肺动脉平滑肌细胞凋亡在大鼠低氧性肺动脉重构自然逆转中的作用及分子机制*
陈 键, 王艳霞, 牛 雯, 李志超
(第四军医大学病理学与病理生理学教研室, 陕西 西安 710032)
肺动脉平滑肌细胞凋亡在大鼠低氧性肺动脉重构自然逆转中的作用及分子机制*
陈 键, 王艳霞, 牛 雯, 李志超△
(第四军医大学病理学与病理生理学教研室, 陕西 西安 710032)
目的: 研究肺动脉平滑肌细胞(pulmonary arterial smooth muscle cells, PASMCs)凋亡在低氧性肺动脉重构自然逆转中的作用,并探讨其可能机制。方法: 24只SD大鼠随机均分为常氧4周组、低氧4周组、低氧4周后复氧1周组及复氧6周组。分别检测右室收缩压(right ventricular systolic pressure, RVSP)、肺动脉中膜厚度(medial thickness, MT)和中膜面积(medial area, MA),以及肺动脉中膜自噬、凋亡等在低氧-复氧中的变化。大鼠原代PASMCs分为常氧48 h组、低氧48 h组、低氧48 h后复氧24 h组及常氧72 h组,观察PASMCs凋亡和自噬在低氧-复氧中的变化。再将PASMCs分为常氧72 h组、低氧48 h后复氧24 h组及低氧48 h+氯喹(自噬抑制剂)干预后复氧24 h组,观察PASMCs低氧阶段的自噬对其复氧阶段凋亡的影响。结果: (1)低氧使大鼠RVSP、右室肥厚指数、MT及MA显著升高(P<0.05);复氧后上述指标逐渐降低。(2)低氧使肺动脉中膜LC3表达升高,P62表达降低,复氧后上述分子的表达逐步恢复正常。低氧显著降低了中膜cleaved caspase-3 的表达,复氧1周其表达显著高于低氧组。(3)低氧期原代PASMCs cleaved caspase-3/PARP 的表达显著低于常氧组,复氧后其表达明显升高(P<0.05);PASMCs LC3和P62的表达在低氧期显著降低(P<0.05)。(4)抑制了PASMCs低氧阶段的自噬后,其复氧阶段 cleaved caspase-3/PARP表达显著降低(P<0.05)。结论: PASMCs的凋亡参与了低氧性肺动脉重构的自然逆转;复氧期PASMCs凋亡的发生可能与其低氧期的自噬有关。
低氧性肺动脉高压; 自噬; 细胞凋亡
低氧性肺动脉高压(hypoxic pulmonary hypertension, HPH)是以肺小动脉收缩及重构为主要特征的慢性渐进性疾病,具有较高的发病率及死亡率[1]。大量的研究表明,HPH中的肺动脉重构在恢复常氧后可以逐步逆转[2]。早先有研究指出,凋亡在低氧性肺动脉重构的逆转中发挥着重要作用[3],但其发生的具体机制尚不清楚。自噬是机体一种进化保守的分解代谢过程,可以降解胞质的蛋白多聚体以及细胞器等[4]。传统的观念认为自噬作为机体在恶劣条件下的一种应激反应,通过清除损伤的蛋白质及细胞器、维持机体代谢稳态等,促进了细胞存活[5];也有研究认为自噬在一定条件下可以诱导凋亡的发生最终导致细胞死亡[6]。在HPH发生发展的过程中常伴有肺动脉平滑肌细胞(pulmonary arterial smooth muscle cells, PASMCs)自噬的发生[7]。是否PASMCs低氧阶段的自噬促进了其复氧阶段凋亡的发生,最终加速了低氧性肺动脉重构的逆转,目前尚无研究报道。本研究分别观察了自噬及凋亡在低氧性肺动脉重构的发生及逆转中的变化,并探讨了PASMCs低氧阶段的自噬对其复氧阶段凋亡的影响。
材 料 和 方 法
1 材料
1.1 实验动物 成年雄性SD大鼠,体重(200±20) g,共24只,购于第四军医大学实验动物中心。
1.2 药物及试剂 水合氯醛(上海山浦化工有限公司);BCA蛋白定量试剂盒(上海碧云天有限公司);LC3兔单克隆抗体(Millipore);P62兔单克隆抗体(Sigma);cleaved caspase-3和cleaved PARP兔多克隆抗体(CST);β-actin兔多克隆抗体(ImmunoWay);免疫组化试剂(北京中衫生物技术有限公司);DAB试剂盒(北京中衫生物技术有限公司);氯喹(chloroquine, CLQ; 购自MCE);CellMax胎牛血清(赛澳美细胞技术有限公司);DMEM高糖培养液(HyClone)。
