Characterization,Photocatalytic Property and Kinetics of ZnO Nanoparticles Synthesized by One Step Solid State Reaction
HE Deng-LiangTAN Zi-XiangTIAN Qi
(School of Chemistry and Chemical Engineering,Mianyang Normal University,Mianyang,Sichuan 621000,China)
Characterization,Photocatalytic Property and Kinetics of ZnO Nanoparticles Synthesized by One Step Solid State Reaction
HE Deng-Liang*TAN Zi-XiangTIAN Qi
(School of Chemistry and Chemical Engineering,Mianyang Normal University,Mianyang,Sichuan 621000,China)
ZnO nanoparticles were synthesized by one step solid state reaction using only zinc sulfate heptahydrate and sodium hydroxide.The synthesized samples were characterized with various analysis methods including of X-ray diffraction(XRD),Fourier transform infrared absorption spectroscopy(FTIR),Thermogravimetric analysis(TG),Scanning electron microscope(SEM),Transmission electron microscopy(TEM),N2adsorpationdesorpation,and Ultraviolet visible diffuse reflectance spectrum(UV-Vis DRS).The experimental results indicate that the dosage of NaOH is an important parameter in the process,which can affect the composition,crystallinity and morphology of the ZnO nanoparticles.Mean while,the formation scheme of ZnO nanoparticles includes that ZnSO4·7H2O and NaOH were grinded to be full contact,then ZnSO4·7H2O reacted with NaOH to form Zn4SO4(OH)6·5H2O.Subsequently,ZnO crystal nucleus would be generated and grown into a ZnO sheets by the sufficient heat of solution from NaOH.In the final,the photocatalytic property and kinetics were evaluated by the photodegradation of methyl orange.The ZnO nanoparticles exhibit excellent photocatalytic activity under ultraviolet light.The photodegradation of methyl orange by ZnO nanoparticles conforms to the pseudo first order kinetics.
ZnO;nanoparticles;one step solid state reaction;photocatalytic property;kinetics
0 Introduction
At present,large amounts of organic pollutants such as organic dyes,organic pesticide and other organic matters had severely polluted the environment throughdifferentways.Theseorganicpollutants discharged into environment are toxic,hazardous, persistent and potentially carcinogenic for human body[1].Hence,the treatment technologies of organic pollutants in the waste water have become a hotspot in the field of environmental protection.As a water purification technology,photocatalytic oxidation was developed in recent decades[1-5].Compared with the traditional water pollution control technology,the photocatalytic oxidation has the advantages of high degradationrate,lowenergyconsumption,low secondary pollution,and low cost[1-5].Semiconductor is the most important photocatalysts,which can be excited by light to generate large amounts of electronhole pairs.These electron-hole pairs can form·OH and·O2-to degrade effectively organic pollutants in the waste water[1-5].
Many photocatalysts including of TiO2,WO3, SnO2,ZrO2,ZnO,CeO2,V2O5,Fe2O3,CdS,ZnS and other semiconductors have been widely studied in the past few decades.Among these semiconductors,TiO2is considered as the most efficient photocatalyst due to its stability,non-toxic,low-cost and renewability[1-5]. ZnO presents wide direct band gap(3.37 eV)and higher exciton binding energy(60 meV),which is an important semiconductor materials[6].Hence,ZnO has been applied in many fields such as transistors[7-9], sensors[10-11],light-emitting[12-14],energy conversion and storage[15],and so on.However,many studies showed that ZnO has also excellent photocatalytic activity on organic pollutants such as acid orange 7,diazinon, metamitron,terephthalicacid,methylorange,rhodamine B,acridine orange,methylene blue,and so on[16]. Among these pollutants,the photocatalytic activities of ZnO on methyl orange have been studied deeply.For example,Souza et al synthesized ZnO nanorods which have photocatalytic activity on methyl orange under UV irradiation more than 10 h[17].Khan et al synthesized ZnO nanoglobules by a facile hydrothermal process, and its degradation rate on methyl orange was 94.3% within 150 min under UV irradiation[18].The study from Tripathy et al shows that methyl orange(~96.3%)can be decomposed by porous ZnO spheres in the 120 min[19].The study from Tripathy et al shows that the ZnO nanoneedles can decompose the methyl orange with a degradation rate of~95.4%within 140 min[20]. In another study[21],the fabricated ZnO powder can removal 80.11%methyl orange from aqueous solution under UV light irradiation.At the same time,many studies were done to improve the photocatalytic activity of ZnO by doped[22],rare metal deposition[23],etc.
Due to the important application of ZnO in many fields,various methods have been developed to prepare ZnO,suchas laser deposition methods[24],vaper transport[25],Sol-Gelmethod[26],electrodepositing method[27],microemulsionmethod[28],hydrothermal method[29]and biological synthesis[30],and so on.However,thesemethodshavethedisadvantagesof complex process,high energy-consumption,high cost, and requiring specific material&equipment[30].One step solid state reaction is novel method,which can preparenanomaterialatroomtemperature[31-32]. Compared with traditional methods,the method has the advantages of simple process,good selectivity,low pollution and low energy consumption.
In this work,one step solid state reaction was employed to synthesiz ZnO nanoparticles without any additives.Mean while,the photocatalytic property of ZnOnanoparticlesonmethylorangewasalso evaluated.
1 Experimental
1.1 Chemicals
Some chemical reagents including zinc sulfate heptahydrate(ZnSO4·7H2O,AR),sodium hydroxide (NaOH,AR),absolute ethanol(C2H5OH,AR)and methyl orange(C14H14N3NaO3S,AR)were all purchased from Chengdu Kelong Chemical Reagent Factory.
1.2 Sample preparation
ZnO nanoparticles were synthesized by one step solid state reaction.In a typical synthesis,0.025 molZnSO4·7H2O and certain quality NaOH(0.025,0.05, 0.075,0.1,0.125 mol)were mixed in agate mortar and grinded for 40 min.Then white products in agate mortar were washed with water and absolute ethanol, respectively.In the final the sample was dried at room temperature and collected for further characterization.
