ZnO Based Recyclable Photocatalyst in The Air
YU Bing-bing, XU Zhi-kun, WANG Fei, WANG Yun-peng, ZHAO Dong-xu*
(1. State Key Laboratory of Luminescence and Applications, Changchun Institute of Optics, Fine Mechanics and Physics, Chinese Academy of Sciences, Changchun 130033, China;2. University of Chinese Academy of Sciences, Beijing 100049, China;3. Harbin Normal University, Harbin 150025, China)
ZnO Based Recyclable Photocatalyst in The Air
YU Bing-bing1,2, XU Zhi-kun3, WANG Fei1, WANG Yun-peng1, ZHAO Dong-xu1*
(1. State Key Laboratory of Luminescence and Applications, Changchun Institute of Optics, Fine Mechanics and Physics, Chinese Academy of Sciences, Changchun 130033, China;2. University of Chinese Academy of Sciences, Beijing 100049, China;3. Harbin Normal University, Harbin 150025, China)
Vertically aligned ZnO nanorods were prepared on sapphire substratesviaa hydrothermal approach. The morphological, structural and optical properties were investigated by the scanning electron microscopy (SEM), X-ray diffraction (XRD) and photoluminescence (PL) spectra. These nanorods on substrates showed a good photocatalytic activity toward the methyl violet in the air atmosphere. The presence of oxygen and water vapor was essential for the photocatalytic oxidation. These nanorods could serve as the effective, convenient and recyclable photocatalysts in the air, which made them a promising photocatalyst candidate for the solid-contaminant treatments.
ZnO; hydrothermal approach; photocatalyst
1 Introduction
One dimensional (1D) nanostructures have been studied extensively owing to their potential applications in optoelectronic, optical, and electric devices[1-3]. Among them, ZnO, with a wide band gap (3.37 eV) and a large exciton binding energy (60 meV) at room temperature, has attracted much attention due to the potential applications in light emission diodes (LEDs), laser diodes (LD), and photodetectors. Recently, many ZnO nanostructures especially 1D nanostructures have been synthesized and widely studied. Due to the high surface-to-volume ratio, 1D nanostructures have potential applications in photocatalytic area[4].
Because the separation of the conventional powder photocatalyst from the reaction aqueous suspension is so difficult, which limits the reuse for the photocatalyst. Various types of 1D ZnO nanostructure arrays on the substrates have been investigated in order to get the recyclable photocatalysts[5-8]. By now, the photocatalytic studies have been mainly focused on the water solution, especially the waste water. Few studies have been performed to investigate the photodecomposition under the air environment, which may have great potential applications for the self-clean of the building surface. In this paper we studied the photodecomposition properties of ZnO nanorods grown on the sapphire substrate. The high efficient photocatalytic property has been observed in the air atmosphere. And it was verified the oxygen and water molecules were essential for the photocatalytic property of ZnO nanorods.
2 Experiments
2.1 Preparation of ZnO Nanorods Array
Before the growth of ZnO nanorods, sapphire substrates with the area of 0.5 cm × 0.5 cm were sonicated in acetone and ethanol for 5 min, respectively. After being dried by the nitrogen stream, a 100 nm thick ZnO film with (002) orientation was deposited on sapphire substrates by the magnetron sputtering method serving as a seeds layer. 0.01 mol/L zinc acetate and 0.01 mol/L hexamethylenetetramine were dissolved in 100 mL deionized water to form the reaction solution. Then, the solution was transferred to a Teflon-lined stainless autoclave of 20 mL capacity. The as-grown ZnO film/sapphire substrates were put into the solution. The tanks were conducted in an electric oven at 90 ℃ for 12 h. After reaction, the samples were washed by deionized water and dried in air at 90 ℃ for several hours.
2.2 Sample Characterizations
The morphology and structure properties of samples were investigated by the field-emission scanning electron microscopy (FESEM, Hitachi S-4800) and the X-ray diffraction (XRD, D/max-RA). The absorption spectra were carried out using a Shimadzu UV-3101 PC spectrophotometer. The photoluminescence (PL) measurement was performed in a JY-630 micro-Raman spectrometer employing the 325 nm line of a He-Cd laser as the excitation source.