1.3 实验仪器 全自动调节低压低氧舱(第四军医大学病理学与病理生理学教研室自主研发);压力检测系统(AD);常氧、低氧细胞培养箱(Thermo);酶联免疫检测仪(BioTek);SDS-PAGE凝胶电泳仪及电转仪(Bio-Rad)。
2 实验方法及步骤
2.1 实验动物分组及处理 24只SD大鼠随机分为:常氧4周(normoxia for 4 weeks, N)组、低氧4周(hypoxia for 4 weeks, H)组、低氧4周后复氧1周(reoxygenation for 1 week after hypoxia for 4 weeks, R1)组以及低氧4周后复氧6周(reoxygenation for 6 weeks after hypoxia for 4 weeks, R6)组,每组6只。低氧组大鼠置于低压低氧舱内(模拟5 500米高空环境,10%O2),每天8 h,连续4周。复氧组大鼠先低氧处理4周,然后在常氧条件下分别恢复1周和6周。常氧组大鼠在常氧条件下饲养。所有大鼠饲养于室温(18~22 ℃)、湿度50%~70%、12 h光照与12 h 黑暗交替的环境中,可自由饮水与摄食。
2.2 血流动力学检测及标本收集 大鼠称重后,10%水合氯醛(3.5 mL/kg)腹腔注射麻醉。经右颈外静脉插管至右心室,压力检测系统记录右心室收缩压(right ventricular systolic pressure, RVSP),可近似视为肺动脉压[8]。测压结束放血处死大鼠,分离出心脏和肺脏。每只大鼠分别取右肺下叶0.3 cm×0.3 cm×0.2 cm大小肺组织,置于4%多聚甲醛中固定,用于后续病理学检测。剩余肺组织保存于液氮中备用。取出心脏,去除心房和大血管,将右心室壁(right ventricle, RV)与左心室及室间隔(left ventricle plus interventricular septum, LV+S)分离。拭去表面的水并称重,计算RV/(LV+S)。
2.3 血管形态学检测 固定的大鼠肺组织经石蜡包埋、切片及HE染色,光镜下观察各处理组外周肺动脉(直径30~100 μm)形态学改变并拍照。使用图像处理软件(Image-Pro Plus),分别计算肺动脉中膜厚度(medial thickness, MT)百分比[MT%=100×MT/(vessel semi-diameter)]以及中膜面积(medial area, MA)百分比[MA%=100×(cross-sectional medial layer area)/(total cross-sectional vessel area)]。
2.4 免疫组化染色 肺组织石蜡切片经梯度二甲苯及乙醇溶液脱蜡后,过氧化氢灭活内源性过氧化物酶、柠檬酸缓冲液进行抗原修复等,最后LC3(1∶100)、P62 (1∶100)及cleaved caspase-3(1∶50)抗体溶液孵育4 ℃过夜。次日,滴加辣根过氧化物酶结合的抗兔抗体、DAB法显色、苏木精复染、脱水、透明、封片后镜下观察并拍照。
2.5 大鼠PASMCs的原代培养 大鼠原代PASMCs通过组织块法进行培养。动物麻醉后(方法同上),放血处死,超净台内分离出肺动脉。在无菌的PBS中除去肺动脉外膜及内膜,将剩余中膜剪至1 mm×1 mm×1 mm大小的组织块,移入培养瓶底面并均匀分布。加入含20%胎牛血清的培养基,将培养瓶倒置于37 ℃、21% O2的细胞培养箱内,3 h后翻瓶。3 d后可见PASMCs从组织块爬出,待细胞长到80%融合后进行传代,使用生长良好的3~5代细胞进行后续实验。
2.6 PASMCs的分组及处理 首先,将PASMCs分为常氧48 h (normoxia for 48 h, N48)组、低氧48 h (hypoxia for 48 h, H48)组、低氧48 h后复氧24 h (reoxygenation for 24 h after hypoxia for 48 h, H48R24)组以及常氧72 h(normoxia for 72 h, N72)组。常氧组PASMCs置于37 ℃、5%CO2、21%O2条件下分别培养48 h和72 h;低氧组在37 ℃、5%CO2、5%O2条件下培养48 h;复氧组先在低氧条件下培养48 h后转至常氧继续培养24 h。再者,将PASMCs分为常氧48 h组、低氧48 h组、低氧48 h+CLQ(hypoxia+CLQ for 48 h, H48+CLQ)组、常氧72 h(N72)组、低氧48 h后复氧24 h(H48R24)组和低氧+CLQ干预48 h后复氧24 h(reoxygenation for 24 h after hypoxia+CLQ for 48 h, HCLQR24)组。低氧及复氧组处理方法同上。CLQ干预组在低氧期间向PASMCs加入CLQ(20 μmol/L)共培养48 h后细胞换液并转至常氧条件继续培养24 h。
