肟型Schiff碱和配合物的合成、晶体结构及光谱性质
郭建强 孙银霞 俞 彬 李 璟 贾浩然
郭建强 孙银霞*俞 彬 李 璟 贾浩然
(兰州交通大学化学与生物工程学院,兰州 730070)
合成了2个Schiff碱Cu/Ni配合物[Cu(L1)2](1)和[Ni(L2)2](2)(HL1=1-(4-(((E)-3,5-dichloro-2-hydroxybenzylidene)amino) henyl)ethanoe O-benzyloxime,HL2=1-(4-(((E)-4-methoxy-2-hydroxybenzylidene)amino)phenyl)ethanone O-benzyloxime),并通过元素分析、红外光谱、紫外光谱及X射线单晶衍射分析进行了表征和分析。X射线结构表明:配合物1和2具有类似的结构,均由1个金属离子和2个配体单元组成。配合物1和2都是单斜晶系,但配合物1空间群为C2/c,而配合物2为P21/c。且中心金属Cu和Ni离子的空间构型均为四配位的扭曲的平面四边形结构。配合物1通过π…π和C-H…π作用形成3D超分子结构,而配合物2通过C-H…π作用形成2D超分子孔道结构。
铜配合物;镍配合物;晶体结构;Schiff碱配体;光谱性质
0 Introduction
The coordination chemistry of transition metal complexes with Schiff base ligands has achieved a considerable attention in the last decades[1-5],because of their redox-chemistry,unusual magnetic and structural properties,as well as their usage as models for metalloproteinase[6-10],and catalysts for oxidation and polymerization reactions[6,8,11].It is important to introduce suitable functional groups into the organic moiety of the ligands in order to improve or tune the properties of these metal complexes[12-16].Oximecontaining Schiff base ligands as a class of strong electron donors,and itis found thatthey can efficiently stabilize high oxidation states of metal ions,such as Cuand Ni[17-20].Herein,in order to further study the supramolecular structures of the transition metal complexes with the oxime-type Schiffbase ligands,two supramolecular Cuand Nicomplexes with oximetype Schiffbase ligands,[Cu(L1)2](1)(HL1=1-(4-(((E)-3, 5-dichloro-2-hydroxybenzylidene)amino)phenyl)ethanoe O-benzyloxime),and [Ni(L2)2](2)(HL2=1-(4-(((E)-4-methoxy-2-hydroxybenzylidene)amino)phenyl)ethanone O-benzyloxime),have been synthesized and characterized by elemental analyses,IR spectra,UV-Vis spectra and X-ray crystallographic analysis.
1 Experimental
1.1 Materials
4-aminoacetophenone,O-benzylhydroxylamine,4-methoxysalicylaldehyde,and 3,5-dichloro-2-hydroxybenzaldehyde were purchased from Aldrich and used without further purification.The other reagents and solvents were analytical grade reagents from Tianjin Chemical Reagent Factory.
1.2 Methods
C,H and N analyses were carried out with a GmbH VariuoEL V3.00 automatic elemental analyzer. FT-IR spectra were recorded on a VERTEX70 FT-IR spectrophotometer,with samples prepared as KBr (400~4 000 cm-1).UV-Vis absorption spectra were recorded on a Shimadzu UV-2550 spectrometer.X-ray single crystal structure was determined on a Bruker Smart 1000 CCD area detector.Melting points were measured by a microscopic melting point apparatus made in Beijing Taike Instrument Limited Company and the thermometer was uncorrected.
1.3 Synthesis of HL1 and HL2
HL1and HL2were synthesized according to the reported method[21-22].The synthetic route is given in Scheme 1.HL1:Yield,81.6%;m.p.399~400 K.Anal. Calcd.for C23H21Cl2N2O2(%):C,64.49;H,4.94;N, 6.54;Found(%):C,64.51;H,4.92;N,6.57.HL2:Yield, 79.6%;m.p.399~401 K.Anal.Calcd.for C23H22N2O3(%):C,73.78;H,5.92;N,7.48;Found(%):C,73.75;H, 5.90;N,7.50.
1.4 Synthesis of[Cu(L1)2](1)
