Photocatalytic Hydrogen Production Based on Cobalt-Thiosemicarbazone Complex with the Xanthene Dye Moiety
YANG Lin-LinJING XuHE ChengDUAN Chun-Ying
(State Key Laboratory of Fine Chemicals,Dalian University of Technology,Dalian,Liaoning 116024,China)
Photocatalytic Hydrogen Production Based on Cobalt-Thiosemicarbazone Complex with the Xanthene Dye Moiety
YANG Lin-LinJING XuHE Cheng*DUAN Chun-Ying
(State Key Laboratory of Fine Chemicals,Dalian University of Technology,Dalian,Liaoning 116024,China)
Two cobalt complexes containing NSP(nitrogen-sulphur-phosphor)chelator prepared from thiosemicarbazone ligands with different terminal functional groups and triphenylphosphine moiety have been synthesized in high yield and characterized.Their photocatalytic activity for hydrogen evolution under visible light irradiation was investigated.The photocatalyst[Co(L2)(L2′)](BF4)2.5·H2O·0.5C2H5OH(2)(L2=2-(diphenylphosphino)benzylidene-2-(3,6-bis(ethylamino)-2,7-dimethyl-9H-xanthen-9-yl)benzothiohydrazide,L2′=2-(diphenylphosphinooxide)benzylidene-2-(3,6-bis(ethylamino)-2,7-dimethyl-9H-xanthen-9-yl)benzothiohydrazide) containing rhodamine groups exhibited activity in light driven hydrogen evolution with the TON and initial TOF reaching to 2 800 molH2·and 930 molH2··h-1,respectively,which is much higher than that of complex [Co(L1)(L1′)](BF4)·0.5H2O(1)(L1=2-(diphenylphosphino)benzylidene hydrazinecarbodithionate,L1′=2-(diphenylphosphinooxide)benzylidene hydrazinecarbodithionate)under similar conditions.The higher catalytic activity was attributed to the potential intermolecular π-π stack interactions between the catalyst 2 and photosensitizer fluorescein(Fl)benefiting the photoinduced electron transfer between the photosensitizer and the photocatalyst. CCDC:1523357;1,1523358,2.
photoinduced electron transfer;cobalt;thiosemicarbazone;light driven H2production
0 Introduction
The global increase in CO2emissions due to uninhibited fossil fuel use has led to explore new renewable and clean energy resources.Photo-induced catalytic splitting of water to non-carbon-contain fuels, i.e.hydrogen,is a promising approach in converting solar energy into storable chemical energy[1-5].As a redox reaction,water splitting can be divided into two reactions:(i)water oxidation,to yield O2and(ii)water reduction,producing H2.In an artificial photosynthetic (AP)system,the reductive side of water splitting is the light-driven generation of hydrogen from aqueous protons.A variety of photocatalytic systems have been broadly investigated for solar hydrogen production[6-13]. MostofthephotocatalyticH2-productionsystems involve three important components:a light-absorbing photosensitizer(PS),a water-reducing catalyst(WRC), and a sacrificial reductant.While noble metal cocatalysts such as Pt and Pd were widely used for photocatalytic H2evolution,but the scarcity and high cost of precious metals pose serious limitations to wide use.Hence,the development of efficient catalysts for water reduction to H2made of inexpensive,earthabundant elements such as Fe,Co,Ni,and Cu has become a prime focus of research on light-driven hydrogen generation.
In an effort to find stable earth-abundant WRCs, a few recent reviews on catalysts made of earthabundant elements for water splitting have been published[14-16].In particular,thiosemicarbazone ligands have been used as versatile molecules,arising from the possibility to act as N,S-donor systems[17-18].And the number of potential donor atoms can be increased by using carbonylic compounds that contain additional donorgroupsinsuitablepositionsforchelation. Moreover,they provide a conventional proton immigration path through the thiolate/thioamide resonance tautomer equilibrium[19-20],which have potential future for multi-electron transfer chemistry in H2reduction. And these cobalt thiosemicarbazone complexes have gainedmuchinterestandmightbepromising candidates for proton reduction,in the case that the redox potential of the complex was well modified[21-22]. In this article,we introduced a triphenylphosphine moietyastheadditionaldonortoenhancethe coordination ability and modify the redox potential of the metal centres(Scheme 1)[23-24].Recent advances have reported Ni-bis(diphosphine)complexes act as effectivereductioncatalystsforelectrochemical photocatalytical hydrogen production in homogeneous system[25-26].We expected that the presence of S and P donors would possibly facilitate the formation of reducing species and increase the catalytic efficiency for the H2evolution[27].Herein,we introduced the rhodamine-modifiedligandstoimprovethelight absorption efficiency and electron transfer ability between the catalyst 2 and photosensitizer Fl.The photocatalytic activity of catalyst 2 was higher than that of 1 under low catalyst concentration.The photoinduced electron transfer pathway mechanisms for the hydrogen evolution in these cobalt-thiosemicarbazone complexes were also proposed.
