大鼠SNI神经痛模型不同时相脊髓背角p-p38MAPK和p-ATF2表达的变化
何晓芬蒋永亮叶佳瑜颜思思杜俊英陈利芳赵文胜方剑乔陈晓军
大鼠SNI神经痛模型不同时相脊髓背角p-p38MAPK和p-ATF2表达的变化
何晓芬1,2蒋永亮1,2叶佳瑜1颜思思1杜俊英1,2陈利芳2赵文胜3方剑乔1,2陈晓军2
目的观察坐骨神经分支选择性损伤(SNI)模型大鼠不同时间点术侧腰段L4~L6脊髓背角神经元磷酸化p38丝裂原活化蛋白激酶(p-p38MAPK)和磷酸化活化转录因子2(p-ATF2)的表达情况,探讨脊髓背角p-p38MAPK和p-ATF2在神经病理性痛模型不同阶段中的作用。方法健康雄性SD大鼠36只,完全随机分为空白对照组、假手术组和手术组,各12只。通过结扎腓总神经及切断胫神经,保留腓肠神经的方法建立SNI大鼠模型。观察造模前、造模后3天和14天术侧足跖缩足阈值(PWT);免疫荧光法检测造模后3天和14天术侧腰段脊髓背角p-p38MAPK和p-ATF2阳性细胞表达情况。结果选模后3天和14天,手术组大鼠术侧足跖PWT较假手术组与空白对照组明显降低(P<0.01),假手术组大鼠与空白对照组大鼠比较差异无统计学意义(P>0.05)。造模后3天和14天,手术组大鼠术侧腰段脊髓背角p-p38MAPK和p-ATF2阳性细胞表达率较假手术组和空白对照组均明显升高(P<0.01),假手术组和空白对照组SNI模型大鼠造模后各时间点,术侧腰段脊髓背角p-p38MAPK和p-ATF2阳性细胞表达率差异均无统计学意义(P>0.05)。结论SNI模型神经病理痛的产生和维持可能与术侧腰段脊髓背角p-p38MAPK和p-ATF2表达上调有关。
大鼠;神经病理痛;坐骨神经分支选择性损伤模型;脊髓背角;p-p38MAPK;p-ATF2
KEY WORDSrats;neuropathic pain;spared nerve injury;spinal cord dorsal horn;p-p38MAPK;p-ATF2
神经病理痛,如中风后遗痛、疱疹后遗痛、三叉神经痛等,是临床上常见、多发又难治的慢性疼痛疾病,发病机制远未明确[1-2],目前临床治疗仍以抗抑郁、抗惊厥和阿片类药物为主,这些药物存在着缺乏针对性以及长期使用伴有严重毒副作用等问题[3]。进一步阐明神经病理痛的发病机制是当前疼痛研究领域的一个热点。神经病理痛主要病理表现为痛敏化,如自发性疼痛、痛觉过敏和痛觉超敏[4-5]。中枢敏化是神经病理痛发生、维持的关键机制之一[3,6-7],主要涉及脊髓背角(spinal cord dorsal horn,SCDH)神经元的超兴奋性。脊髓背角神经元p38丝裂原活化蛋白激酶(p38mitogen-activated protein kinase,p38MAPK)的活化在神经病理痛的中枢敏化中起着重要作用[8-10]。研究[11]表明,转录因子2(activating transcription factor 2,ATF2)的活化参与神经病理痛的调节。p38MAPK和ATF2的活化在神经病理痛过程中均起着重要作用,但在神经病理痛产生与维持的不同阶段,关于脊髓背角神经元p38MAPK和ATF2活化情况,目前尚缺少系统研究。