A methanol solution (5 mL)of Cuacetate monohydrate(1.90 mg,0.01 mmol)was added dropwise to an acetone solution (3 mL)of HL1(8.26 mg,0.01 mmol)at room temperature.The mixing solution turned to red-brown immediately,was filtered and the filtrate was allowed to stand at room temperature for about two weeks.Brown block-shaped single crystals suitable for X-ray structural determination were obtained.Anal.Calcd.for C44H34Cl4CuN4O4(%):C, 59.51;H,3.86;N,6.31.Found(%):C,60.02;H,3.73; N,6.55.
1.5 Synthesis of[Ni(L2)2](2)
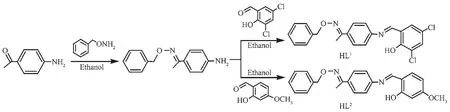
Scheme 1 Synthetic routes of HL1 and HL2
1.6 Crystalstructure determination
The single crystals of complexes 1 and 2 with approximate dimensions of 0.30 mm×0.27mm×0.15 mm and 0.21 mm×0.14 mm×0.08 mm were placed on a Bruker Smart1000 CCD area detector.The diffraction data were collected using a graphite monochromated Mo Kα radiation(λ=0.071 073 nm)at 293(2)K for complex 1 and a graphite monochromated Cu Kα radiation(λ=0.154 178 nm)at 293(2)K for complex 2.Empirical absorption correction was applied to the data using SADABS program[23].The structures were solved by direct methods and refined by full-matrix leastsquares method on F2using the SHELXL-97 program[24].All nonhydrogen atoms were refined anisotropically.All the hydrogen atoms were generated geometrically and refined isotropically using the riding model.Details of the crystal parameters,data collection and refinements for complexes 1 and 2 are summarized in Table 1.
CCDC:1560091,1;1560090,2.
2 Results and discussion
2.1 IR spectra analyses
The FT-IR spectra of HL1,HL2and its corresponding complexes 1 and 2 exhibit various bands in the 400~4 000 cm-1region in Table 2.The free ligand HL1and HL2exhibit characteristic C=N stretching bands at 1 599 and 1 626 cm-1,while the C=N of complex 1 and complex 2 are observed in the 1 595and 1 649 cm-1,respectively.The C=N stretching frequencies shift to lower frequencies by ca.17 and 18 cm-1upon complexation respectively,indicating a decrease in the C=N stretching bond order due to the coordinated bond of the metal atom with the imino nitrogen lone pair[25].In the 1 445~1 564 cm-1region, the observed bands are attributed to aromatic C=C vibrations.Upon coordination these bands shift to lower frequencies for the metal complexes[26].The Ar-O stretching frequency appears as a strong band at 1 171,1 206 cm-1for HL1and HL2,and at1 169,1 213 cm-1for the complexes,respectively[27].The Ar-O stretching frequency shifts to lower frequency, indicating that the M-O bond forms between the metal atoms and oxygen atoms of the phenolic groups[5].The far-infrared spectra of complexes 1 and 2 was also obtained in the region 500~100 cm-1in order to identify frequencies due to the M-O and M-N bonds. The FT-IR spectrum of complex 1 shows(M-N)and (M-O)vibration absorption frequencies at 540 and 503 cm-1(or519 and 463 cm-1forcomplex 2),respectively. These assignments are consistent with the literature frequency values[28].