2.3 Photocatalytic Test
In order to deposit methyl violet on the ZnO nanorods, the as-prepared ZnO nanorods array was pre-irradiated with UV-light (with the wavelength of 365 nm) for 0.5 h to enhance its hydrophility and allow well-distributed adsorption of methyl violet onto the ZnO surface. A droplet with 10-5 L of 0.001 mol/L methyl violet solution in deionized water was dripped onto the samples surface. Samples were then dried at 90 ℃ in air for 10 min.
To examine the photocatalytic activity of the synthesized ZnO nanorods, substrates with the ZnO nanorods of 0.5 cm × 0.5 cm were placed about 2 cm from a mercury lamp. The UV-Vis absorption spectra of the substrates as a function of time were recorded. The spectra were obtained using a Shimadzu UV-3101 PC spectrophotometer. For the examination of the ZnO nanorods on the sapphire substrates as recyclable photocatalysts, the substrate was rinsed with deionized water and irradiated for 1h to completely remove the residual organic species after taking the absorption spectra. Then a droplet of 10-5 L solution at the same concentration was subsequently dripped onto the same substrate for another run of experiment. The same procedure was performed for 5 cycles to evaluate the suitability of the ZnO nanorods for multiple uses in the photodecomposition of common organic dye molecules and environmental contaminants.
3 Results and Discussion
3.1 SEM and X-ray Diffraction Studies
Fig.1(a) and 1(b) show the typical top-view SEM images of the oriented ZnO nanorods array grown on the sapphire substrateviathe hydrothermal process at 90 ℃ for 12 h. Fig.1(c) shows the side-view FESEM image of the ZnO nanorods. From all these images, it can be observed the ZnO nanorods almost aligned vertically on the sapphire substrate. The nanorods have a typical diameter of about 100-500 nm and a length of 2-3 μm. The XRD patterns with an exceptionally strong (002) peak shown in Fig. 1(d) reveals that the hydrothermally grown ZnO nanorods are of the wurtzite structure, which indicates the preferential growth of these nanorods along thec-axis of ZnO.
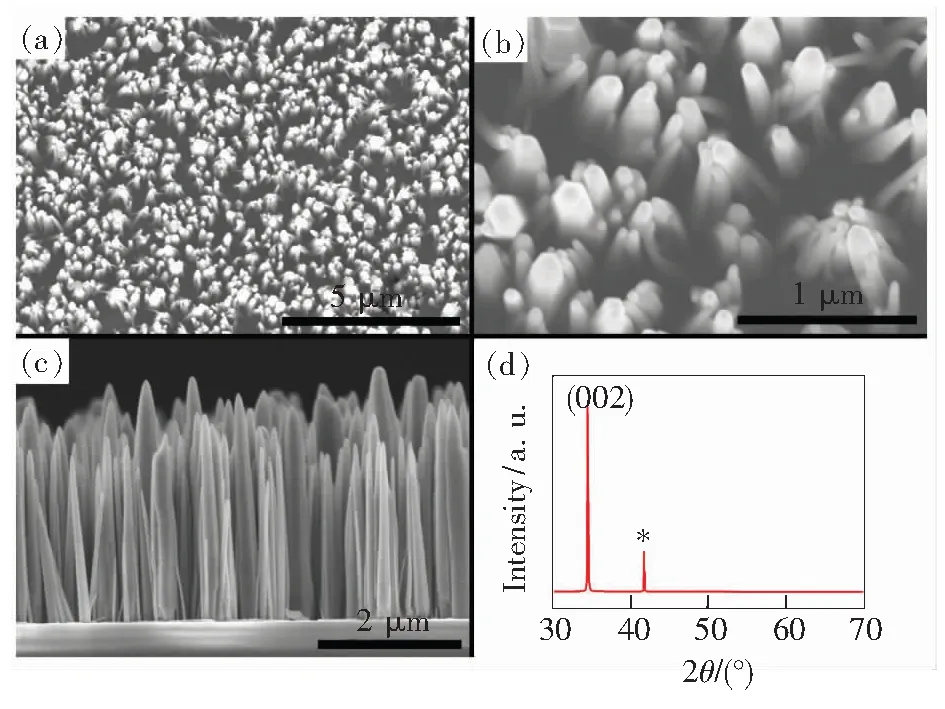
Fig.1 FE-SEM images and XRD pattern of the as-grown ZnO nanorods on sapphire substrates. (a, b) Large and close top view of the nanorods. (c) Side view of the nanorods. (d) XRD patterns of the nanorods. * is the peak of sapphire substrates.