2.7 Western blotting法检测蛋白表达 分别提取组织及细胞总蛋白,BCA法测定蛋白浓度。各组样品取等量蛋白(35 μg)用不同浓度的SDS-PAGE分离蛋白,蛋白经湿转法转移至PVDF膜上,5%的脱脂牛奶封闭1 h, LC3 (1∶4 500)、P62 (1∶4 500)、cleaved caspase-3 (1∶800)、cleaved PARP(1∶800)以及β-actin(1∶2 000)抗体溶液孵育,4 ℃过夜。次日,TBST洗膜后加入辣根过氧化物酶标记的抗兔Ⅱ抗(1∶2 000)敷育1 h,TBST洗膜,ECL进行发光成像。使用Gel-Pro软件,对蛋白条带进行灰度分析。用目的蛋白的灰度值比内参照蛋白的灰度值,统计各蛋白的相对表达。
3 统计学处理
用SPSS 19.0软件进行统计学分析。数据均采用均数±标准差(mean±SD)表示,多组间比较采用单因素方差分析(one-way ANOVA),多重比较采用Bonferroni法。以P<0.05为差异有统计学意义。
结 果
1 肺动脉血流动力学及结构等指标在低氧-复氧过程中的变化
低氧后RVSP、RV/(LV+S)、外周肺小动脉MT及MA与常氧组相比均显著升高(P<0.05);恢复常氧后,上述指标逐步降低至正常水平,见图1。
2 肺动脉中膜的自噬和凋亡在低氧-复氧过程中的变化
低氧后肺动脉中膜LC3的表达与常氧组相比显著升高,复氧后其表达逐步恢复至常氧水平。与常氧组相比,低氧显著降低了肺动脉中膜 P62的表达,复氧后P62的表达逐步恢复。低氧后肺动脉中膜 cleaved caspase-3的表达较常氧组显著降低,复氧1周后cleaved caspase-3水平较低氧及常氧组显著升高,见图2。
3 原代PASMCs的凋亡在低氧-复氧过程中的变化
低氧48 h组原代PASMCs cleaved caspase-3/PARP的表达与常氧48 h组相比均显著降低(P<0.05);低氧48 h后复氧24 h,PASMCs cleaved caspase-3/PARP的表达与常氧72 h及低氧48 h组相比均显著升高(P<0.05),见图3。
4 低氧对原代PASMCs自噬的影响
低氧48 h组原代PASMCs P62及LC3-II的表达与常氧48 h组相比均显著降低(P<0.05),见图4。
5 抑制PASMCs低氧阶段的自噬后其复氧阶段凋亡的变化
低氧48 h,PASMCs P62及LC3-II的表达与常氧48 h组相比均显著降低(P<0.05);低氧阶段用CLQ阻断自噬后,可见PASMCs P62及LC3-II的表达与单纯低氧组相比显著升高(P<0.05)。低氧48 h后复氧24 h,PASMCs cleaved caspase-3/PARP的表达与常氧72 h组相比显著升高(P<0.05);低氧期间用CLQ阻断了PASMCs的自噬后继续复氧24 h,可见PASMCs cleaved caspase-3/PARP的表达与低氧48 h后复氧24 h组相比显著降低(P<0.05),见图5。

Figure 1.The changes of hemodynamic and structural parameters in pulmonary arteries following hypoxia-reoxygenation.N: normoxia for 4 weeks; H: hypoxia for 4 weeks; R1: reoxygenation for 1 week after hypoxia for 4 weeks; R6: reoxygenation for 6 weeks after hypoxia for 4 weeks. Mean±SD.n=3~6.*P<0.05,**P<0.01vsN;#P<0.05,##P<0.01vsH;&&P<0.01vsR1.