Table 1 Crystal data and structure refinement for complexes 1 and 2
Table 2 IR spectrum of HL1,HL2 and theirs corresponding Cuand Nicomplexes cm-1
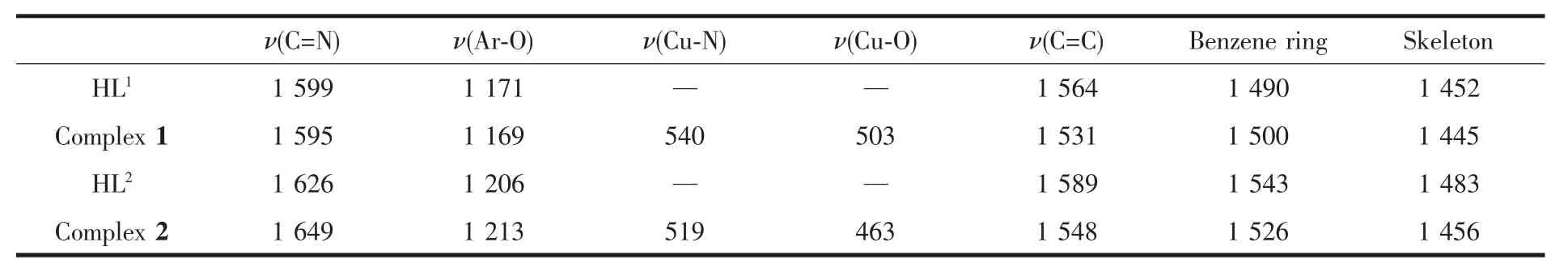
Table 2 IR spectrum of HL1,HL2 and theirs corresponding Cuand Nicomplexes cm-1
ν(C=N) ν(Ar-O) ν(Cu-N) ν(Cu-O) ν(C=C) Benzene ring Skeleton HL1 1 599 1 171 — — 1 564 1 490 1 452 Complex 1 1 595 1 169 540 503 1 531 1 500 1 445 HL2 1 626 1 206 — — 1 589 1 543 1 483 Complex 2 1 649 1 213 519 463 1 548 1 526 1 456
2.2 UV-Vis absorption spectra analyses
The absorption spectra of ligand HL1,HL2and theirs corresponding Cuand Nicomplexes were determined in diluted DMF solution as shown in Fig.1 and 2.UV-Vis spectrum of the free ligand HL1exhibits three absorption peaks at ca.255,293 and 427 nm (Fig.1).The absorption peak of HL1 at 255 nm assigned to theπ-π*transition of the benzene rings shifts to low energy region by ca.1 nm in complex 1,indicating Cuion coordinated with the O and N atoms ofligand units.The absorption peak of HL1at 293 and 427 nm attributed to the intra-ligand π-π*transition of the C=N bonds shift to 272 and 417 nm in complex 1 upon complexation,respectively. The UV-Vis spectrum of the free ligand HL2exhibits two absorption peaks at ca.299 and 353 nm.The former absorption peak at 299 nm can be assigned to theπ-π*transition ofthe benzene rings and the latter one at 353 nm can be attributed to the intra-ligandππ*transition of the C=N bonds[29].Compared with theabsorption peak ofthe free ligand HL2,a corresponding absorption peak at 291 nm is observed in the Nicomplex,which bathochromically shifts for ca.6 nm, indicating the coordination of Niions with the free ligand.Meanwhile,the absorption band at about 353 nm disappears from the UV-Vis spectrum of the Nicomplex,which indicates that the oxime nitrogen atom is involved in the coordination to the Cuatom[30-31]. In addition,the new bands observed at 362 nm for the Nicomplex is assigned to the n-π*charge transfer transition from the filled pπorbital of the phenolic oxygen to the vacant-orbital of the Niions,which are characteristic of the transition metal complexes with N2O2coordination spheres[32].
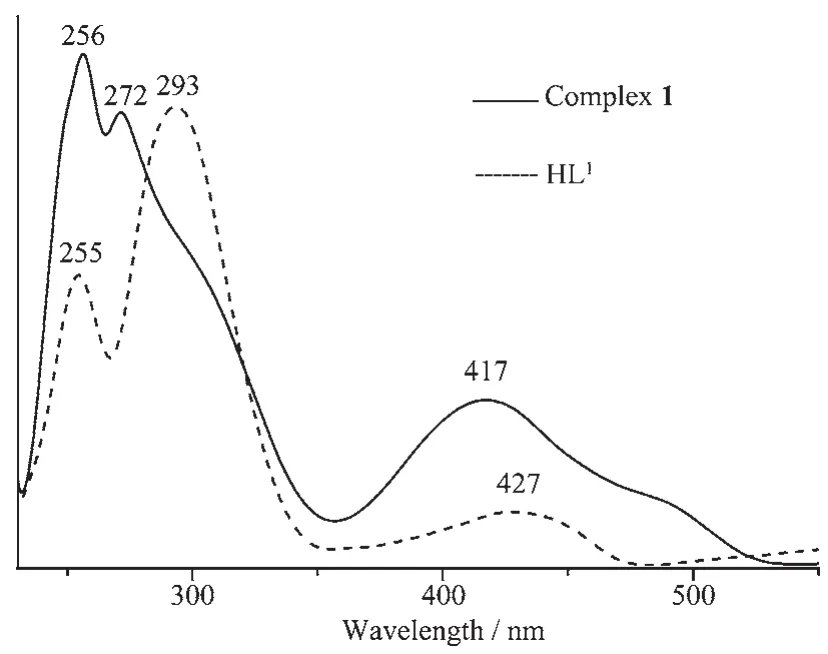
Fig.1 UV-Vis absorption spectra of HL1 and Cucomplex in dilute DMF solution at room temperature