1.3 Characterization
X-ray diffraction patterns were recorded on a D/ max 1400 X-ray diffractometer(Rigaku,Japan)using Cu Kα(λ=0.154 06 nm)radiation at 40 kV and 100 mA(2θ=5°~80°).The thermogravimetry curves of samples in 50~800℃were recorded by SDTA851ethermal analyzer(Mettler Toledo,Switzerland),with the heating rate of 10℃·min-1.The morphology were observed by a FEI Quanta 200F scanning electron microscope and a JEM2100 transmission electron microscope.N2adsorption-desorptionisothermsof samples were obtained on a SSA7000 specific surface area measuring instrument(Builder,China).Ultraviolet visible diffuse reflectance spectrum of samples were recorded by a UV-3700 solid ultraviolet visible near infrared spectrophotometer(Shimadzu,Japan).Infrared absorption spectrums were recorded by a Spectrum One Fourier transform infrared spectrometer(PE,USA). 1.4Photocatalytic experiments
The photocatalytic experiments were done in the photochemical reactor(JOYN-GHX-D,Shanghai Joyn Electronic Co.,Ltd.).In the photocatalytic experiment, 30 mL methyl orange solution of certain concentration was added into 50 mL quartz tube.Then certain amounts ZnO nanoparticles were put into quartz tube at ambient temperature.After that,the suspension containing methyl orange and ZnO nanoparticles were dispersed evenly under ultrasonic for 10 min.Subsequently,quartz tube was placed in photochemical reactor to well mix under dark environment for 20 min,and then began to degrade methyl orange under different light source.After the degradation reaction, supernatant was obtained from suspension in the quartztubebyhighspeedcentrifugation.The concentration of methyl orange in supernatant was measured by UV-visible spectrophotometer(T6,PuXi China).
2 Results and discussion
2.1 Characterization of ZnO nanoparticles
The X-ray diffraction patterns of samples prepared under different condition are presented in Fig.1.XRD patterns show that the sample 1(nZnSO4·7H2O∶nNaOH=1∶1) exhibited intensive diffraction peaks at 7.901°,32.700°, 58.458°,16.021°,21.161°,24.222°,27.363°and 37.255°,which are consistent with the main diffraction peaks of Zn4SO4(OH)6·5H2O(JCPDS Card No.39-0688).Mean while,three weak diffraction peaks at 31.599°,34.283°and 36.121°can be observed in the XRD pattern of sample 1.When the dosage of NaOH increased to 0.05 mol(nZnSO4·7H2O∶nNaOH=1∶2),the diffraction peaks related to Zn4SO4(OH)6·5H2O disappeared, and the diffraction peaks at 31.599°,34.283°and 36.121°became stronger.
