Herbicidal Activity of Toxin from Fusariumavenaceum GD-2 against Wild Oats (Avenafatua L.)
CHENG Liang, GUO Qing-yun*
(1. Key Lab. of Agric. Integrated Pest Management in Qinghai Prov., Inst. of Plant Protect., Qinghai Acad. of Agric. &Forest. Sci.;2. Sci. Observ. & Exper. Stat’n of Crop Pest in Xining, Ministry of Agric., Xining, Qinghai 810016)
Herbicidal Activity of Toxin fromFusariumavenaceumGD-2 against Wild Oats (AvenafatuaL.)
CHENG Liang1, 2, GUO Qing-yun1, 2*
(1.KeyLab.ofAgric.IntegratedPestManagementinQinghaiProv.,Inst.ofPlantProtect.,QinghaiAcad.ofAgric. &Forest.Sci.;2.Sci.Observ. &Exper.Stat’nofCropPestinXining,MinistryofAgric.,Xining,Qinghai810016)
The herbicidal activity potential of toxin fromFusariumavenaceum GD-2 was evaluated against wild oats (AvenafatuaL.) in this study. The toxin was assayedinvitroto evaluate its inhibition against seed germination ofA.fatua. The toxin ofF.avenaceumGD-2 was shown to have an inhibitory effect of around 77.54% at 5 mg/mL against germination ofA.fatuaseeds. The inhibitory effect shown by the toxins against radicle had higher activity than plumule under the same concentration. The toxin ofF.avenaceumGD-2 significantly diminished the plant length the part on the ground with various treatments when treated with the toxin under greenhouse conditions. However, there were no significantly different reductions in plant length and the weed fresh weight with different treatments. In detached leaf injection bioassay, the toxic metabolite was characterized after the culture filtrates crude extraction with petroleum ether, chloroform, ethyl acetate andn-butanol. The residues left after solvent evaporation were evaluated separately for their toxicity against the target weed. Residue (5 mg/mL) obtained from n-butanol fraction showed the highest toxic activity when compared with others. Moreover, a host range experiments on the sensitivity of 10 plant species revealed that barnyard grasses and goosefeet were more sensitive to the toxin of the culture filtrate. Three herbicidal active compounds were isolated and purified from cultural filtrate with the same UV absorption peak. The recent results showed potential for the development of the toxins produced byF.avenaceumGD-2 as a bio-herbicidal source to control and eliminateA.fatuaweed.
herbicidal activity; phytotoxin;Fusariumavenaceum; wild oat (AvenafatuaL.) ; host-range
In cultivated wheat fields of the NW area of China, wild oats (AvenafatuaL.) is an aggressive weed, which competes with the wheat for soil nutrients, water and light. In the mean time, they can be suitable host for numerous pests (aphids, wheat stalks sawfly) and pathogens (wheat head blight, leaf spot and smut diseases). The hazard degree of wild oats might include up to 15.6% and 25.3% in winter and spring wheat fields, respectively[1]. The serious damage area of about 1.6 million per ha. and yield losses of 1.75 billion kg per year have been reported due to wild oats interference[2].
Many measures such as chemical control, crop rotation, manual control and no-tillage have been established for controlling wild oats in wheat fields[3]. The adverse effects of constant use of these chemical compounds have led to the emergence of different resistant weed varieties[4]. Biological control may offer a promising solution. Many pathogenic organisms, especially fungi of the genusFusarium, as biological control agents have received greater attention in recent years.
Fungi are among important microorganisms that produce a variety of bioactive extracellular toxic compounds. Herbicidal properties of such toxic metabolites of fungi have been exploited in weed integrated management[5-9]. SomeFusariumtoxins, such as enniatine and fumonisin, have been evaluated for their herbicidal properties[10-11]. In one of the studies,Fusariumnygamaiwas used for controllingStrigahermonthicawhich is the most common parasitic weeds in sub-Saharan Africa. The results revealed that four toxins produced byFusariumnygamai[12]isolated fromStrigahermonthicawere able to strongly inhibitS.hermonthicaseed germination[13]. Another showed that certain of such products have been patented and a few such as phosphinothricin, bialaphos, hydantocidin, have been commercialized[14-16].
