidemiology of epilepsy[J]. Rev Neurol (Paris), 2016,172(1):27-36.
[2] Shukla GC, Singh J, Barik S. MicroRNAs: processing, maturation target recognition and regulatory functions[J]. Mol Cell Pharmacol, 2011,3(3):83-92.
[3] 李香丹,李今子.miRNAs在人类颞叶癫痫中的作用研究进展[J].延边大学医学学报,2015(1):74-76.
[4] Guerrini R, Parmeggiani L. Topiramate and its clinical applications in epilepsy[J]. Expert Opin Pharmacother, 2006,7(6):811-823.
[5] Chen J, Quan QY, Yang F, et al. Effects of lamotrigine and topiramate on hippocampal neurogenesis in experimental temporal-lobe epilepsy [J]. Brain Res, 2010,1313:270-282.
[6] 李今子,许书翠,尹明姬,等.厄多司坦对癫痫大鼠海马氧化应激及肿瘤坏死因子-α、基质金属蛋白酶-2水平的影响[J].延边大学医学学报,2013(4):250-253.
[7] Ngugi AK, Bottomley C, Kleinschmidt I, et al. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach[J]. Epilepsia, 2010,51(5):883-890.
[8] 李今子,文瑛,许书翠.普罗布考对癫痫大鼠海马神经元的影响[J].延边大学医学学报,2011(1):12-14.
[9] Li MM, Li XM, Zheng XP, et al. MicroRNAs dysregulation in epilepsy[J]. Brain Res, 2014,1584:94-104.
[10] Miller DT, Shen Y, Weiss LA, et al. Microdeletion/duplication at 15q13.2q13.3 among individuals with features of autism and other neuropsychiatric disorders[J]. J Med Genet, 2009,46(4):242-248.
[11] Huang Y, Liu X, Liao Y, et al. MiR-181a influences the cognitive function of epileptic rats induced by pentylenetetrazol[J]. Int J Clin Exp Pathol, 2015,8(10):12861-12868.
[12] Chen L, Zheng H, Zhang S. Involvement of upregulation of miR-210 in a rat epilepsy model [J]. Neuropsychiatr Dis Treat, 2016,12:1731-1737.
[13] He F, Liu B, Meng Q, et al. Modulation of miR-146a/complement factor H-mediated inflammatory responses in a rat model of temporal lobe epilepsy[J]. Biosci Rep, 2016,36(6):00433.
[14] Zhang B, Zhang JW, Wang WP, et al. Effect of lamotrigine on epilepsy-induced cognitive impairment and hippocampal neuronal apoptosis in pentylenetetrazole-kindled animal model[J]. Synapse, 2017,71(2). doi: 10.1002/syn.21945.
[15] Hu Y, Lu Y, Yu W, et al. Long-term retention rate of topiramate as initial monotherapy in Chinese patients with newly diagnosed epilepsy: a prospective, observational study[J]. Epilepsy Res, 2010,90(3):278-284.
[16] Poston S, Dickson M, Johnsrud M, et al. Topiramate prescribing patterns among medicaid patients: diagnosis, comorbidities, and dosing [J]. Clin Ther, 2007,29(3):504-518.
[17] Gurses C, Orhan N, Ahishali B, et al. Topiramate reduces blood-brain barrier disruption and inhibits seizure activity in hyperthermia-induced seizures in rats with cortical dysplasia[J]. Brain Res, 2013,1494:91-100.
[18] Ye F, Chen XQ, Bao GS, et al. Effect of topiramate on interleukin 6 expression in the hippocampus of amygdala-kindled epileptic rats [J]. Exp Ther Med, 2014,7(1):223-227.
Changes of hippocampal injuries in epileptic rats after intraperitoneal injection of topiramate and its mechanism
LIXiangdan1,YINMingji,YANGYang,LINZhenhua,XIANZhemin,LIJinzi
(1TheAffiliatedHospitalofYanbianUniversity,Yanji133002,China)
Objective To observe the changes of hippocampal injuries in epileptic rats after intraperitoneal injection of topiramate (TPM), and to explore its potential mechanisms. Methods Forty rats were randomly divided into the normal group, model group, low-dose TPM group, and high-dose TPM group. The rats in the model group, low-dose TPM group, and high-dose TPM group were treated with 10 mL/kg/d saline, 40 mg/kg/d TPM and 80 mg/kg/d TPM, respectively, for 4 weeks to make the epileptic models. At 5 h after epileptic seizure, rats in the model group, low-dose TPM group, and high-dose TPM group were intraperitoneally injected with 10 mL/(kg·d) normal saline , 40 mg/(kg·d) TPM , and 80 mg/(kg·d) TPM. After 4 weeks of continuous administration, rats were sacrificed to obtain the hippocampus tissues. HE staining was performed to observe the morphological changes of hippocampus. The differentially expressed miRNAs were obtained by miRNA microarray and were identified by real-time fluorescent quantitative PCR. TUNEL assay was used to detect apoptosis of hippocampal neurons and the number of apoptotic cells were counted. The expression levels of Caspase-8 were detected by Western blotting, immunohistochemistry (IHC) and immunofluorescence (ICC). Results The hippocampus was obviously damaged in model group as compared with that of the normal group, whereas the injuries were alleviated in both low-dose TPM group and high-dose TPM group, especially the former one. A total of 12 miRNAs were up-regulated and 14 miRNAs were down-regulated in the model group as compared with those of the normal group. Interestingly, the miR-146a expression was significantly down-regulated both in low- and high-dose TPM groups as compared with that of the model group (allP<0.05). Compared with the normal group, the number of apoptotic cells in the CA1, CA3 and DG regions was significantly higher in the model group (allP<0.05). Meanwhile, the number of apoptotic cells was smaller in the low-dose TPM group and high-dose TPM group than that of the model group (allP<0.05). Western blotting showed that the Caspase-8 protein expression of the model group was higher than that of the normal group, and the Caspase-8 protein expression was successively decreased in the model group, high-dose, and low-dose TPM groups (allP<0.01). Similarly, ICC showed that the number of Caspase-8 positive cells in the CA1, CA3 and DG regions significantly increased in the model group as compared with that of the normal group (allP<0.05). Compared with the model group, the number of Caspase-8 positive cells in the low-dose TPM group and high-dose TPM group were dramatically reduced (allP<0.05). What's more, the results of IHC identified that the integrated option density (IOD) value was significantly higher in the model group as compared with that of the normal group. The value of IOD was successively decreased in the model group, low-dose TPM group, and high-dose TPM group (allP<0.05).Conclusions The intraperitoneal injection of TPM effectively alleviates hippocampal injuries in epileptic rats, inhibits neuronal apoptosis and down-regulates the expression of miR-146a and Caspase-8. Meanwhile, 40 mg/(kg·d) TMP shows greater effect than 80 mg/(kg·d). The anti-epileptic mechanism of TPM may be associated with the regulation of miRNA expression, down-regulation of Caspase-8, and reduction of neuronal apoptosis.
epilepsy; topiramate; hippocampus; miRNAs; miR-146a; apoptosis; Caspase-8
吉林省医药卫生科研计划项目(C2017103)。
李香丹(1987-),女,在读博士,主要研究方向为发作性疾病。E-mail:2015001051@ybu.edu.cn
李今子(1963-),女,博士生导师,主任医师,主要研究方向为发作性疾病。E-mail:yjzli@ybu.edu.cn
10.3969/j.issn.1002-266X.2017.27.004
R742.1
A
1002-266X(2017)27-0014-05
2017-02-19)