Compared with sample 2(nZnSO4·7H2O∶nNaOH=1∶2),the diffraction peaks of others samples at 31.599°,34.283° and36.121°becamemoresharpandstronger. AccordingtotheanalysisresultsfromJade5.0 software,these diffraction peaks were ascribed to ZnO (JCPDS Card No.36-1451).It is clear that the ZnO crystal could be prepared by one step solid state reaction without any additives at room temperature. According to literature[33],the(002)crystal plane is the polar surface of the ZnO crystal,and(100)and(101) is a non-polar surface.According to XRD pattern in Fig.1(1∶5),the diffraction intensity ratio of(002)polarplane to(100)nonpolar plane(I(002)/I(100))is 1.25.It indicates that the surface of ZnO crystal mainly composed of polar surface[34].
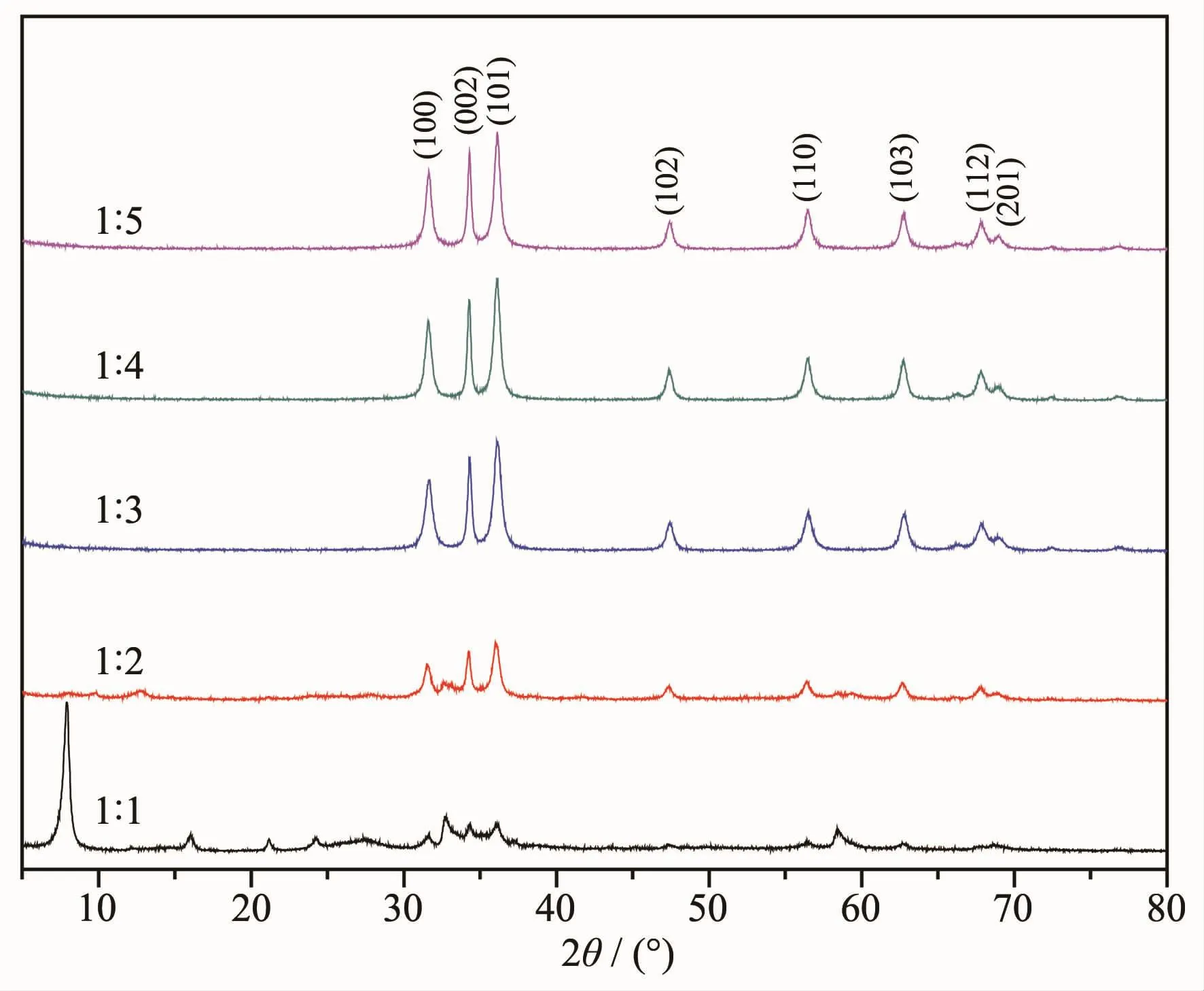
Fig.1XRD patterns of samples prepared at different conditions
Fig.2 is FTIR spectra of samples prepared at different conditions.The FTIR spectra of sample 1 (nZnSO4·7H2O∶nNaOH=1∶1)shows that a broad absorption band at around 3 430 cm-1can be observed,which come from the stretching vibration of hydroxyl group. And the peak at 1 631 cm-1belongs to the bending vibration of hydroxyl groups[35].The strong absorption peak at 1 130 cm-1come from the antisymmetric stretching vibration of[SO4]2-.Meanwhile,a medium intensity absorption peak at 603 cm-1can be observed, which belong to the bending vibration of[SO4]2-[36-37]. According to literature[38],the characteristic absorption peak of ZnO locates around 430 cm-1.However,there only present a very weak peak at 445 cm-1in the FTIR spectra of sample 1(nZnSO4·7H2O∶nNaOH=1∶1).So it is concluded that the main functional group in sample 1(nZnSO4·7H2O∶nNaOH=1∶1)is[SO4]2-groups and OH groups. However,compared with sample 1(nZnSO4·7H2O∶nNaOH=1∶1),two strong absorption peaks can be observed at 443 and 555 cm-1in the FTIR spectra of others samples.As reported by Xiong et al[39],the absorption peak at around 443 cm-1come from the E2mode of hexagonal ZnO,and the peak at 555 cm-1may be caused by the oxygen deficiency and oxygen vacancy defect in ZnO.It is clear that the major reaction product is ZnO when the dosage of NaOH is greater than or equal to 1∶2.Mean while,the absorption peaks at around 3 430 cm-1,1 630 cm-1and 1 384 cm-1can be observed in all the samples.These peaks should belong to the stretching and bending vibration absorption from surface hydroxyl or bridginghydroxyl[40].In addition,the absorption peak from[SO4]2-can still be observed in all the samples.
