Efficacy of endoscopic transantral versus transorbital surgical approaches in the repair of orbital blowout fractures: study protocol for a randomized controlled trial
Nahla Mahmoud Awad*, Ibrahim Ezzat Shindy, Reem Hossameldin
Oral and Maxillofacial Surgery Department, Faculty of Oral and Dental Medicine, Cairo University, Giza, Egypt
Introduction
Orbital blowout is a term used to describe a clinical condition in which there is a fracture of the orbital floor and the medial walls without the orbital rim involvement. In this case it is classi fied as isolated/pure blowout fracture.If the rim fracture occurs, it will be classi fied as complex blowout fracture.1
Repair of orbital blowout fractures faces a lot of complications and dif ficulties to restore and reconstruct the exact position of the orbital bony skeleton especially at the orbital rims, and also a dif ficulty in restoring the functions of the orbital soft contents such as the muscles, the globe and the lacrimal system.2The orbital fractures can cause a wide range of aesthetic problems, functional impairments, and ophthalmic complications like diplopia and enophthalmos due to herniation and dropping of the orbital content into the defect deep to the maxillary sinus.3The main key to proper orbital floor repair is adequate exposure and visualization for the fractures for better anatomic bony and soft tissues reconstruction. The traditional lower eyelid surgical approaches expose the orbital floor, but the posterior edge of the fracture is dif ficult to be explored as the dissection is the most dif ficult due to the complexity of the orbital anatomy and limited surgical space, which is the main cause of unsuccessful repair of orbital blowout fractures.2,4
Traditionally, lower eyelid approaches have been commonly used in the repair of orbital floor fracture. Complete or partial resolution of preoperative diplopia was achieved in 83% of patients, while enophthalmos was improved in 76% of those. But these approaches showed some other complications like ectropions, signi ficant facial scars,extrusion of inserted Medpor, and intra-orbital hematoma.5
With the era of using endoscopic instruments, older techniques for orbital blowout fracture repair (conventional old trans-antral approach through a classic Caldwell-Luc incision) have become new and applicable again. Orbital floor fractures can be easily managed especially through this approach the posterior edge can be clearly visualized to be reduced. Risk of eyelid deformity and orbital floor periosteum torn out is alleviated.6
Hundepool et al.4compared the postoperative outcomes using endoscopic transantral versus external transantral approaches. A signi ficantly better postoperative result,regarding enophthalmos correction and diplopia resolution, was found in the endoscopically controlled group.They concluded that the endoscopically controlled repair of orbital floor fractures seems to be a more accurate, safe,effective, and successful treatment. In 2015, Kim et al.7performed a retrospective study in patients with orbital blow out fractures, they used the transantral approach for the fractures reduction then they inserted silicon membrane under the orbital floor supported with a folded silastic tube in the maxillary sinus. Postoperative improvement of the diplopia and enophthalmos was recorded, but some postsurgical complications occurred such as an overcorrection of the orbital floor, the maxillary sinus infection, and an implant displacement. These minor complications can be managed by revision surgeries.
Objective
The aim of the study is to evaluate the ef ficacy of endoscopic transantral surgical approach versus traditional transorbital surgical approach in the treatment of orbital blowout fractures via postoperative clinical and digital radiographical assessments.
Methods/Design
Study design
A prospective, two-arm, parallel group, randomized controlled trial.
Study setting
This study will be conducted in Oral and Maxillofacial Surgery Department, Faculty of Oral and Dental Medicine,Cairo University, Egypt.
Sample size
To assess the ef ficacy of endoscopic transantral surgical approach (study group) versus traditional transorbital surgical approach (CIC; control group) in orbital blowout fractures. Based on the previous data by Hundepool et al.4and Kim et al.7the probability of diplopia resolution among controls is 0.33. If the true probability among patients is 0.77, we will need to study 19 patients in each group to be able to reject the null hypothesis that the exposure rates for case and controls are equal with probability (power)0.8. The Type I error probability associated with this test of this null hypothesis is 0.05. We will use an uncorrected chi-square test to evaluate this null hypothesis. The sample size was calculated by PS program.