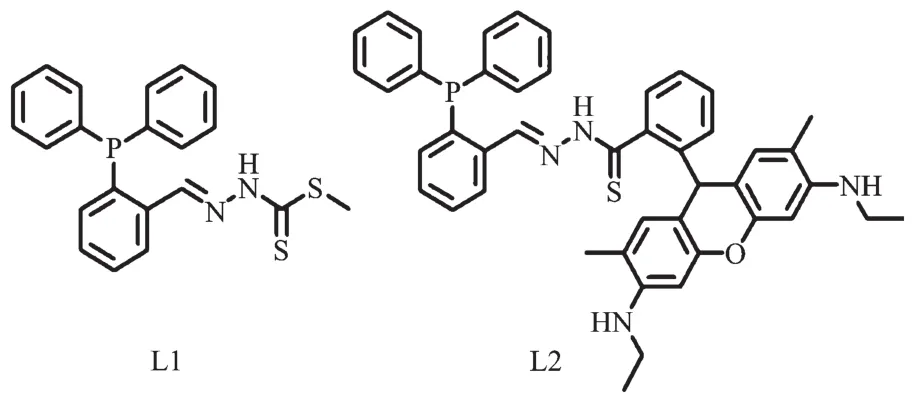
Scheme 1Structures of thiosemicarbazone ligands L1 and L2
1 Experimental
1.1 Materials and physical measurements
All chemicals were of reagent grade quality obtained from commercial sources and used without further purification.The elemental analyses of C,H and N were performed on a Vario ELⅢelemental analyzer.1H NMR spectra were measured on a Varian INOVA 400M spectrometer.ESI mass spectra were carried out on a HPLC-Q-Tof MS spectrometer.The fluorescent spectra were measured on a JASCO FP-6500.
CyclicVoltammogrammeasurements:Electrochemical measurements of catalysts 1 and 2 were performed in CH3CN solutions with 0.1 mol·L-1TBAPF6workingonZAHNERENNIUMElectrochemical Workstation with ahomemadeAg/AgCl electrode as a reference electrode,a platinum silk with 0.5 mm diameter as a counter electrode,and glassy carbon electrode as a working electrode.The addition of NEt3HCl(0.1 mol·L-1in CH3CN)was carried out with syringe.
Fluorescence quenching experiments:A solution (2.0 mL)of Fl at 10 μmol·L-1concentration in a EtOH/H2O solution(1∶1,V/V,pH=11.6)was prepared in a quartz cuvette fitted with a septum cap,and the solution was degassed under N2for 5 min.Aliquots of 20 μL of NEt3and the catalysts(2.5 μmol·L-1)were added,and the intensity of the fluorescence was monitored by steady state fluorescence exciting at 460 nmonaSpexFluoromax-Pfluorimeterwitha photomultiplier tube detector.
Photocatalytic water splitting experiments:Varying amounts of the catalysts 1 and 2,Fl and NEt3in EtOH/H2O solution(1∶1,V/V)were added to obtain a total volume of 5.0 mL in a 20 mL flask.The flask was sealed with a septum and degassed by bubbling argon for 15 min.The pH value of this solution was adjusted to a specific pH value by adding HCl or NaOH and measured with a pH meter.After that,the samples were irradiated by a 500 W Xenon lamp with the 400 nm light filter,and the reaction temperature was maintained at 293 K by using a thermostat water bath.The generated photoproduct of H2was characterized by GC 7890T instrument analysis using a 5A molecular sieve column(0.6 m×3 mm),thermal conductivity detector,and argon used as carrier gas. The amount of hydrogen generated was determined by the external standard method.
1.2 Synthesis of L1 and L2
The ligand L1 was synthesized by using the reported methods[28].Anal.Calcd.for C21H19N2PS2(%): H,4.85;C,63.9;N,7.1;Found(%):H,5.10;C,63.3; N,7.2.1H NMR(CDCl3,400 MHz):10.2(s,1H),8.6 (d,1H),8.2(m,1H),7.1~7.5(m,13H),2.6(s,3H). API-MS m/z:393.07([M-H]+).