3.2 Photoluminescence and UV-Vis Spectroscopic Analysis
Fig.2(a) shows the room temperature photoluminescence (PL) spectrum of the ZnO nanorods excited by the 325 nm line of a He-Cd laser. In the PL spectrum, a strong and sharp UV near-band-edge emission with a peak centered at 377 nm and a weak broad emission band centered at about 550 nm are observed. The UV emission band could be attributed to the recombination of free excitons[9-10]. And the visible emission is usually originated from the oxygen-vacancy-related defect emission. The surface oxygen deficiencies are electron capture centers which can decrease the recombination rate of electrons and holes and thus increase the photocatalytic activity[7,11].

Fig.2 (a)PL spectrum of the ZnO nanorods.(b)UV-Vis absorption spectrum of the as-grown ZnO nanorods on a sapphire substrate. The inset shows a plot of (αhν)2vs.hνfor the determination of the band gap of the nanorods.
The optical absorbance spectrum of the ZnO nanorods arrays in the wavelength region 300-800 nm is presented in Fig.2(b). The sample exhibits a sharp absorption band-edge in the UV region. A clear excitonic nature is visible for the ZnO nanorods in the figure. The presence of the excitonic peak at about 367 nm is attributed to a minimum strain and well crystalline quality[12]. There is also a weak absorbance in the visible regime, which could be ascribed to the large amount of surface defect due to the high specific surface area for the 1D materials. The inset figure shows the optical band gap of ZnO nanorods is about 3.21 eV, which is slightly smaller than the well-known band gap of 3.37 eV for bulk ZnO.
3.3 Photocatalytic Studies
ZnO is a very promising photocatalyst in the area of photodegradation of organic contaminant under UV irradiation[5]. However, because of the difficulty of the recycle for the conventional powder photocatalyst from the aqueous suspension[5], well-aligned 1D ZnO nanostructures on the substrates would be more stable and convenient for reuse especially under the air atmosphere. It will overcome the disadvantage of ZnO powder and extend the application area.
To demonstrate the photocatalytic activity of the as-synthesized ZnO nanorods, the photocatalytic degradation reaction for the methyl violet in the air was examined. The substrate was irradiated with light from a mercury lamp. Time-dependent UV-Vis absorption spectra of the methyl violet on ZnO arrays are shown in Fig.3(a). The characteristic absorption peak of methyl violet at about 586 nm was chosen as a monitored parameter for the photocatalytic degradation process. An apparent decrease of the absorption intensity with a shift of the peak position to shorter wavelengths could be observed after a certain time of irradiation. After being irradiated for 145 min, only 5% methyl violet remained on the sample surface, and the inset in Fig.3(b) shows that almost no change was observed in absorption spectra after irradiation for 1 h without ZnO nanorods, which shows the ZnO nanorods clearly worked as an effective photocatalyst. The plot ofC/C0versustime shown in Fig.3(b) suggests that the photodecomposition reactions follow pseudo-first-order rate law[13]. The calculated rate constant of the degradation is 0.021/min. To demonstrate the photocatalyst stability of these nanorods, a recyclable photocatalysts experiment was further examined. In each cycle, the substrate was first rinsed with deionized water to completely remove the residual molecules. Then, we carried out the photocatalytic test for 30 min. Finally, the absorption spectrum was obtained. As shown in Fig.4(a), after 5 cycles the nanorods do not exhibit an evident decrease in the photodecomposition rate.

Fig.3 (a) Optical absorption spectra of methyl violet as a function of irradiation time. (b) Fraction of remaining molecules with respect to time.
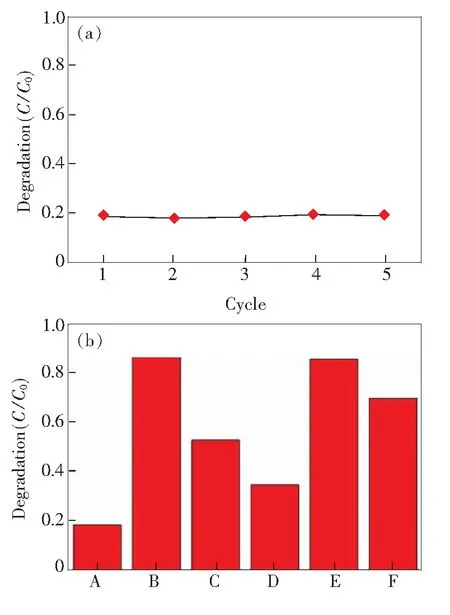
Fig.4 (a) Test of absorbance changes as a function of irradiation time over 5 cycles for methyl violet. Absorbance values at 586 nm was used. In each cycle, spectra were taken before and after irradiation for 1 h. (b) Fraction of remaining molecules after irradiation for 1 h under different atmosphere (A: air. B: vacuum. C: oxygen without water vapor. D: oxygen with water vapor. E: nitrogen without water vapor. F: nitrogen with water vapor.).