FusariumavenaceumGD-2 was isolated from diseased wild oats plants, which showed high potential for biological control of wild oats[17]. However, little information exists on the wild oat and host range of the toxic metabolites produced by pathogenic fungusF.avenaceumGD-2. In this paper, the first objective of this study was to isolate toxic metabolites produced byF.avenaceumand investigate the effects of fungal toxins on wild oat and other plants. The second objective was to purify the toxins in order to determine their chemical properties.
1 Materials and methods
1.1 Materials
1.1.1 Selection of frequently occurring weed of wheat field Mature seeds ofAvenafatuaL. were collected from wheat fields located in the Xining, Institute of plant protection, Qinghai Academy of Agriculture and Forestry Sciences, P. R. China. The seeds specimens were dried under room temperature, packed in paper bags and stored at 4 ℃ until use.
1.1.2 Preparation of cultural filtrate of test fungus Pure culture ofF.avenaceumGD-2 was obtained from Key Laboratory of Agricultural Integrated Pest Management in Qinghai Province, China. Potato dextrose broth (PDB) (2%) was autoclaved at 121 ℃ in 500 mL Erlenmeyer flasks, with 100 mL medium in each flask. Flasks were inoculated with three fungal mycelial disks (5 mm) cut from margins of actively growing fungal colonies. Inoculated flasks were incubated under shaking conditions at 220 r/min for 7 days at 25 ℃ in 12 hours alternation of light and darkness. After 7 days, the cultures were filtered using four layers of cheesecloth and filtrates were preserved at 4 ℃ in a refrigerator. The cultural filtrates were used within 1 week to avoid any contamination or component degradation.
1.1.3 Toxin extraction A volume of 200 mL of original culture filtrate ofF.avenaceumwas extracted three times with 200 mL petroleum. The upper petroleum ether layer was separated off and vacuum dried in a rotary evaporator at 40 ℃ to remove any traces of solvents and to obtain the final residues. The remaining filtrate was extracted similarly in succession with chloroform, ethyl acetate andn-butanol. The final residues obtained from culture filtrate ofF.avenaceumwere stirred in vials for further experiments.
1.2 Methods
1.2.1 Characterization of herbicidal activity of culture filtrate extracts The following biological assays were conducted to characterize the herbicidal activity of the extracts.①Seed germination bioassay:TheA.fatuaseed-germination bioassay was conducted in 90-mm diameter Petri dishes. For surface sterilization,A.fatuaseeds were immersed in 1% sodium hypochlorite (NaOCl) solution for 3 min and thoroughly washed three times with sterile water, and air dried under room temperature. Disinfected seeds ofA.fatuawere distributed on the discs at a density of 30 seeds per dishes. A 200 μL aliquot of 2% methanol solution containing 200 μg of the extract was applied gently to each paper dishes, and two control treatments were performed with sterilized distilled water and only methanol under the same conditions as above. Four replicates were conducted for each treatment. The Petri dishes were arranged in a completely randomized design in a growth room maintained at 25 ℃ with 12 h light period daily. Plants ofA.fatuawere harvested 5 days after the start of germination (radicle emerged through seed coat). Data were expressed as percentage germination and radicle/plumule length. Seed germination was determined by counting the number of germinated seeds. Each measurement was repeated three times.②Greenhouse experiments:A.fatuaseeds were surface-sterilized in 1% NaOCl for 5 min, rinsed three times with sterile distilled water, and germinated on moistened filter paper in Petri dishes. After the seeds germinated (~72 h), they were planted in a commercial potting mix contained in peat strips. Each pot contained 10 plants. The potting mix was supplemented with a controlled-release(14∶14∶14, N∶ P∶K) fertilizer. The plants were placed in subirrigated trays that were mounted on greenhouse benches. Plants were grown at a temperature regime of (25/15±5)℃ (day/night) with supplemental light provided by 400 W Philips lamps 12 h per day.All the pots were arranged in a completely randomized design in greenhouse conditions. The extracts of culture filtrates of fungus were sprayed on 10 days weed seedlings. Treatment in a similar manner with distilled water and methanol only spray served as two control treatments. Each treatment was replicated four times. Plant height and biomass reductions were determined after 14 days. The experiment was repeated three times.