本实验通过建立坐骨神经分支选择性损伤(spared nerve injury,SNI)大鼠模型,通过观察SNI神经痛大鼠早期与维持期腰段脊髓背角磷酸化p38MAPK(p-p38MAPK)和磷酸化ATF2(p-ATF2)的表达情况,明确p38MAPK和ATF2在神经病理痛模型中产生与维持的不同阶段的活化情况,为找寻神经痛不同时期更具有针对性的疼痛治疗靶点提供理论依据。
1 材料与方法
1.1 实验动物选用健康清洁级雄性SD大鼠36只,体质量(180±20)g,购自中国科学院上海实验动物中心,实验动物合格证:SCXK(沪)2013-0016,由浙江中医药大学实验动物中心[合格证号:SYXK(浙)20 13-0184]饲养。饲养期间给予标准饲料及自由饮水,12h黑白循环灯光,恒定温度和湿度。本实验所有操作均符合中华人民共和国《实验动物管理条例》。
1.2 主要仪器和试剂仪器:0.25mm×13mm华佗牌无菌针灸针(苏州医疗用品厂有限公司)、HANS-200A穴位神经刺激仪(联创科技南京济生医疗科技有限公司)、动态足底测量仪(意大利UGO Basile公司)、冰冻切片机(美国Thermo公司)、激光共聚焦显微镜(日本Nikon公司)。试剂:兔抗大鼠p-p38MAPK抗体(美国Cell Signaling公司)、兔抗大鼠p-ATF2多克隆抗体(美国Cell Signaling公司)。
1.3 分组与造模采用完全随机法将大鼠随机分为空白对照组、假手术组与手术组,各12只。手术组:将SD大鼠用10%水合氯醛(0.35mL/100g)腹腔注射麻醉后,俯卧位固定,充分暴露右侧臀区,剃毛消毒,在大鼠股骨中点下约0.5cm处平行坐骨神经大鼠方向剪开皮肤,钝性分离臀部肌肉、股二头肌,暴露坐骨神经干,玻璃分针分离周围粘连组织,分离出坐骨神经的3个分支:胫神经、腓总神经和腓肠神经,用5.0丝线把胫神经和腓总神经紧紧结扎,在靠近结扎的远侧端并且距离神经干远侧端大约2~ 4mm处切断;保持腓肠神经完整,然后分层缝合肌肉和皮肤。肌注青霉素4~5U以预防感染。单笼饲养1天后用于实验。假手术组:大鼠仅暴露神经,不结扎和切断,其余方法同手术组。空白对照组:大鼠不做任何处理。
1.4 机械痛检测采用动态足底测量仪检测大鼠术侧缩足阈值(paw withdrawal threshold,PWT),作为大鼠机械性痛觉超敏的评价指标。测量前,各组大鼠适应环境2天;将大鼠置于铁丝网上的透明有机玻璃箱内(20cm×20cm×15cm),足部暴露于铁丝网眼中,安静后(即停止梳理毛发和探索性活动,15~20min),将类似Von Frey丝的金属丝(直径0.5mm)置于大鼠足底外侧(腓肠神经的支配区域),刺激力量从0g开始以2.5g/s递增,直至大鼠产生缩腿反应;此时电子记录器会自动记录下大鼠逃避时所接受的刺激力量,此力量数值即为大鼠PWT。最大刺激力量为50g,避免大鼠足爪损伤;连续测量3次,间隔5min,取平均值。每组选取8只大鼠,分别于造模前及造模后第3天、第14天测量PWT。