The ligand L2 was synthesized according to procedures reported previously[29].Anal.Calcd.for C45H43N4SOP(%):H,6.03;C,75.2;N,7.8;Found(%): H,6.11;C,74.9;N,8.0.1H NMR(CDCl3,400 MHz): 9.37(s,1H),8.4(t,1H),8.11(d,1H),8.08(m,1H), 7.6~7.7(m,4H),7.4~7.6(m,10H),7.38(m,3H),7.3 (m,2H),6.5(s,2H),6.2(s,2H),3.1(m,2H),1.13(m, 3H);API-MS m/z:715.0([M-H]+).
1.3 Synthesis of complex 1
The ligand L1(0.6 mmol,0.236 g)and Co(BF4)2· 6H2O(0.3 mmol,0.1 g)was dissolved in 10 mL ethanol.The solution was refluxed for 2 h,and then slowly evaporated at room temperature.The red block crystals were obtained in several days.Yield:~55%. ESI-MS m/z:845.23 for the+1 charge cation.Anal. Calcd.for CoC42H37N4OP2S4BF4·0.5H2O(%):H,3.99;C, 52.6;N,5.84;Found(%):H,4.02;C,52.1;N,5.89. IR(KBr,cm-1):3 424(br),3 056(m),1 630(s),1 586 (s),1 480(m),1 460(m),1 436(m),1 313(s),1 127 (m),1 063(m),1 012(m),875(s),754(m),727(m), 696(m),562(m),526(m),498(m).
1.4 Synthesis of complex 2
Co(BF4)2·6H2O(0.05 mmol,0.017 g)and the ligand L2(0.05 mmol,0.035 g)was dissolved in 15 mL of dichloromethane/ethanol(1∶1,V/V).The solution was stirred at boiling temperature for 2 h to obtain a clear black red solution and allowed to stand at room temperature.Theredblockcrystalssuitablefor single-crystal X-ray diffraction were obtained.Yield:~40%.ESI-MS m/z:(2L2+Co)3+,497.16;(2L2+Co+BF4)2+, 789.76.Anal.Calcd.for CoC91H85.5N8O3P2S2B2.50F10·H2O ·0.5C2H5OH(%):H,5.15;C,61.7;N,6.33;Found(%): H,5.20;C,61.1;N,6.37.IR(KBr,cm-1):3 243(br), 2 973(s),1 647(m),1 606(m),1 560(m),1 527(m), 1 499(m),1 446(s),1 366(s),1 306(m),1 243(m), 1 186(m),1 124(m),1 083(m),944(s),882(s),748 (s),693(m),611(m),522(s).
1.5 X-ray crystallography
The data were collected on a Bruker Smart APEXⅡX-diffractometer equipped with graphite monochromatedMo Kα radiation(λ=0.071 073 nm) using the SMART and SAINT[30]programs at 296 K for complexes 1 and 2.Final unit cell parameters were based on all observed reflections from integration of all frame data.The structures were solved in thespace group by direct method and refined by the fullmatrix least-squares using SHELXTL-97 fitting on F2[31]. For complexes 1 and 2,all non-hydrogen atoms were refinedanisotropically.Exceptthesolventwater molecules,the hydrogen atoms of organic ligands were located geometrically and fixed isotropic thermal parameters.The BF4-groups in complex 2 were disordered; therefore,large thermal displacement parameters were foundfortheseatomsandrefinedwithpartialoccupancy. One of the benzene rings was disordered into two parts with the S.O.F.(sites occupied factor)of each part being fixed as 0.5.One ethylamine moiety and ethyl groups in complex 2 were disordered into two parts with the S.O.F.of each part being fixed as 0.5. The crystal data and details of the structure refinement of complexes 1 and 2 are summarized in Table 1.
CCDC:1523357;1,1523358,2.