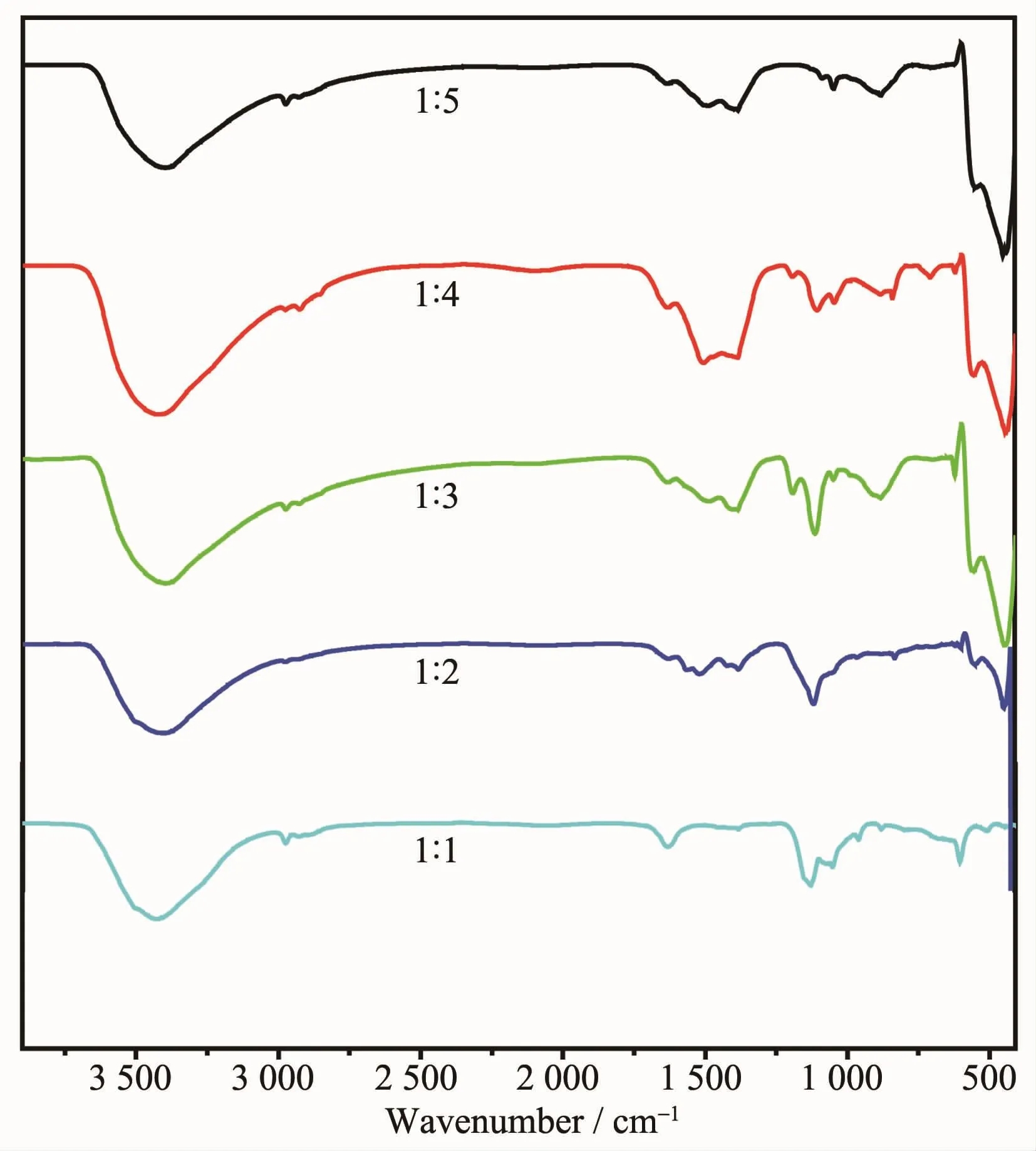
Fig.2FTIR spectra of samples prepared at different conditions
Fig.3 is the thermal weight loss curves(TG)of samples from 50 to 800℃.As shown in Fig.3,TG curve of sample 1 was obviously different from other samples.According to our observation and previous study[14,41],the weight loss at around 50~145℃were primarily resulted from the loss of adsorbed water. The weight loss at around 145~250℃might originate from the loss of the crystal water in the Zn4SO4(OH)6· 5H2O.The weight loss peak at about 250~300℃might come from loss of structural hydroxyls in the Zn4SO4(OH)6·5H2O.The weight loss peak above 300℃may come from the decomposition of ZnSO4in the Zn4SO4(OH)6·5H2O.The thermogravimetric analysis further confirms that the major product is Zn4SO4(OH)6·5H2O when dosage of NaOH is 0.025 mol.
When the dosage of NaOH increased to 0.05 mol (nZnSO4·7H2O∶nNaOH=1∶2),similar TG curve still can be observed.However,compared with sample 1(nZnSO4·7H2O∶nNaOH=1∶1),the TG curves of others samples become gentler and the weight loss decreased significantly.It indicates that the content of Zn4SO4(OH)6·5H2O in theproduct gradually decreased,and the purity of ZnO is higher with the continuing increase of NaOH dosage. According to the results from XRD,FTIR and TG,it is concluded that the ZnO prepared by this method contain a small amount of impurities and defects.
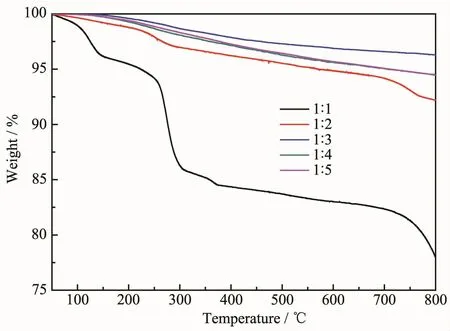
Fig.3TG curves of the samples prepared at different conditions
Fig.4 shows that the dosage of NaOH has obvious influence on the morphology of ZnO particles.As shown in Fig.4,ZnO nanoparticles(nZnSO4·7H2O∶nNaOH=1∶2)consists of aggregated nano particles.However,the images in Fig.4 show that the particles size of ZnO particles would grow and became larger with the increase of NaOH dosage.Combining Fig.4 and Fig.5, it is clear that ZnO nanoparticles are formed by the accumulation of hexagonal ZnO single crystal.Andthe morphology of ZnO single crystal presents nano sheets.
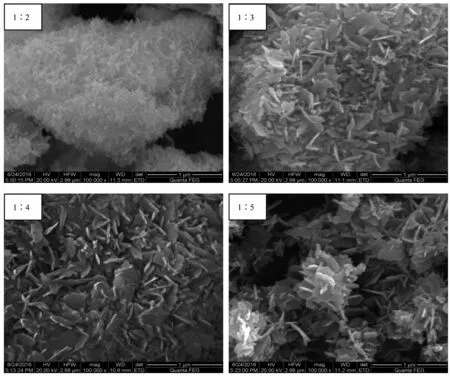
Fig.4Effect of NaOH dosage on the morphology of ZnO particles
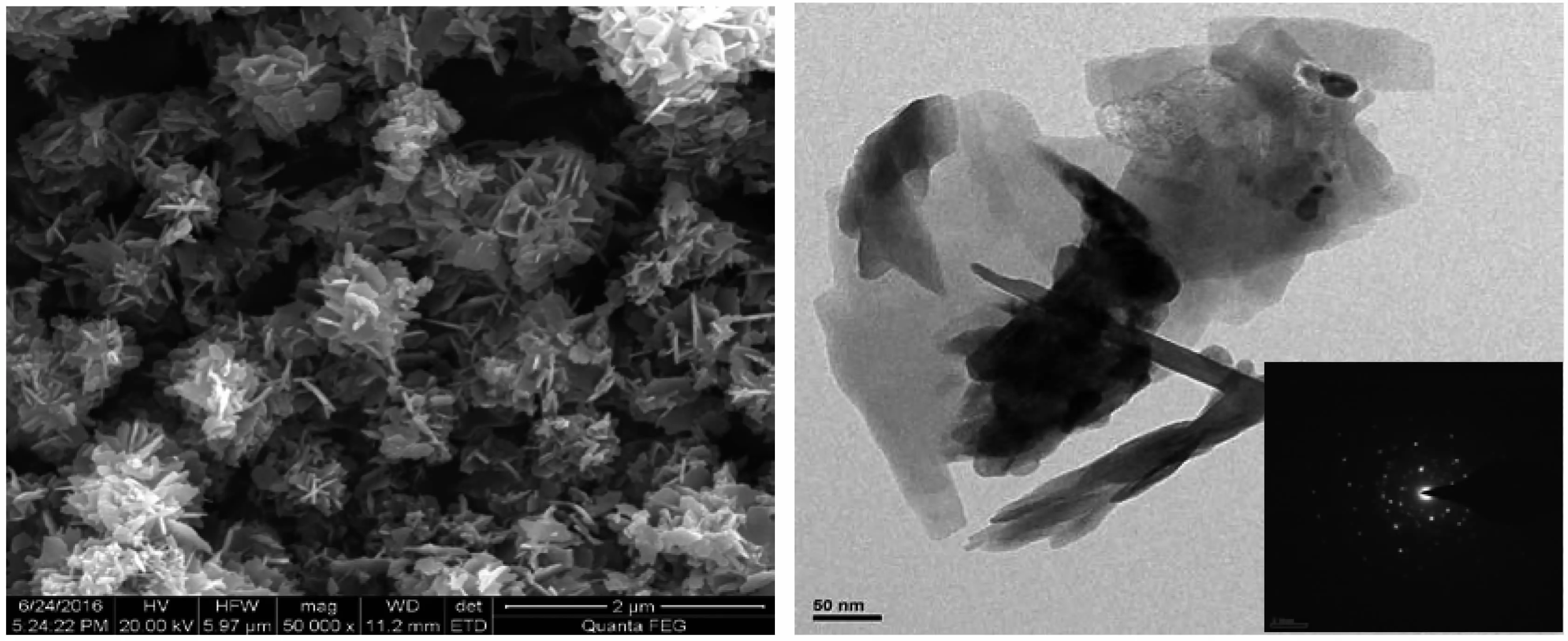
Fig.5SEM and TEM photograph of ZnO nanoparticles
Fig.6 shows that the specific surface area(SBET)of ZnO nanoparticles presented a clear dependence on NaOH dosage(18,29,25 and 23 m2·g-1,respectively). It is clear that tendency of specific surface area(SBET) correspond to their morphology and stacking states.