Inclusion criteria
In order to be eligible to participate in this study, a subject must meet all of the following criteria:
· No age or gender restriction
· Unilateral/bilateral orbital blowout fractures
· Positive forced-duction test
· Extra-ocular muscle entrapment on face computed tomography (CT) scan
Exclusion criteria
Exclusion criteria are indicated as follows:
· Medical condition affecting bone healing
· Medically compromised conditions, not proper candidate for general anesthesia
· Tumor case encroaching on the orbital floor
· Pathological orbital blowout fractures
· Allergy/metal hypersensitivity
Recruitment and screening
Patients will be recruited from outpatient clinic of Department of Oral and Maxillofacial Surgery, Faculty of Oral and Dental Medicine-Cairo University, Egypt, where there is a continuous and high patient flow from which eligible patients will be recruited to ful fill the eligibility criteria 1 week before intervention.
Screening of patients will be performed until the target population is achieved (Table 1).
Randomization
After signing written informed consent, study participants who meet the eligibility criteria will be randomly assignedto two groups (the endoscopic transantral surgical approach group or the traditional transorbital surgical approach group)in a 1:1 ratio. Randomization will be conducted using a computer-generated random allocation sequence, and the random code will be kept in a sealed envelope by Nahla Mahmoud Awad (NMA) for the whole duration of the study who will not contact patients.
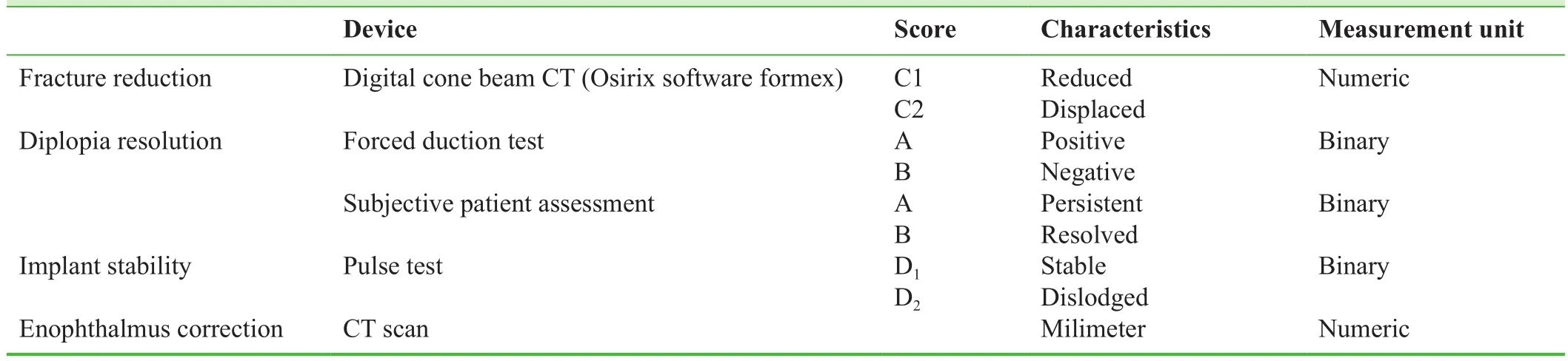
Table 1: Patient screening and outcome analysis
Blinding
The outcome assessor involved in neither allocation nor surgical procedures is blind to treatment allocation. Due to the nature of the intervention, NMA cannot be blinded to
allocation but is firmly instructed not to disclose the allocation status of the participant at the follow-up assessments.They must know either the participant will be involved in the study group or the other one to be able to manage the expected complications. Participants will be blinded till they choose one sealed envelope (one for endoscopic approach and the other one for the transorbital one). NMA will be the care provider and responsible for patient enrollment and allocation. An employee out of the research team will perform computer-related data entry in separate datasheets.Thus, the researchers can analyze data without having access to information about the allocation.