Table 1Crystal data and structure refinements for complexes 1 and 2
2 Results and discussion
2.1 Crystal structural description
Single-crystalstructurerepresentationforthe complexes 1 and 2 are shown in Fig.1.In the crystal of complex 1,each central cobalt environment can be described as a distorted octahedron,comprising two N, S atoms from thiosemicarbazone groups,one P atoms from the triphenylphosphine group and the O atom originated from oxidized P atom.One of the P atoms in the ligand L1 was oxidized under the synthesis process.The two ligands bind to a cobalt(Ⅱ)in a mer configurationasfoundintherelatedcobalt thiosemicarbazone complexes[32-33].The C-S,C-N and N-N bond distances are agreed well with the normal range of single and double bonds,resulting in the extensiveelectrondonationenvironmentoverthe entire molecular skeleton[34-35].Similar to the complex 1,in the crystal of complex 2,the metal center was octahedrally coordinated by two N,S atoms and a P or O chelator atom that originated from the different ligands L2 and L2′.And the distance between two xanthene rings of rhodamine ligands in adjacent complex was 0.36 nm.The presence of intermolecular strongπ-πstackinginteractionsbetweenthe rhodaminegroupsimpliedthepossibilityontheformation of π-π interaction between complex 2 and the xanthene rings within the photosensitizer Fl.As a result,the photogenerated electron would be easily immigratedbetweenthephotosensitizerandthe photocatalyst during the photoreduction processes, which was beneficial to achieve the high activity in the photocatalytic system[36].

Fig.1 Single-crystal structure representations of 1(a)and 2(b)showing the coordination geometry of the metal ion
2.2 Cyclic voltammetry of the complexes
The cyclic voltammetry shows that the redox potential of Co(Ⅱ)/Co(Ⅰ)occurs at-1.0 V and-0.87 V for 1 and 2,respectively.And the complex 2 exhibits one inreversible Co(Ⅱ)/Co(Ⅰ)redox process at-0.6 V. To investigate the role of the Co complex as a WRC catalyst,cyclic voltammetry was performed in the presence of increasing amounts of NEt3HCl.Addition of NEt3H+with increasing amounts triggers the appearance of a new irreversible cathodic wave near the Co(Ⅱ)/Co(Ⅰ)response[37-38].Increasing the concentrationof NEt3H+raises the height of the new wave and shifts it to more negative potentials,indicating that complexes 1 and 2 are able to reduce the proton with a catalysis process.This observation indicates direct protonation of the reduced Co(Ⅰ)center and also shows that the protonation process is fast in the overall catalytic rate.

Fig.2Cyclic Voltammogram of 1(a)and 2(b)(1 mmol·L-1)in CH3CN with 0.1 mol·L-1TBAPF6upon addition of NEt3HCl with different concentrations
2.3 Complexes 1 and 2 as efficient quencher for the photosensitizer Fl
Complexes 1 and 2 also serves as an efficient quencher for the photosensitizer Fl.To verify this hypothesisfurther,theStern-Volmerquenching constant Kqin this system was calculated as 1.9×104L·mol-1upon the addition of 1 into Fl solution in EtOH/H2O(1∶1,V/V).The quenching behavior can be considered as a photoinduced electron transfer process from excited state of Fl*to 1,providing possibilities for Fl to activate complex 1 producing H2in solution. Although the Kqfor the catalyst 1 was higher than that reported for NEt3(0.44 L·mol-1),the concentration of NEt3(1.08 mol·L-1,15%)in the actual photocatalytic reactions is much higher than the catalyst 1(10 μmol·L-1).A photoinduced electron transfer from the NEt3to the excited state of Fl(reduction quenching) dominated the homogeneous photolysis of the reaction mixture instead of direct quenching by 1.As the Stern-Volmer quenching constant of 2(9.85×104L· mol-1)is higher than that of complex 1(Fig.3).It indicates that the modified rhodamine group was in favor of the electronic transport between the catalyst 2 and photosensitizer Fl.When the concentration of NEt3was fixed at 0.5 mol·L-1(7%,V/V)and catalyst 2 was fixed at 10 μmol·L-1under the photocatalytic condition,the direct luminescence quenching of Fl by the catalyst 2 should be largely dominated by the oxidative quenching.The initial photochemical step was the formation of Fl+caused by 2[39-40].Because both the photosensitizer Fl and the rhodamine-based catalyst contain highly conjugated xanthene moieties, Fl molecules might have strong π-π stacking interact with the catalyst 2,which is beneficial to transfer electrons from the photoexcited Fl*to 2[41].This process led to a more efficient separation of the photogeneratedelectrons,thussuppressingthe combinationofelectronsthatwouldresultin undesired radiative transition.Therefore,the photoinduced electron transfer pathway obtained by the catalyst2couldpotentiallybebenefitforthe construction of efficient photocatalytic systems.