1.5 免疫荧光法检测脊髓背角p-p38MAPK和p-ATF2阳性细胞表达
1.5.1 样本处理每组选取3只大鼠,分别于造模后3天和14天分批处死大鼠:以10%水合氯醛以3.5mL/kg的剂量腹腔注射麻醉,小心解剖胸腔暴露心脏,经左心室升主动脉予生理盐水(4℃)灌注冲洗,再用4%多聚甲醛500mL灌注。快速取出大鼠患侧腰段脊髓(即腰膨大),置4%多聚甲醛溶液中后固定4h。依次置于15%、30%蔗糖溶液梯度脱水,经液氮速冻后,置入-80℃冰箱保存,用于免疫荧光法的检测。
1.5.2 免疫荧光法检测SCDH p-p38MAPK阳性细胞表达取出大鼠脊髓,用OCT包埋后,固定于冰冻切片机的冻头上,以50μm的厚度修片至所需组织部位,以30μm切取组织待用。采用漂片法染色,具体步骤如下:(1)小心取出切片,TBST漂洗10min× 3次。(2)10%驴血清(TBST稀释),37℃孵育1h,以增加细胞通透性和封闭非特异性结合位点,切片勿洗。(3)分别加入p-p38MAPK和p-ATF2的一抗:兔抗大鼠p-p38MAPK单克隆抗体(1:400)、兔抗大鼠p-ATF2多克隆抗体(1:200),4℃孵育过夜,TBST漂洗10min×3次。(4)加入相应的二抗:p-p38MAPK、p-ATF2均加入Alexa Fluor 488驴抗兔IgG(H+L)(1:400),进行免疫荧光标记,37℃孵育(避光)1h,TBST漂洗10min×5次(避光)。(5)捞片:将漂洗干净的切片转移至处理好的载玻片上,擦干水渍,滴加抗荧光淬灭封片液封片。(6)拍片:共聚焦显微镜下观察并摄取图片。由Image ProPlus6.0病理图像分析系统计算阳性细胞率。每组选取3只大鼠,每只大鼠取5张不连续切片,分别记录大鼠患侧腰段脊髓背角浅层(Ⅰ~Ⅱ层)内p-p38MAPK和p-ATF2阳性像素点和区域总像素点,并计算其阳性百分率[12-13]。
2 结果
2.1 各组大鼠不同时间点痛阈变化比较造模前,三组大鼠PWT无显著差异。造模后3天和14天,与空白对照组比较,假手术组大鼠术侧足跖PWT无显著变化(P>0.05),手术组大鼠术侧PWT显著降低(P< 0.01);与假手术组比较,手术组大鼠术侧PWT显著降低(P<0.01),见图1。
2.2 各组大鼠腰段脊髓背角p-p38MAPK和p-ATF2阳性细胞表达比较造模后3天和14天,与空白对照组比较,假手术组大鼠术侧腰段脊髓背角浅层内p-p38MAPK和p-ATF2无显著变化(P>0.05),手术组大鼠术侧腰段脊髓背角浅层内p-p38MAPK和p-ATF2显著升高(P<0.01);与假手术组比较,手术组大鼠术侧腰段脊髓背角浅层内p-p38MAPK和p-ATF2显著升高(P<0.01)。见图2~3(封三)。
3 讨论

图1 各组SNI模型大鼠不同时间点痛阈变化情况(n=8)