Interventions
Preoperative preparation
(1) Preoperative cone beam CT.
(2) A clearance from the ophthalmology department after examination of papillary re flexes, motility restriction and measurement of the visual acuity.
(3) Laboratory investigations: complete blood picture(CBC), liver functions, kidneys functions, prothrombin time (PT), partial thromboplastin time (PTT) and international normalized ratio (INR).
(4) Informed consent signed by the patient.
(5) NMA & Reem Hossameldin (RH) will perform all procedures under general anesthesia with orotracheal intubation.
(6) With the patient supine, general anesthesia will be induced. A cuffed, oral, right angle endotracheal tube(RAE) will be placed in the nose. The tube is further immobilized with a mouth pack.
(7) Head ring and shoulder rolls are placed. Sterile tapes will be placed over the closed eyelids. The face is prepared and draped.
(8) A forced suction test will be done to reveal if the inferior rectus was entrapped between fractured segments.
Endoscopic transantral surgery (study group)
(1) 1% or 2% lidocaine with 1:100,000 dilution of epinephrine will be injected into the upper gingivobuccal sulcus above the canine area.
(2) A 3- to 4-cm horizontal incision will be made just superior to the sulcus extending from the canine to the first molar area,
(3) The periosteum and overlying soft tissue will be gently elevated from the underlying maxillary bone, not reaching to the level of the infraorbital foramen using a periosteal elevator.
(4) Two holes (wide enough to house the 4 mm sinus scope One for the instrument and the other one for the suction)will be made into the maxillary face bone with an electric fissure bur, and care should be taken to avoid injury to dental roots, infraorbital vessels or the nasal aperture.
(5) With the aid of sinus instruments (i.e., ostium finder,Blakesley forceps, periosteal elevator, and gauze packer), the maxillary mucosa is stripped circumferentially around the fracture site.
(6) The maxillary sinus and prolapsed orbital contents will be visualized employing a 30-degree endoscope (KARL STORZ, Germany) (sinus scope 4 mm, 30 degrees short one 17 cm).
(7) The herniated orbital contents will be reduced by digital manipulation or by using surgical instruments like an elevator, without removing the mucosa with periosteal orbital preservation.
(8) A silicone sheet will be cut larger than the defect size and inserted subperiosteally below the fracture margins,is introduced into the sinus via the osteotomy. Once in the sinus, the sheet is inserted above the posterior stable bony shelf. The instruments are then “walked” forward on the sheet, seating it on the anterior stable bony shelf(orbital rim), then its stabilization tested by pulse test(performed to assess the fracture).
(9) The test is performed by applying gentle pressure on the globe while visualizing the transmitted movement of the orbital floor from below pressure on the eyeball through the maxillary sinus).
(10) A forced duction test will be performed to con firm correct positioning of the orbital floor and to avoid entrapment of the orbital contents before closing.
(11) The sinus is then irrigated and the vestibular incision will be closed with 3-0 vicryl suture (polyglactin 910;J218H: 3-0 VICRYLTM UNDYED 27 SH-1 TAPER,© Ethicon, USA).
Transorbital surgery (control group)
(1) Transorbital incision will be performed in patients.
(2) Polymyxin/oxytetracycline (Tetramycin: 1,000 IU/g Polymyxin and 0.5% oxytetracycline, P fizer, Egypt)eye ointment will be applied to both eyes.
(3) Local anesthesia with 1:100,000 epinephrine as a vasoconstrictor will be injected at the planned incision lines.(4) Temporary traction sutures will be applied to the lower eyelid using 4-0 black silk.
(5) After exposure of the fracture lines, the mobilized segments will be reduced then the orbital floor reconstruction will be made with silicone membrane sheet larger than the defect size.
(6) A forced duction test will be performed to con firm correct positioning of the orbital floor and to avoid entrapment of the orbital contents before closing.