2.4 Complex 1 as a WRC
Protonreductioncatalyticactivityof1was evaluated by coupling it with Fl as a photosensitizer (PS)upon irradiation with visible light(λ>400 nm)in an EtOH/H2O(1∶1,V/V)solvent mixture containing NEt3at room temperature[42-44].The optimum pH value of the reaction mixture was maintained at 11.5, decreasing or increasing pH values resulted in both a lower initial rate and shorter system lifetime for hydrogen evolution(Fig.4a).The efficiency of thevisible light induced hydrogen evolution depends on the concentration of sacrificial reagent NEt3.The optimal concentration is 15%,with a decrease of activity at lower or higher concentration.Furthermore, the addition of two of the three components of the homogeneous systems could not give any further H2evolution which indicated that both the 1 and Fl were decomposed during the photolysis.

Fig.3Family of the emission spectra of fluorescein solution(10 μmol·L-1)in EtOH/H2O(1∶1,V/V)solution upon addition of catalyst 1(a)and 2(b)with various concentration
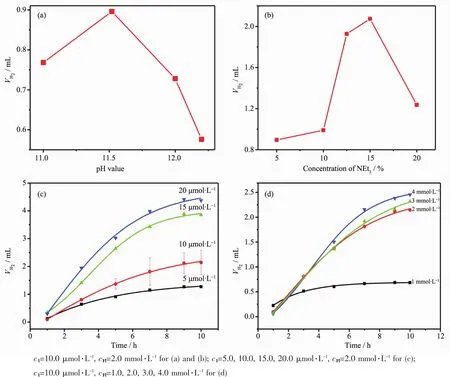
Fig.4Initial rates of H2production in systems containing 1,Fl and 15%(V/V)of NEt3with different pH values(a)and containing 1,Fl at pH 11.5 with different concentrations of NEt3(b);Photocatalytic hydrogen evolution of systems containing Fl,15%(V/V)NEt3with different concentrations of 1(c)and containing 1,15%(V/V)NEt3with different concentrations of Fl(d)
In the system with Fl and NEt3at fixed concentrations,the initial rate of H2generation exhibits a first-order dependence on catalyst 1.At 10.0 μmol·L-1catalysts 1 and 2 mmol·L-1Fl,this system exhibits a TON of 2 267 molH2·after 10 hours and an initial TOF of 220 molH2··h-1.At higher catalyst concentrations,even though a larger amount of H2is evolved,the TON does not scale linearly with catalyst concentration due to the limited lifetime of the system. To determine the limit of this system,an additional experiment was carried out at different Fl concentrations shown in Fig.4d.At higher Fl concentration as 4 mmol·L-1,there is no continuous increased H2generation detected in 10 h.The TOF reaches a maximum at 2.0 mmol·L-1Fl,which indicates that the system is limited by the concentration of the catalyst.The system has a longer life time at higher Fl,thus suggesting that Fl decomposes during photolysis.This phenomenon also demonstrates that the reductive quenching path way dominates in the system of catalyst 1.
2.5 Complex 2 as a WRC
Through investigating the influence of different pH values and concentration of NEt3,we found thehighest efficiency of H2production could be achieved at pH 11.6 and NEt37%.For initial experiments,the Fl concentration in the reaction vials was kept at 2 mmol·L-1,while a 5 μmol·L-1catalyst concentration was chosen.The initial TOF calculated was 346.5 molH2··h-1in 5 h with the TON of about 1 732 molH2·To further probe the photocatalytic process, concentrations of both the Fl and the catalyst 