Fig.2 UV-Vis absorption spectra of HL2 and Nicomplex in dilute DMF solution at room temperature
2.3 Crystalstructures of the Cuand Nicomplexes
The single crystal structures of complexes 1 and 2 were confirmed by X-ray crystallography(Fig.3 and 4).The selected bond lengths and angles of complexes 1 and 2 are listed in Table 3.X-ray crystallographic analysis reveals thatboth complexes 1 and 2 crystallize in the monoclinic system with space group C2/c for complex 1 and P21/c for 2.The complexes 1 and 2 can be described as mononuclear Mcomplexes(M= Cu or Ni),consist of one Mion and two L-units,in which the Mion is four-coordinated in a trans-N2O2square-planar geometry,with two phenolic O and two imino N atoms from two N,O-bidentate oxime-type ligands(HL1and HL2).

Fig.3 Molecular structure of complex 1 showing 30% probability displacement ellipsoids

Fig.4 Molecular structure of complex 2 showing 30%probability displacement ellipsoids

Table 3 Selected bond lengths(nm)and bond angles(°)for complexes 1 and 2

Fig.5 Intramolecular hydrogen bonds ofcomplex 1

Fig.6 View of3D supramolecular structure ofcomplex 1 showing the formation offourπ…πstacking interaction
The crystal structure of the complex 1 is stabilized by four intramolecular non-classic hydrogen bonds(Fig.5,Table 4).Meanwhile,the Cucomplex is linked byπ…πstacking interactions(Fig.6,Table 5)between benzene rings or/and chelate ring of the adjacent Cucomplex molecules into an infinite 3D layer supramolecular structure.This linkage is further stabilized by three C-H…πstacking interactions(Fig. 7,Table 6).Thus,the crystal packing of complex 1 shows that a 3D supramolecular network structure form through intermolecularC-H…πhydrogen bonding andπ…πstacking interactions(Fig.8).Consequently, the intermolecular non-classical hydrogen-bonding plays a very important role in the construction of supramolecular networks structure.