图1 肺动脉血流动力学及结构等指标在低氧-复氧过程中的变化

Figure 2.The changes of autophagy and apoptosis in the pulmonary arterial medial layer following hypoxia-reoxygenation(×400). Representative images of immunostaining for LC3, P62, or cleaved caspase-3 in the pulmonary arterial medial layer. N: normoxia for 4 weeks; H: hypoxia for 4 weeks; R1: reoxygenation for 1 week after hypoxia for 4 weeks; R6: reoxygenation for 6 weeks after hypoxia for 4 weeks.
图2 肺动脉中膜的自噬和凋亡在低氧-复氧过程中的变化
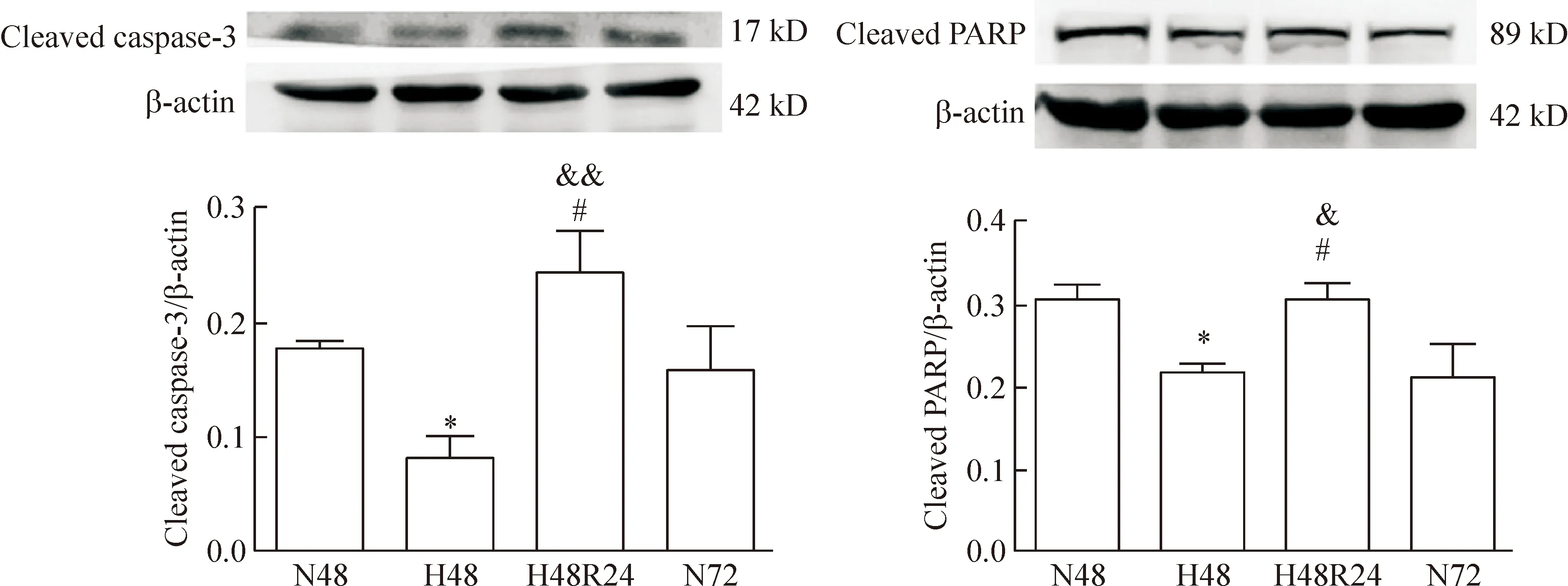
Figure 3.The changes of primary PASMC apoptosis following hypoxia-reoxygenation. Western blotting analysis of the cleaved caspase-3 and cleaved PARP expression. Densitometry analysis of protein abundance was conducted by normalizing to that of β-actin. N48: normoxia for 48 h; H48: hypoxia for 48 h; H48R24: reoxygenation for 24 h after hypoxia for 48 h; N72: normoxia for 72 h. Mean±SD.n=3.*P<0.05vsN48;#P<0.05vsN72;&P<0.05,&&P<0.01vsH48.