2 were varied.Details of the effect of reactant concentration on H2evolution are depicted in Fig.5.In terms of catalyst turnovers,it was observed that the photocatalytic system performs better at high Fl concentration and low catalyst concentration.These results pointed toward high degradation degree of the catalyst 2,which was more notable at higher concentrations. As shown in Table 2,in the same reaction condition, catalyst 2 has a higher TON and TOF than catalyst 1 in the concentration of 5 μmol·L-1for catalyst. However,in the higher catalyst concentration of 20 μmol·L-1,the catalyst 1 showed the better photocatalytic activity than catalyst 2.It was attribute to the self-stacking interaction of rhodamine group in high concentration which reduced the electronic transfer ability between the catalyst 2 and Fl.And in the same catalyst concentration,catalyst 2 exhibited higher TON and TOF than catalyst 1 in different Fl concentration in 5 h.These results demonstrated that the fast electronic transfer in catalyst 2 was benifit for the photocatalytic system in low catalyst concentration. When the concentration of the catalyst was lowered from 5 μmol·L-1to 1 μmol·L-1,keeping the Fl at the optimalconcentration,thehydrogenevolution continued within 3 hours.Although the quantity of hydrogen production decrease,it ledtoalargeincrease in turnovers yielding up to 2 800 molH2·and the highest TOF 930
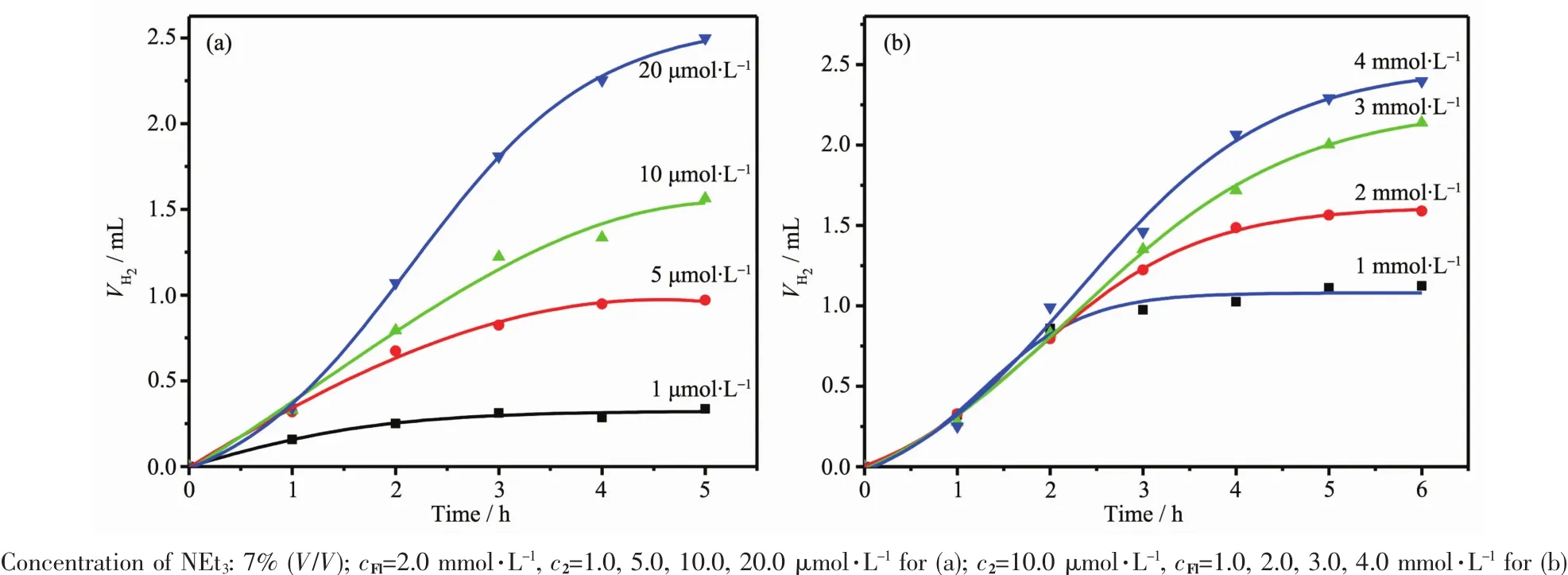
Fig.5Photocatalytic hydrogen evolution of systems containing Fl,NEt3with different concentrations of 2(a)and containing 2, NEt3with different concentrations of Fl(b)

Table 2Data of hydrogen production test in 5 h for complexes 1 and 2
The light induced H2evolution varied on the concentration of Fl,and the increasing of Fl amount caused the increasing of TON.These results suggest both the 2 and Fl were decomposed during the photolysis,but the decompose rate of Fl was much higher than that of the catalyst 2.The photocatalytic activity of catalyst 2 was decreased after 5 h,while catalyst 1 showed better activity in 10 h.The life time of the hydrogen evolution system was shorten,which could be due to the cooperation effect between rhodamine groups and Fl.This fast energy transfer process resulted the quick decompose of Fl and catalyst 2.Relative to catalyst 1,catalyst 2 has a higher hydrogen production rate in the beginning of the reaction process which also demonstrated the fast electron transfer in rhodamine-modified systems.A tentative mechanism proposed for the high activity of catalyst 2 for the production of H2has been deduced. Photogenerated electrons in Fl firstly transfer to the rhodamine groups in 2 under illumination with visible lightthroughthepossibleintermolecularπ-π interactions,and then the ligands transfer electrons to the Co center,where H2evolution reactions occur.At last,the electron donor NEt3restore the excited Fl+to the ground state to complete the catalytic cycle.