③Detached leaf bioassay:Aqueous solutions of petroleum ether, chloroform, ethyl acetate andn-butanol residues were prepared in sterilized distilled water to obtain a final concentration of 5 mg/mL. Surface sterilized (2% NaOCl) leaves detached from 15 to 20 days old seedlings of the weed were treated with different organic extracts solutions and then were incubated for 24 h in moist chambers at room temperature. They were incubated at (28±2)℃ under constant fluorescent illumination (2×104erg/cm2/s).F.avenaceumwas further evaluated regarding its potential biological activity towards important plants. All organic solvent extracts obtained after cultural filtrate were combined, the combined extract was tested by the puncture and wilt bioassays. The following plants were tested: wheat (Triticumaestivum), rapeseed (Brassicanapus), barley (Hordeumvelgare), broadbean (Viciafaba), pea (Pisumsativum), carrot (Daucuscarota), mung bean (Vignaradiate), barnyardgrass (Echinochloacrus-galli), goosefoot (Chenopodiumalbum) and cabbage (Brassicaoleracea). Herbicidal activity was assessed after 24 h of incubation following the procedures outline above.In all the bioassays, sterilized methanol was used for control and sterilized distilled water served as control over control. All the treatments were carried out in quadruplicate and all the bioassays were repeated at least thrice.
1.2.2 Isolation of toxic compounds from cultural filtrate Thirty hundred milliliters of cultural filtrate was fractionated with an equivalent volume ofn-butanol to obtain aliquot layer. This material wad applied to a silica gel column (800 mm×40 mm internal diameter) and eluted with dichloromethane, dichloromethane/methanol mixtures, and methanol. Each active fraction eluted wad subsequently applied to HPLC analysis using an Athena 120A C18 column (250 mm×4.6 mm i.d.×5 μm) in a equal participation mode using 5∶95 methanol/water as eluent (v/v) and a flow rate 1 m/min with an injection volume ("loop") of 10 μL to yield five separate compounds (compounds A to C). Yield of each isolated compound was appropriately 0.1 mg. 0.1 mg of compound A-C were dissolved by in 0.1 mL methanol and diluted with sterilized distilled water to obtain a final concentration of 100 μg/mL. Seed germination bioassay was conducted as described above.
1.2.3 Statistical analysis All the data were subjected to standard analysis of variance procedures for a randomized complete design using the SPSS for windows (SPSS 2006). Treatment means were compared using the least significant difference (LSD) multiple range tests atP≤0.05.
2 Results
2.1 Herbicidal activity of culture filtrate extracts
2.1.1A.fatuaseed-germination bioassay Data depicted in table 1 clearly indicate that the toxic compound(s) produced byF.avenaceumGD-2 had significant influence on germination and early seedling growth ofA.fatua.Seed-germination bioassay demonstrated that germination ofA.fatuaseeds was reduced by 6.74% due to methanol treatment. The effect of methanol treatment was not significant as compared to sterilized water treatment. All the organic extracts treatments significantly reduced germination by 58.55%~77.54%, as compared to the negative control. All the fungal cultural filtrate extracts significantly reduced shoot length as compared to sterilized water and methanol treatments. The highest adverse effect was recorded for then-butanol extracts, where a 91.37% reduction in plumule length was recorded over the sterilized water treatment. Similarly, 5 mg/mL concentration ofn-butanol extracts significantly reduced the length of radicle. All the organic extracts treatments significantly suppressed the length of radicle as compared to sterilized water treatment.