To further understand the mechanism of the photocatalyst reactions in the solid condition, we designed a group of experiments carried out under different atmospheres (as shown in Fig.4(b)). First, the degradation experiments under the air atmosphere and in vacuum were carried out. 18.5% and 87.3% residue remained after the irradiation of 1 h, respectively. It is proposed that the small fraction photodegradated in vacuum can be attributed to the residual air in the vessel. As we know, the major components in the air are nitrogen and oxygen. Therefore, the degradation experiments were continued under the nitrogen and oxygen atmospheres, respectively. Under the oxygen atmosphere only 47.2% methyl violet was decomposed and under the nitrogen atmosphere 14.4% methyl violet was decomposed after the irradiation of 1 h. The results from the nitrogen experiment are consistent with the vacuum experiment. The photocatalytic activity was much higher under the oxygen atmosphere than that under the nitrogen atmosphere.
Generally, the photocatalytic experiments were carried out in the water solution, and the —OH group was essential in the degradation process. Considering this point we continue to carry out our experiments under different atmospheres with or without water vapor, since the water vapor is the precursor of hydroxyl radical. As shown in Fig.4(b), the photocatalytic activity is enhanced after adding the water vapor. With introducing the water vapor, 65.4% and 30.3% methyl violet are decomposed under the oxygen and nitrogen atmosphere after the irradiation of 1 h, respectively. All the above experiments demonstrate that the water vapor plays an important role in the photodecomposition process.
Based on the above experiments, the possible mechanism of the photodecomposition in the air could be described as follows[14-15]. The photogenerated electrons in the conduction band of ZnO nanorods could be captured by the molecular oxygen O2to form the superoxide radical anion O2-and the hydrogen peroxide H2O2. Then the hydroxyl radical OH· could be produced, which is a powerful oxidizing agent to the organic pollutions. Because the oxygen and the water take part in these reactions, both of them are needed to the photodecomposition process in the air for ZnO nanorods.
e-+ O2→ O2*-,
O2*-+ H2O → HO2*+ OH-,
HO2*+ H2O → H2O2+ OH*,
H2O2→ 2OH*,
OH*+ organic dye → CO2+ H2O.
4 Conclusion
Insummary,ZnOnanorodswerefabricatedbythehydrothermalapproach.TheXRDpatternsandSEMimagesillustratedthehighlyorientedarraysonthesubstrates.TheresultsfromthePLspectrashowtheoxygen-vacancy-relateddefectwhichisbeneficialforphotocatalyticactivity.Thephotocatalyticpropertieshavebeenstudiedundertheairatmosphereconditionandthesenanorodshavebeendemonstratedaseffectiveandrecyclablephotocatalysts.Thisstudyshowsthatthephotocatalyticactivitywaseffectbythecomponentsofvapor,especiallyoxygenandwatervaporwhichcouldeffectivelyenhancethephotodecompositionrate.
[1] YAN R X, GARGAS D, YANG P D. Nanowire photonics [J].Nat.Photon., 2009, 3(10):569-576.
[2] LAW M, GREENE L E, JOHNSON J C,etal.. Nanowire dye-sensitized solar cells [J].Nat.Mater., 2005, 4(6):455-459.
[3] MCALPINE M C, FRIEDMAN R S, JIN S,etal.. High-performance nanowire electronics and photonics on glass and plastic substrates [J].NanoLett., 2003, 3(11):1531-1535.
[4] ZHOU W M, YAN L J, WANG Y,etal.. SiC nanowires: a photocatalytic nanomaterial [J].Appl.Phys.Lett., 2006, 89(1):013105.
[5] YANG J L, AN S J, PARK W I,etal.. Photocatalysis using ZnO thin films and nanoneedles grown by metal-organic chemical vapor deposition [J].Adv.Mater., 2004, 16(18):1661-1664.