Fig.7 View ofthe C-H…πstacking interaction of complex 1

Table 4 Putative Hydrogen-bonding for complexes 1 and 2

Table 5 Putativeπ…πstacking interactions for complex 1

Table 6 Putative C-H…πstacking interactions for complex 1
In complex 2,the crystal structure of complex 2 is stabilized by pair of intramolecular C1-H1C…O1 hydrogen bonds generating five-membered ring motifs (Fig.9,Table 4).This linkage is further stabilized by two pairs of C15-H15…π (Cg7)and C22-H22…π (Cg8)interactions to form the 2D channel supramolecular structure along the b axis(Fig.10,Table 6).
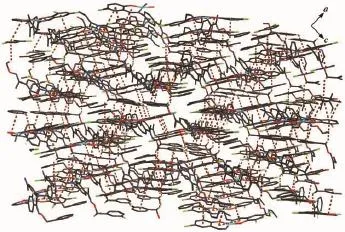
Fig.8 View of the 3D supramolecular structure of complex 1
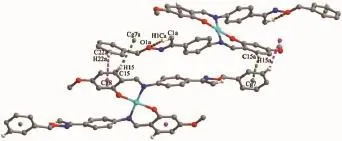
Fig.9 Putative intramolecular hydrogen bonds and intermolecular C-H…πstacking interaction of complex 2