3 Conclusions
In summary,we reported a simple but effective method to gain cobalt-thiosemicarbazone complexes 1 and2astheefficientwaterreductivecatalyst. Structures of these complexes were determined by single crystal X-ray analysis.Electrochemical analysis demonstrated their WRC activity in presence of a protonsourceofNEt3HCl.Thewaterreduction properties of the catalyst were evaluated using Fl as PS with NEt3as the sacrificial donor.Introducing the rhodamine group which could cooperate with the photosensitizer makes the catalyst 2 more efficient with the TON and initial TOF reaching to 2 800 molH2·and 930 molH2··h-1.The higher photocatalytic activity in lower catalyst concentration and short time indicates the fast electron transfer ability between the catalyst 2 and photosensitizer Fl.The photocatalytic process is dominated by the oxidative quenching with the formation of Fl+caused by 2 which is benefit for the hydrogen evolution.All these workdeclarethebrightfutureofthethiosemicarbazone complex in hydrogen evolution.
[1]Cook T R,Dogutan D K,Reece S Y,et al.Chem.Rev., 2010,110:6474-6502
[2]Bard A J,Fox M A.Acc.Chem.Res.,1995,28:141-145
[3]Chen X,Liu L,Yu P Y,et al.Science,2011,331:746-750
[4]Wang F,Wang W G,Wang X J,et al.Angew.Chem.,Int. Ed.,2011,50:3193-3197
[5]Yuhas A D,Smeigh A L,Douvalis A P,et al.J.Am.Chem. Soc.,2012,134:10353-10356
[6]Kluwer A M,Kapre R,Hartl F,et al.PNAS,2009,106:10460 -10465
[7]Zhang P,Wang M,Li C,et al.Chem.Commun.,2010,46: 8806-8808
[8]McNamara W R,Han Z,Alperin P J,et al.J.Am.Chem. Soc.,2011,133:15368-15371
[9]McCormick T M,Calitree B D,Orchard A,et al.J.Am. Chem.Soc.,2010,132:15480-15483
[10]McLaughlin M P,McCormick T M,Eisenberg R,et al.Chem. Commun.,2011,47:7989-7991
[11]Han Z,McNamara W R,Eum M S,et al.Angew.Chem.Int. Ed.,2012,51:1667-1670
[12]Zhang W,Hong J,Zheng J,et al.J.Am.Chem.Soc.,2011, 133:20680-20683
[13]WEN Fu-Yu(温福宇),YANG Jin-Hui(杨金辉),ZONG Xu (宗旭),et al.Prog.Chem.(化学进展),2009,21(11):2285-2302
[14]Artero V,Chavarot-Kerlidou M,Fontecave M.Angew Chem, Int Ed.,2011,50:7238-7266
[15]Lin Y,Yuan G,Sheehan S,et al.Energy Environ.Sci.,2011, 4:4862-4869
[16]Lobana T S,Sharma R,Bawa G,et al.Coord.Chem.Rev., 2009,253:977-1055
[17]Beraldo H,Gambino D.Mini Rev.Med.Chem.,2004,4:31-39
[18]Milunovic M N M,Enyedy E A,Nagy N V,et al.Inorg. Chem.,2012,51:9309-9321
[19]Peng H,Liu G F,Liu L,et al.Tetrahedron,2005,61:5926-5932
[20]AliM A,Bernhardt P V,Brax M A H,et al.Inorg.Chem., 2013,52:1650-1657
[21]Chang T M,Tomat E.Dalton Trans.,2013,42:7846-7849
[22]Credico A,de Biani F F,Gonsalvi L,et al.Chem.Eur.J., 2009,15:11985-11998
[23]Zhang L Y,Xu L J,Zhang X,et al.Inorg.Chem.,2013,52: 5167-5175
[24]Han Z J,Shen L X,Brennessel W W,et al.J.Am.Chem. Soc.,2013,135:14659-14669
[25]Goff A L,Artero V,Jousselme B,et al.Science,2009,326: 1384-1387
[26]Kilgore U J,Roberts J A S,Pool D H,et al.J.Am.Chem. Soc.,2011,133:5861-5872
[27]Du P,Eisenberg R.Energy Environ.Sci.,2012,5:6012-6021
[28]Jing X,Wu P,Liu X,et al.New J.Chem.,2015,39:1051-1059
[29]Huang W,Song C,He C,et al.Inorg.Chem.,2009,48:5061 -5072
[30]SMART,SAINT and XPREP,Bruker Analytical Instruments Inc.,Madison,WI,1995.