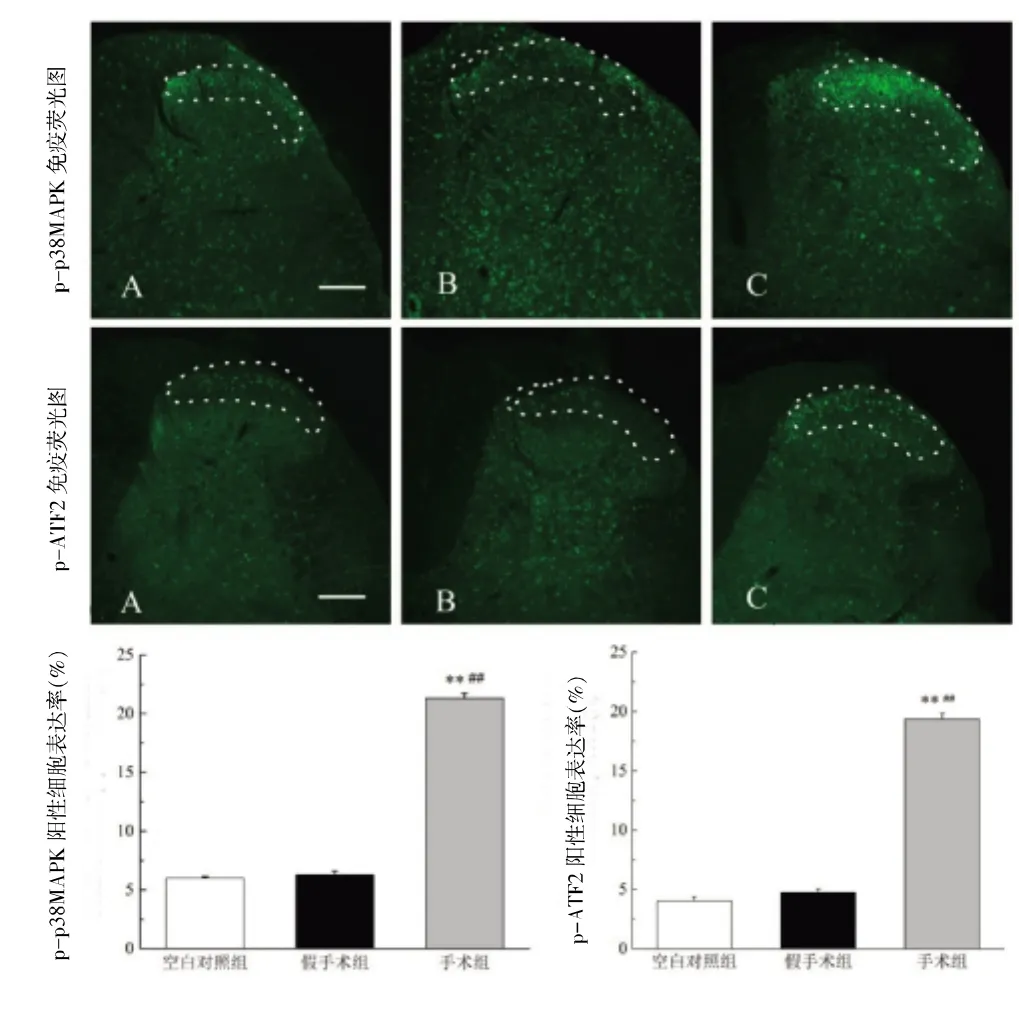
图2 各组SNI模型大鼠造模后3天术侧L4~L6脊髓背角pp38MAPK和p-ATF2表达情况(n=3)

图3 各组SNI模型大鼠造模后14天术侧L4~L6脊髓背角p-p38MAPK和p-ATF2表达情况(n=3)
目前常用的神经病理痛动物模型主要包CCI模型[14]、坐骨神经部分结扎模型[15]、SNL模型[16]、SNI模型[17]4种。SNI模型是相对较新型的外周神经损伤导致的长时程神经病理痛模型,因其造模方法简单、重复性高[17],在基础研究中得到广泛应用。在本实验中,SNI模型大鼠造模后3天机械痛阈明显下降并伴有舔足、抬脚等自发性疼痛现象,造模后14天痛阈仍显著低于空白对照组大鼠(P<0.01),表明神经病理痛模型成功建立。
研究[18]发现,MAPK是信号从细胞表面传导至细胞核内部的重要传递者,通过对转录因子的磷酸化来调节参与细胞反应基因的转录表达,包括p38MAPK、细胞外信号调节激酶(extracellular signalregulated kinase,ERK)和c-jun氨基末端激酶(c-junN-terminal kinase,JNK)。p38MAPK通过对细胞转录、蛋白合成和受体表达等的调节,在神经元的可塑性变化及痛觉信息转导中起重要作用[19]。近年研究发现,脊髓背角神经元p-p38MAPK在神经病理痛的中枢敏化的产生和维持中发挥着关键作用[8];研究[10]表明,脊神经结扎12h后,p-p38MAPK表达开始增加,在3天达高峰,术后3周一直维持在一个较高的水平。研究表明鞘内注射p38MAPK抑制剂能减轻伤害性行为学变化[20];脊髓背角是接受外周伤害性信息传入,并将此信息向上位脑结构传递的中继站,传递伤害性信息的细纤维,主要终止于脊髓背角Ⅰ、Ⅱ层[21]。本结果显示,SNI造模后3天、14天,SNI大鼠腰段脊髓背角p-p38MAPK阳性细胞表达均明显升高。
研究[11]表明,活化的p38MAPK进入细胞核,使ATF2活化后作用于靶基因启动子,进而参与疼痛的调节。已有研究表明,在多种疼痛模型中,脊髓背角p-ATF2水平明显升高[22]。本实验免疫荧光结果显示,在神经病理痛的早期和维持期脊髓背角p-ATF2阳性细胞表达明显升高。
本研究表明,SNI神经痛大鼠早期和维持期的脊髓背角p-p38MAPK和p-ATF2均明显升高,以上结果提示SNI神经病理痛的发生、维持均与脊髓背角p38MAPK和ATF2的活化有关。
[1]Serpell M,Gater A,Carroll S,et al.Burden of postherpetic neuralgia in a sample of UK residents aged 50 years or older:findings from the Zoster Quality of Life(ZQOL)study[J].Health Qual Life Outcomes,2014,12(1):1-14.