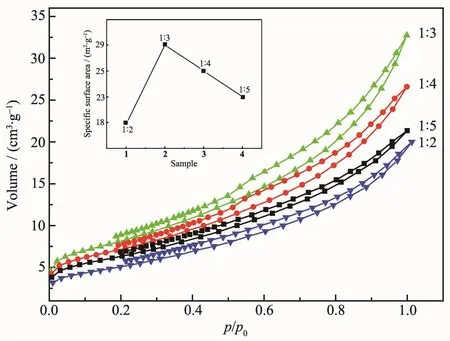
Fig.6N2adsorption-desorption isotherms of ZnO nanoparticles
TheexperimentalresultsindicatethatZnO nanoparticles can be synthesized by one step solid state reaction using only zinc sulfate heptahydrate and sodium hydroxide.In this process the dosage of NaOH is an important parameter,which can affect the composition,crystallinity and morphology of the ZnO nanoparticles.Based on the above results and other′s studies[14,42],some reactions are concluded as follows. When the dosage of NaOH was not enough,the main reaction between ZnSO4·7H2O and NaOH was showed in Eq.1.The main products is Zn4SO4(OH)6·5H2O from reaction 1.At the same time,there is only a small amount of ZnO generated in the process of reaction.AlthoughwiththeincreasesofNaOH dosage,thereactionsinEq.1andEq.2would occurred between ZnSO4·7H2O and NaOH.In the reaction 2,large amounts of ZnO crystal nucleus would be generated and grown into ZnO sheets by the sufficient heat of solution from NaOH.According to the above analysis,the formation mechanism of ZnO nanoparticles prepared by one step solid state reaction was proposed in Fig.7.
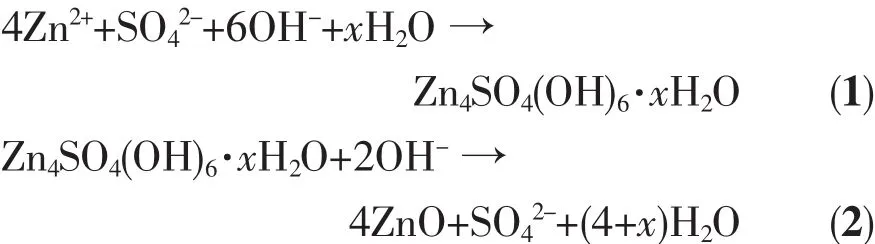
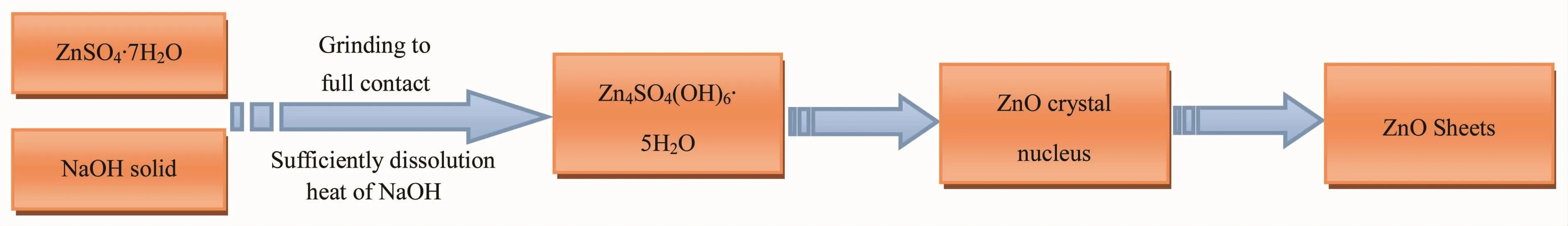
Fig.7Formation scheme of ZnO nanoparticles by one step solid state reaction
2.2 Photocatalytic activity of ZnO nanoparticles on methyl orange
As stated in the introduction,ZnO nanoparticles have been wildly applied in the filed of photocatalysis. Hence,the photocatalytic activity of ZnO nanoparticles was evaluated by the degradation of methyl orange under different light source(dark environment,xenon lamp and high pressure mercury lamp).Fig.8a is UVVis absorption spectrum of methyl orange which was adsorbedbyZnOnanoparticlesinthedark environment.As shown in Fig.8a,ZnO nanoparticles have almost no absorption for methyl orange,except for the sample 2(nZnSO4·7H2O∶nNaOH=1∶2).Fig.8b shows that ZnO nanoparticles have very low photocatalytic activity on methyl orange irradiated by xenon lamp. Meanwhile,all the samplesbothexhibitedgood photocatalytic activity on methyl orange irradiated by high pressure mercury lamp(Fig.8c).