[31]Sheldrick G M.SHELXS-97,Program for X-ray Crystal Structure Solution and Refinement,University of Göttingen, Germany,1997.
[32]Duan C Y,Liu Z H,You X Z,et al.Chem.Commun.,1997: 381-382
[33]Li M X,Chen C L,Zhang D,et al.Eur.J.Med.Chem., 2010,45:3169-3177
[34]Katti K V,Singh P R,Barnes C L.J.Chem.Soc.,Dalton Trans.,1993:2153-2159
[35]ZhaoY G,Guo D,Liu Y,et al.Chem.Commun.,2008:5725-5727
[36]Stewart M P,Ho M H,Wiese S,et al.J.Am.Chem.Soc., 2013,135:6033-6046
[37]Razavet M,Artero V,Fontecave M.Inorg.Chem.,2005,44: 4786-4795
[38]Kasunadasa H I,Chang C J,Long J R.Nature,2010,464: 1329-1333
[39]Zhang P,Wang M,Dong J,et al.J.Phys.Chem.C,2010, 114:15868-15874
[40]Li L,Duan L L,Wen F Y,et al.Chem.Commun.,2012,48: 988-990
[41]Dong X Y,Zhang M,Pei R B,et al.Angew.Chem.Int.Ed., 2016,55:2073-2077
[42]Lazarides T,McCormick T,Du P W,et al.J.Am.Chem. Soc.,2009,131:9192-9194
[43]HAN A-Li(韩阿丽),DU Ping-Wu(杜平武).Chinese J.Inorg. Chem.(无机化学学报),2013,29(8):1703-1709
[44]Zhang P,Wang M,Na Y,et al.Dalton Trans.,2010,39:1204 -1206
氧杂蒽染料修饰钴-硫脲配合物的光解水放氢性能
杨林 林景旭 何成*段春迎
(大连理工大学精细化工重点实验室,大连116024)
将具有不同端基的硫脲基团与三苯基磷组分结合,利用所得到的配体合成了2个具有NSP(氮硫磷)鳌合位点的钴-硫脲化合物,并研究了其光解水产氢性能。配合物[Co(L2)(L2′)](BF4)2.5·H2O·0.5C2H5OH(2)(L2=(2-二苯基膦-苯烯基)-氨基硫脲腙-罗丹明6G,L2′=(2-二苯基膦氧-苯烯基)-氨基硫脲腙-罗丹明6G)通过引入罗丹明荧光团与光敏剂分子协同作用,其产氢TON值可以达到2 800 molH2·molcat-1,其初始TOF值可达到930 molH2·molcat-1·h-1。相同条件下,相比于配合物[Co(L1)(L1′)](BF4)·0.5H2O(1) (L1=(2-二苯基膦-苯烯基)-氨基硫脲腙-硫甲基,L1′=(2-二苯基膦氧-苯烯基)-氨基硫脲腙-硫甲基),提高了体系的催化活性,可能是由于荧光素分子与配合物2之间的分子间π-π堆积作用有利于光敏剂和光催化剂之间的光致电子转移。
光致电子转移;钴;硫脲;光催化产氢
O614.81+2
A
1001-4861(2017)06-0913-10
2016-12-21。收修改稿日期:2017-04-25。
10.11862/CJIC.2017.126
国家自然科学基金(No.21531001)资助项目。
*通信联系人。E-mail:hecheng@dlut.edu.cn
- 无机化学学报的其它文章
- 过渡元素掺杂Fe3O4(001)表面磁电性能的理论研究
- 熔盐法合成电化学性能优异的富锂层状正极材料Li1.5Ni0.25Mn0.75O2.5
- Syntheses,Crystal Structures and Properties of Two d10Metal Complexes Constructed from 1,5-bis(2-ethyl-imidazolyl)pentane
- Efficient Synthesis and Application in Heck Reaction of Pd/Fe3O4Magnetic Nanoparticles
- Syntheses,Crystal Structures and Catalytic Activity of Rhenium Carbonyl Complexes Containing Aryl-Substituted Tetramethylcyclopentadienyl Ligands
- Characterization,Photocatalytic Property and Kinetics of ZnO Nanoparticles Synthesized by One Step Solid State Reaction