[2]Jaggi AS,Singh N.Role of different brain areas in peripheral nerve injury-induced neuropathic pain[J].Brain Res,2011,1381(1381):187-201.
[3]Dworkin RH,Backonja M,Rowbotham MC,et al.Advances in neuropathic pain:diagnosis,mechanisms,and treatment recommendation[J].Arch Neurol,2003,60(11):1524-1534.
[4]Wang W,Gu J,Li YQ,et al.Are voltage-gated sodium channels on the dorsal root ganglion involved in the development of neuropathic pain[J].Mol Pain,2011,79(1):16.
[5]Austin PJ,Moalem-Taylor G.The neuro-immune balance in neuropathic pain:involvement of inflammatory immune cells,immune-like glial cells and cytokine[J].J Neuroimmunol,2010,229(1-2):26-50.
[6]Baron R.Mechanisms of disease:neuropathic pain a clinical perspective[J].Nat Clin Pract Neurol,2006,2(2):95-106.
[7]Costigan M,Scholz J,Woolf CJ.Neuropathic pain:a maladaptive response of the nervous system to damage[J].Annu Rev Neurosci,2009,32(32):1-32.
[8]Zhuang ZY,Kawasaki Y,Tan PH,et al.Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkin[J].Brain Behav Immun,2007,21(5):642-651.
[9]Jin SX,Zhuang ZY,Woolf CJ,et al.p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain[J].J Neurosci,2003,23(10):4017-4022.
[10]Chu H,Xia J,Xu H,et al.Melanocortin 4 receptor mediates neuropathic pain through p38MAPK in spinal cord[J].Can J Neurol Sci,2012,39(4):458-464.
[11]Ji RR,Suter MR.p38 MAPK,microglial signaling,and neuropathic pain[J].Mol Pain,2007,3(1):33.
[12]Liang Y,Du JY,Qiu YJ,et al.Electroacupuncture attenuates spinal nerve ligation-induced microglial activation mediated by p38 mitogen-activated protein kinase[J].Chin J Integr Med,2016,22(9):704-713.
[13]Liang Y,Fang JQ,Du JY,et al.Effect of Electroacupuncture on Activation of p38MAPK in Spinal Dorsal Horn in Rats with Complete Freund's Adjuvant-Induced Inflammatory Pain[J].Evid Based Complement Alternat Med,2012,2012(1741-427X):568273.
[14]Bennett GJ,Xie YK.A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man[J].Pain,1988,33(1):87-107.
[15]Seltzer Z,Dubner R,Shir Y.A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury[J].Pain,1990,43(2):205-218.
[16]Kim SH,Chung JM.An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat[J].Pain,1992,50(3):355-363.
[17]Decosterd I,Woolf CJ.Spared nerve injury:an animal model of persistent peripheral neuropathic pain[J].Pain,2000,87(2):149-158.