[6] ZOU C W, RAO Y F, ALYAMANI A,etal.. Heterogeneous lollipop-like V2O5/ZnO array: a promising composite nanostructure for visible light photocatalysis [J].Langmuir, 2010, 26(14):11615-11620.
[7] KUO T J, LIN C N, KUO C L,etal.. Growth of ultralong ZnO nanowires on silicon substrates by vapor transport and their use as recyclable photocatalysts [J].Chem.Mater., 2007, 19(21):5143-5147.
[8] WU Y J, YAN H Q, YANG P D. Semiconductor nanowire array: potential substrates for photocatalysis and photovoltaics[J].Top.Catal., 2002, 19(2):197-202.
[9] BAGNALL D M, CHEN Y F, ZHU Z,etal.. High temperature excitonic stimulated emission from ZnO epitaxial layers[J].Appl.Phys.Lett., 1998, 73(8):1038-1040.
[10] JUNG S W, PARK W I, CHEONG H D,etal.. Time-resolved and time-integrated photoluminescence in ZnO epilayers grown on Al2O3(0001) by metalorganic vapor phase epitaxy [J]. Appl.Phys.Lett., 2002, 80(11):1924-1926.
[11] WAN Q, WANG T H, ZHAO J C. Enhanced photocatalytic activity of ZnO nanotetrapods [J].Appl.Phys.Lett., 2005, 87(8):083105.
[12] MRIDHA S, BASAK D. Effect of thickness on the structural, electrical and optical properties of ZnO films [J].Mater.Res.Bull., 2007, 42(5): 875-882.
[13] BASU M, SINHA A K, PRADHAN M,etal.. Evolution of hierarchical hexagonal stacked plates of CuS from liquid-liquid interface and its photocatalytic application for oxidative degradation of different dyes under indoor lighting [J].Environ.Sci.Technol., 2010, 44(16):6313-6318.
[14] XIAO M W, WANG L S, WU Y D,etal.. Preparation and characterization of CdS nanoparticles decorated into titanate nanotubes and their photocatalytic properties [J].Nanotechnology, 2008, 19(1):015706.
[15] WANG J Y, LIU Z H, ZHENG Q,etal.. Preparation of photosensitized nanocrystalline TiO2hydrosol by nanosized CdS at low temperature [J].Nanotechnology, 2006, 17(18):4561-4566.

余冰冰(1992-),女,江西上饶人,硕士研究生,2014年于吉林大学获得学士学位,主要从事ZnO基纳米材料与器件的研究。
E-mail: ybbcareer@163.com

赵东旭(1974-),男,辽宁新民人,研究员,博士生导师,2002年于中科院长春光机所获得博士学位,主要从事ZnO基纳米材料与器件的研究,2006年获得第六届长白青年科技奖特别奖。
E-mail: zhaodx@ciomp.ac.cn
2016-10-22;
2016-11-28
国家自然科学基金重点项目(50532050); “973” 国家重点基础研究发展计划(2006CB604906); 国家自然科学基金青年科学基金(60506014)资助项目 Supported by Key Project of National Natural Science Foundation of China(50532050); “973”National Basic Research Program of China(2006CB604906); National Natural Science Foundation of China for Youth(60506014)
空气中氧化锌基可循环使用光催化剂
余冰冰1,2, 徐志堃3, 王 飞1, 王云鹏1, 赵东旭1*
(1. 发光学及应用国家重点实验室 中国科学院长春光学精密机械与物理研究所, 吉林 长春 130033;2. 中国科学院大学, 北京 100049; 3. 哈尔滨师范大学, 黑龙江 哈尔滨 150025)
采用水热方法在蓝宝石衬底上制备出垂直衬底生长的氧化锌纳米柱阵列。分别通过扫描电镜、X射线衍射和光致发光光谱等表征了氧化锌纳米柱阵列的形貌、结构和光学性质。这种纳米柱在空气中对甲基紫显示出良好的光催化性能。空气中的氧气和水蒸气对光降解性能有重要的影响。这种纳米柱对于固态污染物的光降解处理有着潜在的应用前景,是一种高效、可循环使用的光催化剂。
氧化锌; 水热方法; 光催化剂
1000-7032(2017)07-0911-06
O484.4 Document code: A
10.3788/fgxb20173807.0911
*Corresponding Author, E-mail: zhaodx@ciomp.ac.cn