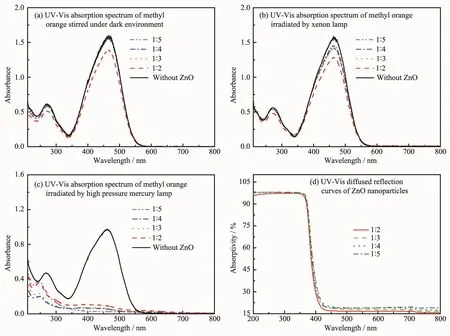
Fig.8Photoresponse characteristics and photocatalytic property of ZnO nanoparticles on methyl orange
Comparison between Fig.8a,Fig.8b and Fig.8c,it is clear that photocatalytic activity of the sample (nZnSO4·7H2O∶nNaOH=1∶2)is the lowest under ultraviolet light.Among these samples,the ZnO nanoparticles (nZnSO4·7H2O∶nNaOH=1∶5)exhibits the best photocatalytic activity under ultraviolet light.And furthermore,the photocatalytic activity of ZnO nanoparticles has a weak improvement with the increase of NaOH dosage (Fig.8c).As shown in the UV-Vis/DRS spectra(Fig. 8d),ZnO nanoparticles prepared by one step solid state reaction can effectively absorb UV light,the absorption wavelength of ZnO nanoparticles is<360 nm.In contrary,ZnO nanoparticles only have a very weak absorption capacity for visible light.At the same time,there is a progressive increase in absorptivity with the increase of NaOH dosage,which corresponds to their photocatalytic activity.The literature[33]reported that the more polar planes in ZnO,the more oxygen vacancies,and the stronger catalytic activity.Hence, it is concluded that the enhancement of photochemical degradation on methyl orange can be ascribed to an obvious improvement in the crystallinity,particle size and defects in ZnO nanoparticles.
2.3 Photocatalytic kinetics of ZnO nanoparticles on methyl orange
At present,there are a lot of researches on the photocatalytic performance and kinetics of ZnO.In photocatalytic degradation reaction of methyl orange, the first order kinetics model is widely used to describe dynamic process of photocatalyst.In this experiment,first-order-kinetics of ZnO nanoparticles synthesized by one step solid state reaction was preliminary investigated.Then Eq.3 was employed to estimate dynamic relationship for the degradation of methyl orange[43-44].

The photocatalytic experiments were done at different pH values of 3.0,5.0,7.0 and 9.0.The pH valuesofsolutionswereadjustedwithdiluted hydrochloric acid and sodium hydroxide solution.The first-order-kinetics plots for the degradation of methyl orange at different pH value were showed in Fig.9. The kinetic parameters were shown in Table 1.The data shows that photodegradation of methyl orange by ZnO nanoparticles prepared by one step solid state reaction conforms to the pseudo first order kinetics.
TheexperimentresultsindicatethatZnO nanoparticles prepared by one step solid state reaction maybeusedtophotocatalyticdegradeorganic compounds in the waste water.Furthermore,the method is simpler than traditional methods.
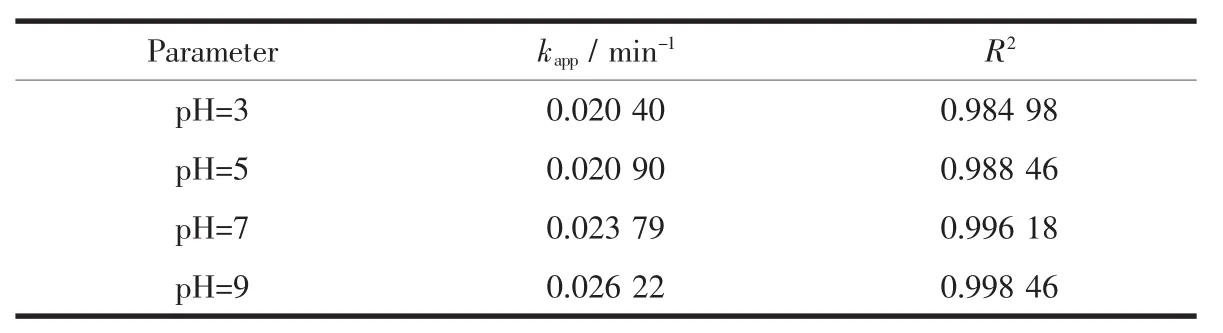