图3 原代PASMCs的凋亡在低氧-复氧过程中的变化

Figure 4.The effect of hypoxia on primary PASMC autophagy. Western blotting analysis of the LC3-II and P62 expression. Densitometry analysis of protein abundance was conducted by normalizing to that of β-actin. N48: normoxia for 48 h; H48: hypoxia for 48 h. Mean±SD.n=3.*P<0.05,**P<0.01vsN48.
图4 低氧对原代PASMCs自噬的影响
讨 论
HPH是一种原发或继发性的肺血管阻力进行性升高的疾病,如未及时有效地接受治疗常常导致病人发生右心衰竭直至死亡[9]。目前治疗HPH药物的种类逐渐增多,但大多都不能有效地抑制或逆转肺动脉重构。大量的研究证实,以往认为是不可逆性改变的低氧性肺动脉重构,在恢复常氧后可以逐步逆转[2, 10],但其机制尚不清楚。曾有研究表明,凋亡在低氧性肺血管重构的逆转中发挥着重要作用,然而此研究并未就凋亡发生的细胞类型及机制作详细的探讨[3]。本研究证实,低氧性肺动脉重构在恢复常氧后逐步逆转至正常水平,进一步探明PASMCs特异性的凋亡参与了低氧性肺动脉重构复氧逆转的过程。
关于PASMCs恢复常氧之后凋亡增加的机制,目前尚无报道。从恶劣的低氧条件转至相对优越的常氧条件,细胞的凋亡反而较前增多,我们推测PASMCs发生复氧凋亡最根本的动因可能存在于低氧阶段。自噬作为机体的一种适应性分解代谢反应,可以清除胞内损伤的蛋白质及细胞器等。在大多数应激条件下,自噬序贯性的分解代谢过程通常可以促进细胞存活[11],然而也有研究报道细胞自噬在一定程度上可以诱导凋亡的发生[12]。那么低氧期PASMCs是否发生了自噬,我们的结果显示低氧期大体肺动脉中膜P62表达降低,LC3 的表达升高,提示PASMCs自噬增加。但低氧阶段,原代培养的PASMCs P62及LC3-II的表达均显著降低。在自噬发生的过程中,LC3-II的主要作用是形成自噬体双层膜,P62可特异性地识别和选择待降解的目标分子并将其运至自噬体[13]。最终此两者在自噬体与溶酶体融合后,而被降解掉。因此,只有当细胞发生过度自噬时,大量的自噬体被溶酶体降解掉后,细胞中P62和LC3-II表达才会同时减少[14]。我们后续在低氧条件下应用了自噬抑制剂CLQ(升高溶酶体内的pH,抑制自噬体与溶酶体融合)后,可见PASMCs P62及LC3-II的表达均显著增多。由此我们可以得出,低氧期原代培养的PASMCs发生了过度自噬。低氧期,大体肺动脉中PASMCs的自噬只是适度增加,而离体培养的PASMCs却发生了过度自噬,我们推测此差异可能与在体情况下神经体液等因素的调节有关。

Figure 5.The effect of PASMC autophagy under hypoxia on its apoptosis during reoxygenation. To ensure the effect of chloroquine (CLQ) on the inhibition of PASMC autophagy, Western blotting analysis of the P62 and LC3-II expression of different groups was performed (A). To further examine the effect of PASMC autophagy under hypoxia on its apoptosis during reoxygenation, the expression of cleaved caspase-3/PARP was also examined (B). N48: normoxia for 48 h; H48: hypoxia for 48 h; H48+CLQ: hypoxia+CLQ for 48 h; H48R24: reoxygenation for 24 h after hypoxia for 48 h; N72: normoxia for 72 h; HCLQR24: reoxygenation for 24 h after hypoxia+CLQ for 48 h. Mean±SD.n=3.*P<0.05,**P<0.01vsN48 or N72;&&P<0.01vsH48;#P<0.05,##P<0.01vsH48R24.