[18]Krishna M,Narang H.The complexity of mitogenactivated protein kinases(MAPKs)made simple[J].Cell Mol Li-fe Sci,2008,65(22):3525-3544.
[19]Kumar S,Boehm J,Lee JC.p38 MAP kinases:key signalling molecules as therapeutic targets for inflammatory diseases[J].Nat Rev Drug Discov,2003,2(9):717-726.
[20]Mizushima T,Obata K,Katsura H,et al.Intensitydependent activation of extracellular signal-regulated protein kinase 5 in sensory neurons contributes to pain hyper-se nsitivity[J].J Pharmacol Exp Ther,2007,321(1):28-34.
[21]Yang K,Wang D,Li YQ.Distribution and depression of the GABA(B)receptor in the spinal dorsal horn of adult rat[J].Brain Res Bull,2001,55(4):479-485.
[22]Fang JQ,Du JY,Liang Y,et al.Intervention of electroacupuncture on spinal p38 MAPK/ATF-2/VR-1 pathway in treating inflammatory pain induced by CFA in rats[J].Mol Pain,2013,9(1):1-14.
(收稿:2016-11-20修回:2016-12-19)
Changes of p-p38MAPK and p-ATF2 Expression in Spinal Cord Dorsal Horn at Different Periods of Spared Nerve Injury Induced Neuropathic Pain in Rats
HE Xiaofen1,2,JIANG Yongliang1,2,YE Jiayu1,YAN Sisi1,DU Junying1,2,CHEN Lifang2,ZHAO Wensheng3,FANG Jianqiao1,2,CHEN Xiaojun2.1 Department of Neurobiology and Acupuncture Research,the Third Clinical Medical College,Zhejiang Chinese Medical University, Hangzhou(310053),China;2 Department of Acupuncture and Moxibustion,the Third Affiliated Hospital of Zhejiang Chinese Medical University,Hangzhou(310053),China;3 Department of Pain Treatment,the TCM&WM Hospital of Zhejiang Traditional Chinese Medicine University,Hangzhou(310003),China
ObjectiveTo investigate the changes of p-p38MAPK and p-ATF2 expression in ipsilateral L4-L6 spinal cord dorsal horn(SCDH)of ratsat different times of the spared nerve injury(SNI),and to probe into the effect of central p-p38MAPK and p-ATF2 in neuropathic pain.MethodsThirty-six healthy male SD rats were randomly divided into control group,sham surgery group and surgery group.The spared nerve injury model was established by ligating the common peroneal and the tibial nerves and then cutting off the nerves but keeping the sural nerve intact. Ipsilateral paw withdrawal threshold(PWT)were measured.The levels of p-p38MAPK and p-ATF2 in ipsilateral SCDH were tested by immunofluorescence.ResultsRats in surgery group developed spontaneous pain and showed a significant reduction in PWT on D3 and D14(P<0.01),while that of sham surgery did not show a significant reduction(P>0.05).P-p38MAPK and p-ATF2 expression in ipsilateral L4-L6 SCDH of spared nerve injury rats increased on D3 and D14 after injury(P<0.01,P<0.01).There was no difference of p-p38MAPK and p-ATF2 expression in ipsilateral L4-L6 SCDH between control group and sham surgery group(P>0.05).ConclusionThe induction and maintainence of spared nerve injury-induced neuropathic pain were associated with the activation of p-p38MAPK and p-ATF2 in ipsilateral SCDH.
浙江省自然科学基金(No.LY14H270002,No.LY14H270007);浙江省重点科技创新团队计划资助(No.2013TD15)
1浙江中医药大学第三临床医学院针灸神经生物学实验室(杭州310053);2浙江中医药大学附属第三医院针灸科(杭州310005);3浙江中医药大学附属浙江省中西医结合医院疼痛科(杭州310003)
陈晓军,Tel:15967120599;E-mail:cxj1019@sina.com;方剑乔,Tel:0571-86673000;E-mail:fangjianqiao7532@163.com