Table 1 Effect of cultural filtrate extracts fromF.avenaceumGD-2 on germination and growth ofAvenafatuaL. in seed germination bioassay
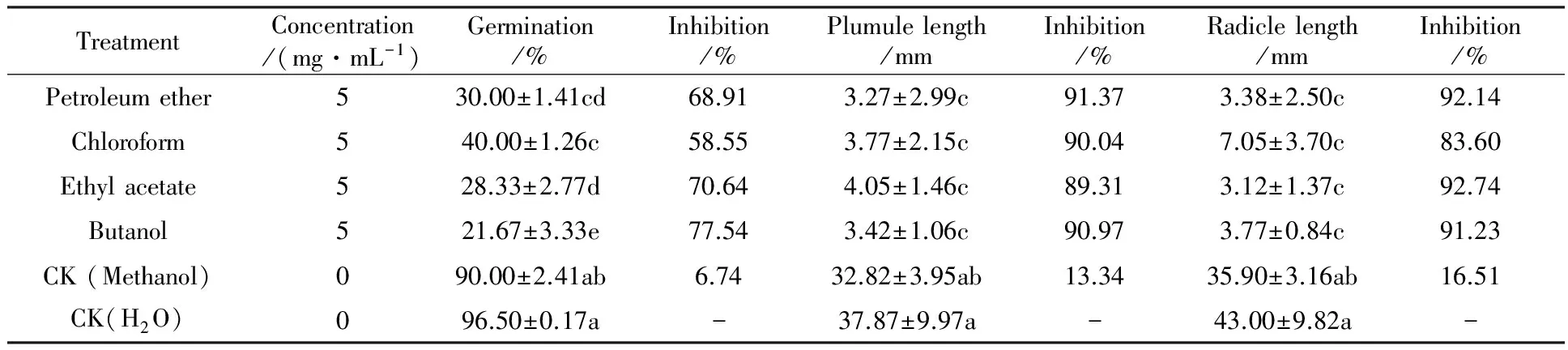
TreatmentConcentration/(mg·mL-1)Germination/%Inhibition/%Plumulelength/mmInhibition/%Radiclelength/mmInhibition/%Petroleumether530.00±1.41cd68.913.27±2.99c91.373.38±2.50c92.14Chloroform540.00±1.26c58.553.77±2.15c90.047.05±3.70c83.60Ethylacetate528.33±2.77d70.644.05±1.46c89.313.12±1.37c92.74Butanol521.67±3.33e77.543.42±1.06c90.973.77±0.84c91.23CK(Methanol)090.00±2.41ab6.7432.82±3.95ab13.3435.90±3.16ab16.51CK(H2O)096.50±0.17a-37.87±9.97a-43.00±9.82a-
Notes: In the columns, values with different letters show significant difference (P≤0.05) as determined by Duncan’s multiple range tests,the same as in Table 2
2.1.2 Foliar spray bioassay Weed growth inhibitory activity of all the organic extracts treatments was determined at 5 mg/mL againstA.fatuaplants. Among the all extracts examined, the inhibitory effect of these extracts was not significantly difference for length and biomass ofA.fatuaplumule. Similarly, the extracts of culture filtrates ofF.avenaceumGD-2 significantly reduced plumule length and biomass by 44.57%~50.33% and 72.34%~74.49% in 25-day-oldA.fatuaplants, respectively (Table 2). The plumule length was significantly reduced inA.fatuaplants by foliar spray of cultural filtrate extracts ofF.avenaceum. The adverse effect of foliar spraying on plumule length was recorded for then-butanol extract, where a 50.33% reduction in plumule length was recorded over the negative control. All the foliar spray treatment significantly reduced plumule fresh biomass in 25-day-oldA.fatuaplants. Similarly, foliar spraying of n-butanol extracts significantly reduced the fresh biomass of plumule by 74.49% in 25-day-oldA.fatuaplants.