图5 PASMCs低氧阶段的自噬对其复氧阶段凋亡的影响
通常低氧阶段会有大量的线粒体活性氧簇(mitochondrial reactive oxygen species, mROS)产生,而mROS会引起细胞器尤其是线粒体损伤[15],损伤的线粒体常常会启动线粒体自噬[16]。所以低氧结束时,细胞中线粒体的数量处于相对较低的水平,加之恢复常氧后PASMCs处在一个相对高氧的环境,因此PASMCs在复氧早期会产生过量的ROS[17]。而过量ROS常会引起胞浆内蛋白及细胞器等发生氧化损伤,最终导致细胞凋亡[18]。据此我们推测可能是低氧阶段增加的自噬,诱导了复氧阶段PASMCs凋亡的发生。我们的结果显示抑制了PASMCs低氧阶段的自噬后,其复氧阶段凋亡的水平显著降低,该结果说明PASMCs低氧阶段的自噬促进了其复氧阶段凋亡的发生。
综上所述,本研究提示PASMCs的凋亡参与了低氧性肺动脉重构复氧逆转的过程,且PASMCs凋亡的发生可能与其低氧阶段发生的自噬有关,这为低氧期应用自噬诱导药物来治疗低氧相关肺动脉高压提供了新的理论基础。
[1] Wilkins MR, Ghofrani HA, Weissmann N, et al. Pathophysiology and treatment of high-altitude pulmonary vascular disease[J]. Circulation, 2015, 131(6):582-590.
[2] Sluiter I, van Heijst A, Haasdijk R, et al. Reversal of pulmonary vascular remodeling in pulmonary hypertensive rats[J]. Exp Mol Pathol, 2012, 93(1):66-73.
[3] Riley DJ, Thakker-Varia S, Wilson FJ, et al. Role of proteolysis and apoptosis in regression of pulmonary vascular remodeling[J]. Physiol Res, 2000, 49(5):577-585.
[4] Fuchs Y, Steller H. Live to die another way: modes of programmed cell death and the signals emanating from dying cells[J]. Nat Rev Mol Cell Biol, 2015, 16(6):329-344.
[5] Das G, Shravage BV, Baehrecke EH. Regulation and function of autophagy during cell survival and cell death[J]. Cold Spring Harb Perspect Biol, 2012, 4(6):a008813.
[6] Fuchs Y, Steller H. Live to die another way: modes of programmed cell death and the signals emanating from dying cells[J]. Nat Rev Mol Cell Biol, 2015, 16(6):329-344.
[7] Lee S, Smith A, Guo L, et al. Autophagic protein LC3B confers resistance against hypoxia-induced pulmonary hypertension[J]. Am J Respir Crit Care Med, 2011, 183(5):649-658.
[8] Zimmer HG, Zierhut W, Seesko RC, et al. Right heart catheterization in rats with pulmonary hypertension and right ventricular hypertrophy[J]. Basic Res Cardiol, 1988, 83(1):48-57.
[9] Rubin LJ. Primary pulmonary hypertension[J]. N Engl J Med, 1997, 336(2):111-117.
[10]Weisel FC, Kloepping C, Pichl A, et al. Impact ofS-adenosylmethionine decarboxylase 1 on pulmonary vascular remodeling[J]. Circulation, 2014, 129(14):1510-1523.
[11]Geng Y, Zhang C, Shi Y, et al. Icariside II-induced mitochondrion and lysosome mediated apoptosis is counterbalanced by an autophagic salvage response in hepatoblastoma[J]. Cancer Lett, 2015, 366(1):19-31.
[12]Nezis IP, Shravage BV, Sagona AP, et al. Autophagy as a trigger for cell death: autophagic degradation of inhibitor of apoptosis dBruce controls DNA fragmentation during late oogenesis in Drosophila[J]. Autophagy, 2010, 6(8):1214-1215.