(7) The subciliary incision will be sutured in three layers,periostial and subcutical layers will be closed with 6/0 vicryl interrupted then the skin will be sutured with 6/0 prolene (PROLENE®Polypropylene Suture, Ethicon)interrupted sutures.
(8) A frost suture will be applied for the lower eyelid suspension to the forehead using black silk and will be left for 3 days.
Postoperative care
(1) Ice pack application to the surgical area for 10 minutes every 30 minutes will be done during the first 24 hours.
(2) The vision (light perception) will be checked after the patient recovery from the general anesthesia.
(3) Use of sterile tape as simple coverage for the wound for 5 days.
(4) Medications:
- Sulbactam/ampicillin 1.5 g (intramuscular; Unictam 1.5 g vial: 1,000 mg ampicilin and 500 mg sulbactam,Pfeizer, Egypt)/12 hours for 5 days postoperatively and Diclofenac Sodium (intramuscular; Voltaren 75 mg vial,Novartis, Egypt) every 12 hours for 48 hours.
- Dexamethazone (Epidron ampoule: 8 mg/2 mL,E.I.P.I.C.O, Egypt) 8 mg/mL will be given intramuscularly every 6 hours in the first 24 hours and half of the dose in the next 24 hours.
- Long-acting corticosteroid methylprednisolone acetate(Depo-medro; 40 mg ampoule, P fizer) 80 mg will be given intramuscularly with the final dose of dexamethazone.
- Oxymetazoline Hydrochloride (Afrin nasal, 0.05% nasal spray, E.I.P.I.C.O) nasal spray will be given three times daily for 3 days.
- Telekast (Montelukast Sodium Tablets: Levocetirizine (5
mg), Montelukast (10 mg), Lupin, Egypt) orally for 5 days.
Follow-up
Clinically
The patients will be followed up at the 3rdday after surgery for the removal of the Frost suture (control group).
The 2ndclinical follow-up visit will be scheduled on the 7thday for clinical assessment for the soft-tissue healing.
The patients (subjective assessment) will be followed up on a monthly basis for 6 months following surgery for the evaluation of the eye movement, double vision resolution,enophthalmos correction, and esthetics.
Radiographically
A cone beam CT will be requested at the 2ndday after surgery for the assessment of the implant stability and the proper reduction of the bony fracture segments (no displacement).Volume of the orbit will be calculated by the separation of the orbital contents on a slice by slice basis in the cuts.
Outcomes
The detailed time points of each outcome assessment index are listed in Table 2.
Primary outcome
The primary outcome will be the diplopia resolution(gradual clearing of double vision caused by interruption and dropping of the orbital floor membrane downward into the sinus) detected intraoperatively via forced duction test.
Secondary outcomes
Secondary outcomes will include the enophthalmos correction measured on axial CT scan, and preservation oforbital floor periosteum and exacting/equalization of the injured bony level measured on CT scans on the noninjured side.

Table 2: Time schedule of enrollment, treatment, and assessments
Data collection, management, and analysis
NM will be responsible for data collection. The collected data whether personal or statistical will be stored on paper& excel sheets. Plans to promote participant retention and complete follow-up Telephone numbers and address of all subjects in the study will be recorded as a part of the signed consent. All subjects will receive a phone call at the time of the pre-determined follow-up dates.
Auditing
In this trial auditing will be done by the main and cosupervisors to inspect , survey, systematically examine and assure quality of the research methods, surgical techniques and post-trial care. Also they have has the right to modify or discontinue the trail.
Participant retention
The phone number of the patient will be recorded in the patient's chart. The patient will receive a phone call to remind him of the time of his visit. If the patient did not reply for any reason, another visit will be scheduled within a week. The rate of loss to follow-up for patients on an annual basis will be at most 5%. To ensure participant retention,appointments should be scheduled at 2, 7 days, and 1, 3,and 6 months after surgical procedures. Study site staff is responsible for developing and implementing local standard operating procedures to achieve this level of follow-up.