Table 2 Effect of cultural filtrate extracts fromF.avenaceumGD-2 on the growth ofAvenafatuaL. in greenhouse bioassay
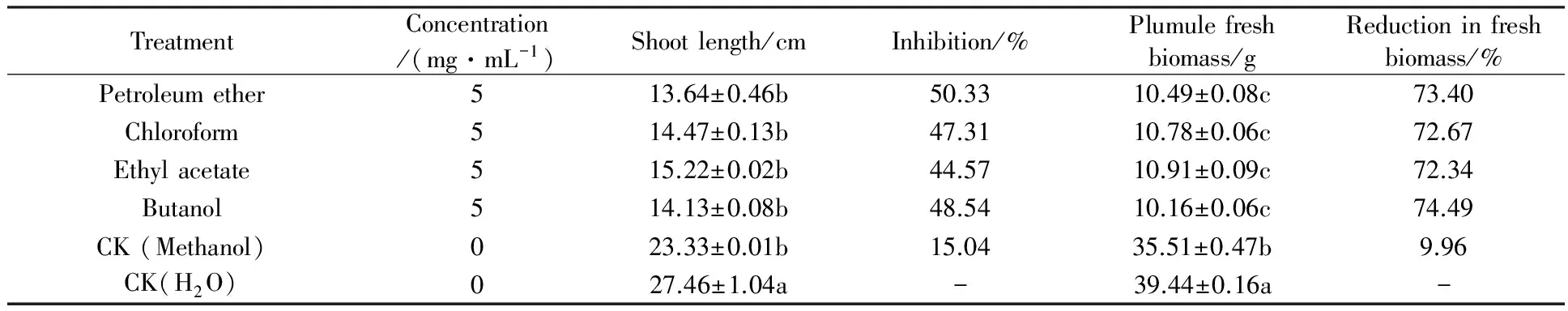
TreatmentConcentration/(mg·mL-1)Shootlength/cmInhibition/%Plumulefreshbiomass/gReductioninfreshbiomass/%Petroleumether513.64±0.46b50.3310.49±0.08c73.40Chloroform514.47±0.13b47.3110.78±0.06c72.67Ethylacetate515.22±0.02b44.5710.91±0.09c72.34Butanol514.13±0.08b48.5410.16±0.06c74.49CK(Methanol)023.33±0.01b15.0435.51±0.47b9.96CK(H2O)027.46±1.04a-39.44±0.16a-
2.1.3 Detached leaf bioassay The metabolites extracts ofF.avenaceumGD-2 was found to induce necrotic spots of varying sizes on the detached leaves as shown in table 3. Among the four organic solvent fractions tested, the highest toxic activity of then-butanol extract was obtained. The necrotic reaction covered the whole leaf area and the leaf was completely destroyed. The leaves ofA.fatuaexhibited some reduced sensitivity to the ethyl acetate extract and showed intermediate reactions to the chloroform and petroleum ether extracts of the culture filtrate and no toxic activity was found in methanol and water treatment.
A range of plants was tested for their susceptibility toF.avenaceum, the possible active ingredient in a biological control agent for use against the weedA.fatua. The tested plant response to crude extract showed that 50% of the tested plants were slightly susceptible (Table 4).
ChenopodiumalbumandEchinochloacrus-galliwere highly susceptible to the crude extract resulting in necrosis and heavy leaf necrosis with more than
Table 3 Necrotic area resulting fromFusariumavenaceumGD-2 extract in the detached leaf bioassay
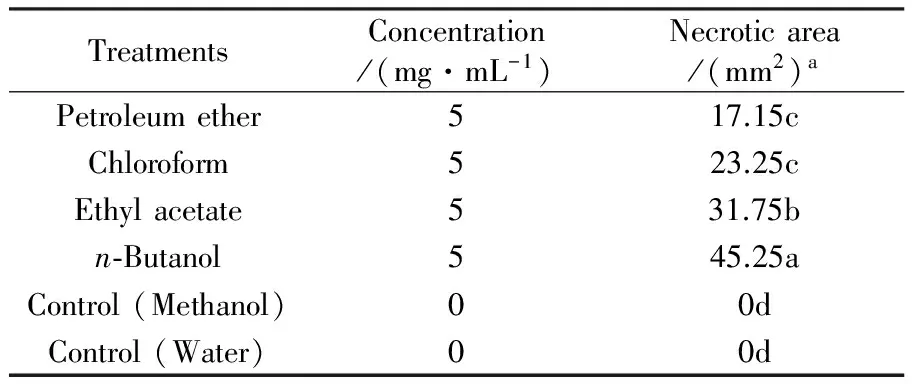
TreatmentsConcentration/(mg·mL-1)Necroticarea/(mm2)aPetroleumether517.15cChloroform523.25cEthylacetate531.75bn⁃Butanol545.25aControl(Methanol)00dControl(Water)00d
Note:aNecrotic area in the detached leaf bioassay was calculated according to the formula S=Π*d2/4, where d is the diameter of the necrotic area;Means with the same letter for each column are not significantly different after the Tukey’s LSD test atP≤0.05,the same below
Table 4 Reaction of various plant species toFusariumavenaceumGD-2 crude extract in the detached leaf bioassay 70% of leaf area coalescing with a resultant death. The dicotyledonPisumsativumandViciafabawere slightly susceptible to the crude extract resulting in leaf spotting.Daucuscarotaexhibited some reduced sensitivity and the monocotyledonTriticumaestivumandHordeumvelgareshowed intermediate reactions to the crude extract, indicating selectivity of the toxic metabolites towards some plant species.