Table 1Fitting results of degradation according to pseudo first order kinetic models
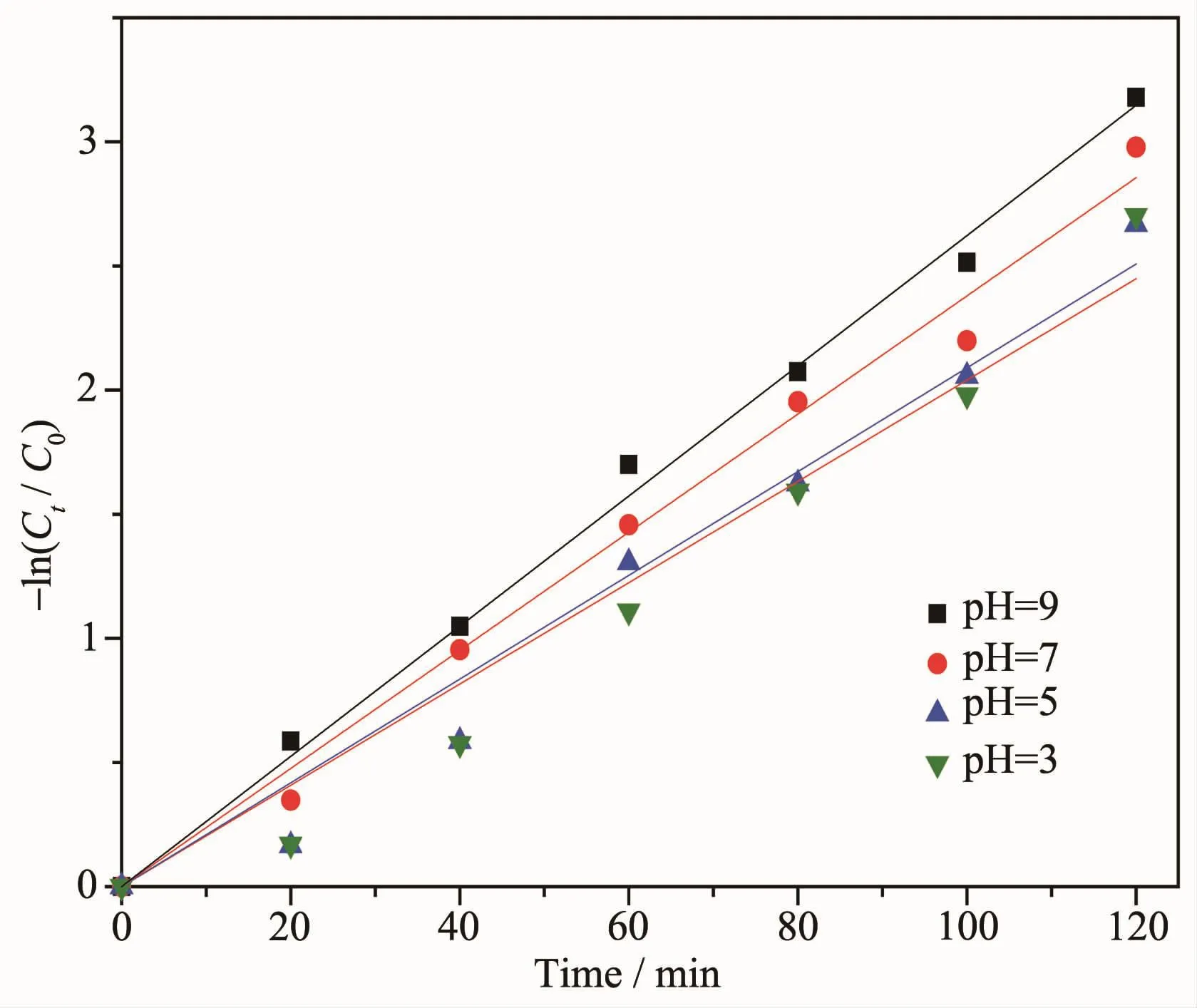
Fig.9First-order-kinetics plots for the degradation of methyl orange at different pH values
3 Conclusions
ZnO nanoparticles can be prepared by one step solid state reaction without any additives at room temperature.TheformationschemeofZnO nanoparticleswasrevealedbyvariousanalytical methods,and the dosage of NaOH is an important parameter in the synthesis process.ZnO nanoparticles prepared by one step solid state reaction may be used to photocatalytic degrade organic compounds in the waste water.
[1]Ramirez R J,Arellano C A P,Varia J C,et al.Curr.Org. Chem.,2015,19(6):540-555
[2]Zangeneh H,Zinatizadeh A A L,Habibi M,et al.J.Ind. Eng.Chem.,2015,26:1-36
[3]Chong M N,Jin B,Chow C W K,et al.Water Res.,2010,44 (10):2997-3027
[4]YU Chang-Lin(余长林),YANG Kai(杨凯),YU Jimmy C(余济美),et al.Acta Phys.-Chim.Sin.(物理化学学报),2011, 27(2):505-512
[5]Zhao Z,Song J L,Zheng J H,et al.Trans.Nonferrous Met. Soc.China,2014,24(5):1434-1439
[6]ZHANG Hui(张辉),CHEN Gui-Feng(陈贵锋),MO Zhao-Jun (莫兆军),et al.Chinese J.Lumin.(发光学报),2015,36(6): 628-633
[7]Hong W K,Sohn J I,Hwang D K,et al.Nano Lett.,2008,8 (3):950-956
[8]Kang T,Kim T,Lee G,et al.J.Mater.Chem.C,2014,2(8): 1390-1395
[9]Wang X D,Zhou J,Song J H,et al.Nano Lett.,2006,6(12): 2768-2772
[10]Hjiri M,Mir L E,Leonardi S G,et al.Sens.Actuators,B, 2014,196:413-420
[11]Chiang K C,Wu W Y,Ting J M.J.Am.Ceram.Soc.,2011, 94(3):713-716
[12]Liu J S,Shan C X,Shen H,et al.Appl.Phys.Lett.,2012, 101(1):011106-011106-4
[13]Liu Y,Xu H,Liu C,et al.Chin.Sci.Bull.,2014,59(12): 1219-1227
[14]HE Peng(何朋),GAO Xiang-Dong(高相东),WU Li-Bin(吴历斌),et al.Acta Phys.-Chim.Sin.(物理化学学报),2013, 29(4):874-880
[15]Bandyopadhyay P,Dey A,Basu R,et al.Energy Sources Part A,2016,38(13):1833-1839
[16]Adnan M A M,Julkapli N M,Hamid S B A.Rev.Inorg. Chem.,2016,36(2):77-104
[17]Souza D A R,Gusatti M,Sanches C,et al.Chem.Eng. Trans.,2013,32:2275-2280
[18]Khan R,Hassan M S,Uthirakumar P,et al.Mater.Lett., 2015,152:163-165
大观园是隐逸者的“世外桃源”。中国古代农耕社会是自给自足的小农经济生活方式,我国古代文化中一直以隐逸之士作为理想人格的象征,如:《诗经·卫风·考槃》对隐士的歌之,颂之,陶渊明只要“结庐在人境”就能“心远地自偏”,白居易“隐在留司官”,尽管各个时代隐士隐逸的方式不同,但对隐士隐逸行为的认同逐渐成为中国士大夫高雅的文化范式。所以人们为自己设计山水园林,以山水象征山林江湖,在自己创造的“第二自然”里构建精神上的“世外桃源”。
[19]Tripathy N,Ahmad R,Kuk H,et al.J.Photochem.Photobiol. B,2016,161:312-317
[20]Tripathy N,Ahmad R,Song J E,et al.Mater.Lett.,2014, 136:171-174
[21]Alzahrani E.Curr.Anal.Chem.,2016,12(5):465-475
[22]Zong Y Q,Li Z,Wang X M,et al.Ceram.Int.,2014,40(7): 10375-10382
[23]HAN Jing(韩婧),SHI Li-Yi(施利毅),CHENG Rong-Ming (成荣明),et al.Chinese J.Inorg.Chem.(无机化学学报), 2008,24(6):950-955