Participant withdrawal
We will educate and inform every patient the new trend in using endoscope in the repair of the blowout fractures(esthetical, complications rate). Periodic follow-up visits will be performed to ensure the eye functions satisfy the patients, and periodic telephone calls will be done to ensure everything after the surgery is going well.Participants may withdraw from the study process for any reason at any time. According to the time of patient withdrawal, we will decide to eliminate him/her from sample size estimates or not.
Harms
Some postsurgical complications occurred such as the maxillary sinus infection (antibiotics + anti-in flammatory medications), and an implant displacement (surgical replacement at its place).4Mild orbital pain and hypoesthesia of the infra-orbital nerve may be occurred in both groups but can be improved with time. No adverse effects were reported for both techniques.4We will terminate the study if adverse effects (permanent blindness related procedure,numbness) occur.
Discussion
In this clinical trial, we attempt to answer the following question: endoscopic transantral surgical approach will result in better and satisfactory clinical and radiological postoperative outcomes in patients suffering from orbital blowout fractures in comparison with traditional transorbital surgical approach? Maybe answers to this question will lead to a revolution in choosing a proper surgical approach for the repair and treatment of orbital blowout fractures.
Trial Status
We are currently recruiting participants.
Declaration of patient consent
The authors certify that they will obtain all appropriate patient consent forms. In the form the patients will give their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Conflicts of interest
None declared.
Author contributions
Auditing and main surgical supervision: IES. Clinical outcome assessment, reporting the study protocol: RH. Randomization and data collection, writing the manuscript and the final reporting of the study: NMA. All authors approved the final version of the manuscript for publication.
Plagiarism check
This paper was screened twice using CrossCheck to verify originality before publication.
Peer review
This paper was double-blinded and stringently reviewed by international expert reviewers.
1. Zubair FH, Touseef M, Holland I. Orbital trauma: the blow out fracture. 2005.
2. Zhang S, Li Y, Fan X. Application of endoscopic techniques in orbital blowout fractures. Front Med. 2013;7:328-332.
3. Shi W, Jia R, Li Z, He D, Fan X. Combination of transorbital and endoscopic transnasal approaches to repair orbital medial wall and floor fractures. J Craniofac Surg. 2012;23:71-74.
4. Hundepool AC, Willemsen MA, Koudstaal MJ, van der Wal KG. Open reduction versus endoscopically controlled reconstruction of orbital floor fractures: a retrospective analysis. Int J Oral Maxillofac Surg. 2012;41:489-493.
5. Jin HR, Yeon JY, Shin SO, Choi YS, Lee DW. Endoscopic versus external repair of orbital blowout fractures. Otolaryngol Head Neck Surg. 2007;136:38-44.
6. Ducic Y, Verret DJ. Endoscopic transantral repair of orbital floor fractures. Otolaryngol Head Neck Surg. 2009;140:849-854.
7. Kim JY, Choi G, Kwon JH. Transantral orbital floor fracture repair using a folded silastic tube. Clin Exp Otorhinolaryngol.2015;8:250-255.
 Clinical Trials in Orthopedic Disorder2017年2期
Clinical Trials in Orthopedic Disorder2017年2期
- Clinical Trials in Orthopedic Disorder的其它文章
- Platelet-rich plasma combined with conventional surgery in the treatment of atrophic nonunion of femoral shaft fractures: study protocol for a prospective, randomized, controlled clinical trial
- Effects of lumbar plexus-sciatic nerve block combined with sevoflurane on cognitive function in elderly patients after hip arthroplasty: study protocol for a prospective, single-center,open-label, randomized, controlled clinical trial
- Effects of two different tranexamic acid administration methods on perioperative blood loss in total hip arthroplasty: study protocol for a prospective, open-label, randomized, controlled clinical trial
- Supercapsular percutaneously-assisted total hip approach for the elderly with femoral neck fractures: study protocol for a prospective, open-label, randomized, controlled clinical trial
- Should angiography be done in suspected tuberculosis of spine in an endemic country? – Hemangioma masquerading as tuberculosis of spine