Fig.10 View of the 2D channel supramolecular structure within complex 2 along the b axis
3 Conclusions
Two oxime-type Schiff base mononuclear Cu/ Nicomplexes 1 and 2 have been synthesized and characterized structurally.The complexes 1 and 2 are all tetra-coordinated by two nitrogen atoms and two oxygen atoms of two deprotonated Lˉunits defining the N2O2 basal plane.The coordination environment around center metal ion is regarded as the distorted square-planar geometry.Complexes 1 and 2 also form a 3D and 2D supramolecular structures via different intermolecularinteraction C-H…πandπ…πstacking interactions,respectively.Consequently,the intermolecular non-classical hydrogen-bonding plays a very important role in the construction of supramolecular networks structure.
[1]Wu H L,Wang H,Wang X L,et al.New J.Chem.,2014,38: 1052-1061
[2]Song X Q,Liu P P,Xiao Z R,etal.Inorg.Chim.Acta,2015, 438:232-244
[3]Yu T Z,Zhang K,Zhao Y L,et al.Inorg.Chim.Acta,2008,361:233-240
[4]Ma J C,Dong X Y,Dong W K,et al.J.Coord.Chem.,2016, 69:149-159
[5]Song X Q,Cheng G Q,Liu Y A.Inorg.Chim.Acta,2016, 450:386-394
[6]Holm R H,Solomon E I,Majumdar A,et al.Coord.Chem. Rev.,2011,255:993-1015
[7]Klein L J,Alleman K S,Peters D G,et al.J.Electroanal. Chem.,2000,481:24-33
[8]Wu H L,Pan G L,Bai Y C,et al.Res.Chem.Intermed., 2015,41:3375-3388
[9]Wang L,Ma J C,Dong W K,et al.Z.Anorg.Allg.Chem., 2016,642:834-839
[10]Sun Y X,Wang L,Dong X Y,etal.Synth.React.Inorg.Met. Org.Nano Met.Chem.,2013,43:509-513
[11]Gibson V C,Spitzmesser S K.Chem.Rev.,2003,103:283-316
[12]Rigamonti L,Demartin F,Forni A,etal.Inorg.Chem.,2006, 45:10976-10989
[13]SUN YIN-XIA(孙银霞),DONG Wen-Kui(董文魁),WANG Li(王莉),et al.Chinese J.Inorg.Chem.(无机化学学报), 2009,25(8):1478-1482
[14]Xu L,Zhu L C,Ma J C,et al.Z.Anorg.Allg.Chem.,2015, 641:2520-2524
[15]Zhao L,Wang L,Sun Y X,et al.Synth.React.Inorg.Met. Org.Nano Met.Chem.,2012,42:1303-1308
[16]Wang P,Zhao L.Spectrochim.Acta,Part A,2015,135:342-350
[17]Dong W K,Zhu L C,Ma J C,et al.Inorg.Chim.Acta,2016, 453:402-408
[18]Dong W K,Zhu L C,Dong Y J,et al.Polyhedron,2016,117: 148-154
[19]DONG Wen-Kui(董文魁),WANG Li(王莉),SUN Yin-Xia (孙银霞),et al.Chinese J.Inorg.Chem.(无机化学学报), 2011,27(2):372-376
[20]Dong W K,Ma J C,Zhu L C,et al.New J.Chem.,2016,40: 6998-7010
[21]Dong W K,Ma J C,Dong X Y,et al.Polyhedron,2016,115: 228-235
[22]SUN Yin-Xia(孙银霞),LU Rui-E(陆瑞娥),LI Xin-Ran(李新然),etal.Chinese J.Inorg.Chem.(无机化学学报),2015,31 (5):1055-1062
[23]Sheldrick G M.SADABS,University of Göttingen,Germany, 1996.
[24]Sheldrick G M.SHELXL-97,Program for the Refinement of Crystal Structures,University of Göttingen,Germany,1997.
[25]Asadi M,Jamshid K A,Kyanfar A H.Inorg.Chim.Acta, 2007,360:1725-1730
[26]Tümer M,Koksal H,Sener M K,etal.Transition Met.Chem., 1999,24(4):414-420
[27]Majumder A,Rosair G M,Mallick A,et al.Polyhedron, 2006,25:1753-1762
[28]Akine S,Taniguchi T,Nabeshima T.Inorg.Chem.,2004,43: 6142-6144
[29]Ghosh T,Mondal B,Ghosh T,et al.Inorg.Chim.Acta,2007, 360:1753-1761
[30]Smith H E.Chem.Rev.,1983,83:359-377
[31]Dong W K,Sun Y X,Liu G H,et al.Z.Anorg.Allg.Chem., 2012,638(9):1370-1377
[32]Akine S,Taniguchi T,Nabeshima T.Chem.Lett.,2001,30: 682-683
Syntheses,Crystal Structures and Spectroscopic Properties of Copperand NickelComplexes with Oxime-Type Schiff Base Ligands
GUO Jian-Qiang SUN Yin-Xia*YU Bin LI Jing JIA Hao-Ran
(School of Chemical and Biological Engineering,Lanzhou Jiaotong University,Lanzhou 730070,China)
Two copperand nickelcomplexes,[Cu(L1)2](1)(HL1=1-(4-(((E)-3,5-dichloro-2-hydroxybenzylidene) amino)henyl)ethanoe O-benzyloxime)and[Ni(L2)2](2)(HL2=1-(4-(((E)-4-methoxy-2-hydroxybenzylidene)amino) phenyl)ethanone O-benzyloxime),have been synthesized and characterized by elemental analyses,IR and UV-Vis spectra and X-ray single crystal diffraction method.X-ray structure showed that complexes 1 and 2 have the similar structure,consisting of one metal ion,two ligand units.But the complex 1 crystallize in the monoclinic space group C2/c with the unit cell parameters:a=2.718 77(13)nm,b=1.321 99(6)nm,c=2.225 56(12)nm,β= 92.069 0(10)°;whereas the complex 2 crystallize in the monoclinic space group P21/c,with the unitcellparameters: a=1.423 89(18)nm,b=0.601 22(8)nm,c=2.467 7(2)nm,β=113.353(2)°.The center Cuor Niatoms are all four-coordinated in a trans-N2O2slightly distorted square-planar geometry by two phenoxy O atoms and two imine N atoms from two symmetry-related N,O-bidentate Schiff base ligands.And the supramolecular structures of the two complexes 1 and 2 are widely different by obvious intermolecular interaction.The complex 1 links some other molecules into an infinite 3D layer supramolecular structure via intermolecular C-H…π andπ…π stacking interactions between neighboring benzene rings,while complex 2 formed 2D supramolecular channel structure by intermolecular C-H…πhydrogen bond interactions.CCDC:1560091,1;1560090,2.
Cucomplex;Nicomplex;crystal structure;Schiff-base ligand;spectroscopic property
O614.121;O614.81+3
A
1001-4861(2017)08-1481-08
10.11862/CJIC.2017.181
2017-03-21。收修改稿日期:2017-05-22。
兰州交通大学研究生教改项目(No.160012)资助。
*通信联系人。E-mail:sun_yinxia@163.com