[13]He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy[J]. Annu Rev Genet, 2009, 43:67-93.
[14]Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting[J]. Autophagy, 2007, 3(6):542-545.
[15]Sena LA, Chandel NS. Physiological roles of mitochon-drial reactive oxygen species[J]. Mol Cell, 2012, 48(2):158-167.
[16]Pua HH, Guo J, Komatsu M, et al. Autophagy is essential for mitochondrial clearance in mature T lymphocytes[J]. J Immunol, 2009, 182(7):4046-4055.
[17]Holmstrom KM, Finkel T. Cellular mechanisms and phy-siological consequences of redox-dependent signalling[J]. Nat Rev Mol Cell Biol, 2014, 15(6):411-421.
[18]Braunersreuther V, Jaquet V. Reactive oxygen species in myocardial reperfusion injury: from physiopathology to therapeutic approaches[J]. Curr Pharm Biotechnol, 2012, 13(1):97-114.
(责任编辑: 卢 萍, 罗 森)
Effect of PASMC apoptosis on reversal of hypoxic pulmonary arterial remodeling during reoxygenation and its related molecular mechanism
CHEN Jian, WANG Yan-xia, NIU Wen, LI Zhi-chao
(DepartmentofPathologyandPathophysiology,TheFourthMilitaryMedicalUniversity,Xi’an710032,China.E-mail:lizhic@fmmu.edu.cn)
AIM: To explore the effect of pulmonary arterial smooth muscle cell (PASMC) apoptosis on the reversal of hypoxic pulmonary arterial remodeling during reoxygenation and its possible mechanism. METHODS: Male SD rats (n=24) were randomly divided into normoxia for 4 weeks group, hypoxia for 4 weeks group, reoxygenation for 1 week after hypoxia for 4 weeks group and reoxygenation for 6 weeks after hypoxia for 4 weeks group. Right ventricular systolic pressure (RVSP), right ventricular hypertrophy index, pulmonary arterial medial thickness (MT) and medial area (MA) as well as autophagy and apoptosis in the pulmonary arterial medial layer were examined during hypoxia-reoxygenation. The rat primary PASMCs were divided into normoxia for 48 h group, hypoxia for 48 h group, reoxygenation for 24 h after hypoxia for 48 h group and normoxia for 72 h group to explore the changes of PASMC autophagy and apoptosis following hypoxia-reoxygenation. Finally, primary PASMCs were divided into normoxia for 72 h group, reoxygenation for 24 h after hypoxia for 48 h group and reoxygenation for 24 h after hypoxia for 48 h + chloroquine (inhibitor of autophagy) group to investigate the effect of PASMC autophagy during hypoxia on the apoptosis during reoxygenation. RESULTS: After hypoxia for 4 weeks, the RVSP, during right ventricular hypertrophy index, MT and MA increased significantly compared with normoxia group (P<0.05), and gradually decreased during reoxygenation. The expression of LC3 in the pulmonary arterial medial layer increased evidently after hypoxia and gradually reversed during reoxygenation. Moreover, the P62 and cleaved caspase-3 expression decreased after hypoxia compared with normoxia group, and increased markedly following reoxyge-nation. The expression of cleaved caspase-3/PARP in rat primary PASMCs decreased significantly under hypoxia (P<0.05), and increased evidently during reoxygenation. The expression of P62 and LC3-II decreased markedly under hypoxia (P<0.05). After inhibition of PASMC autophagy under hypoxia, the expression of cleaved caspase-3/PARP decreased remarkably during reoxygenation (P<0.05). CONCLUSION: The PASMC apoptosis participates in the reversal of hypoxic pulmonary arterial remodeling, and the PASMC autophagy under hypoxia might facilitate its apoptosis during reoxygenation.
Hypoxic pulmonary hypertension; Autophagy; Apoptosis
1000- 4718(2017)04- 0583- 07
2016- 12- 06
2017- 02- 15
国家自然科学基金资助项目(No. 81471816; No. 81270328)
R363.21; R541.3
A
10.3969/j.issn.1000- 4718.2017.04.002
△通讯作者 Tel: 029-84772705; E-mail: lizhic@fmmu.edu.cn