[24]Jamal R K,Hameed M A,Adem K A.Mater.Lett.,2014,132: 31-33
[25]Huang M H,Wu Y Y,Feick H,et al.Adv.Mater.,2001,13 (2):113-116
[26]Rani S,Suri P,Shishodia,P K,et al.Energy Mater.Sol. Cells,2008,92(12):1639-1645
[28]Altntas Y Ö,Durucan C.J.Alloys Compd.,2010,506(2):944 -949
[29]LI Li(李丽),LIU Xiao-Ming(刘晓明),ZHOU Shu-Ting(周舒婷),et al.Chinese J.Inorg.Chem.(无机化学学报),2016,32 (2):241-249
[30]Z˙elechowska K.J.Biotechnol.,Comput.Biol.Bionanotechnol., 2014,95(2):150-159
[31]Sun Z P,Liu L,Zhang L,et al.Nanotechnology,2006,17 (9):2266-2270
[32]Ye X R,Jia D Z,Yu J Q,et al.Adv.Mater.,1999,11(11): 941-942
[33]Li G R,Hu T,Pan G L,et al.J.Phys.Chem.C,2008,112 (31):11859-11864
[34]YANG Shou-Bin(杨守斌),DI Wang-Ming(邸琬茗),NING Wen-Sheng(宁文生),et al.Mod.Chem.Ind.(现代化工), 2014,34(6):101-104
[35]Wu L,Wu Y,Pan X,et al.Opt.Mater.,2006,28(4):418-422
[36]YAN Wei(闫蔚),ZENG Bo-Lin(曾柏淋),MENG Jiang(孟江),et al.Spectrosc.Spectral Anal.(光谱学与光谱分析), 2016,36(7):2098-2103
[37]PENG Yu-Xuan(彭玉旋).Xinjiang Geol.(新疆地质),2015, 33(1):130-133
[38]CHEN Xiao-Gang(陈小刚),HE Yun-Qiu(贺蕴秋),ZHANG Qiong(张琼),et al.Chinese J.Inorg.Chem.(无机化学学报), 2009,25(11):1953-1959
[39]Xiong G,Pal U,Serrano J G,et al.Phys.Status Solidi C, 2006,3(10):3577-3581
[40]WU Zhi-Fu(武志富),LI Su-Juan(李素娟).Chinese J. Spectrosc.Lab.(光谱实验室),2012,29(4):2172-2715
[41]Chen G P,Sang S B,Huang K L,et al.Trans.Nonferrous Met.Soc.China,2004,14(3):456-458
[42]Hou Q,Zhu L Q,Chen H N,et al.Electrochim.Acta,2012, 85:438-443
[43]YANG Yue(杨悦),ZHAO Cui-Zhen(赵翠真),YU Chen-Guang(于晨光),et al.Chinese J.Lumin.(发光学报),2014, 35(12):1449-1454
[44]LI Xiu-Yan(李秀艳),WANG Jian(王健).J.Jilin Normal Univ.:Nat.Sci.Ed.(吉林师范大学学报:自然科学版), 2011,32(4):13-16
室温一步固相反应合成纳米氧化锌的表征、光催化性能及动力学
何登良*谭自香 田奇
(绵阳师范学院化学与化学工程学院,绵阳621000)
以七水硫酸锌、氢氧化钠为原料,采用室温一步固相反应合成ZnO纳米粒子,并分别利用X射线衍射分析(XRD)、傅里叶变换红外光谱分析(FTIR)、热重分析(TG)、扫描电子显微分析(SEM)、透射电子显微分析(TEM)、N2吸附-脱附、紫外可见漫反射光谱分析(UV-Vis DRS)等方法对ZnO纳米粒子进行表征。实验结果表明:不需任何添加剂,室温下可通过一步固相反应合成ZnO纳米粒子,其形成过程首先是ZnSO4·7H2O和NaOH充分接触,然后反应形成Zn4SO4(OH)6·5H2O,最后NaOH的溶解热可使Zn4SO4(OH)6·5H2O转变为ZnO并逐渐长大形成纳米粒子。同时以甲基橙为降解对象评价了ZnO纳米粒子的光催化活性,实验结果表明:紫外光照射下,该方法合成的ZnO纳米粒子对甲基橙具有较好的光催化活性,且光催化动力学方程符合准一级反应动力学。
氧化锌;纳米粒子;一步固相反应;光催化性能;动力学
O614.24+1
A
1001-4861(2017)06-1065-09
2017-01-08。收修改稿日期:2017-04-01。
10.11862/CJIC.2017.142
国家自然科学基金(No.51004066)和绵阳师范学院科研启动项目(No.QD2013A02)资助。
*通信联系人。E-mail:449011902@qq.com,Tel:+86-13458443537
- 无机化学学报的其它文章
- CoAl2O4/蜂窝陶瓷催化剂的制备及其催化臭氧化性能
- Photocatalytic Hydrogen Production Based on Cobalt-Thiosemicarbazone Complex with the Xanthene Dye Moiety
- 两种金属-有机钙钛矿材料的负热膨胀性质
- Pyrazolate-Based Dipalladium(Ⅱ,Ⅱ)Complexes:Syntheses,Characterization and Catalytical Performance in Suzuki-Coupling Reaction
- 以滤纸为模板合成新型介孔生物活性玻璃微管材料
- Br-掺杂Bi2WO6的水热法合成及其可见光催化性能