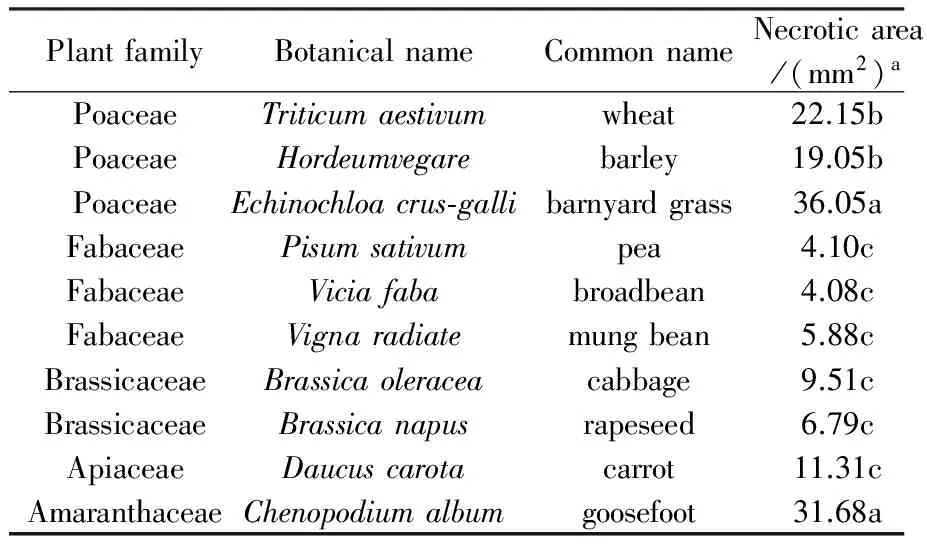
PlantfamilyBotanicalnameCommonnameNecroticarea/(mm2)aPoaceaeTriticumaestivumwheat22.15bPoaceaeHordeumvegarebarley19.05bPoaceaeEchinochloacrus⁃gallibarnyardgrass36.05aFabaceaePisumsativumpea4.10cFabaceaeViciafababroadbean4.08cFabaceaeVignaradiatemungbean5.88cBrassicaceaeBrassicaoleraceacabbage9.51cBrassicaceaeBrassicanapusrapeseed6.79cApiaceaeDaucuscarotacarrot11.31cAmaranthaceaeChenopodiumalbumgoosefoot31.68a
2.2 Isolation of toxic compounds from cultural filtrate
Among the 3 compounds tested at 100 μg/mL concentration on the inhibition ofA.fatuaseed seedling growth, compound B proved to be much more toxic than compound A and C, being able to reduce plumule and radicle length by 81.76% and 91.37%, respectively, compare with 81.18% and 85.49% with compound A and 80.58% and 83.53% with compound C. The inhibitory effects of compound A-C on radicle length were greater than those on radicle length. This showed that there were strong destructive effects on the germination growth ofA.fatua.
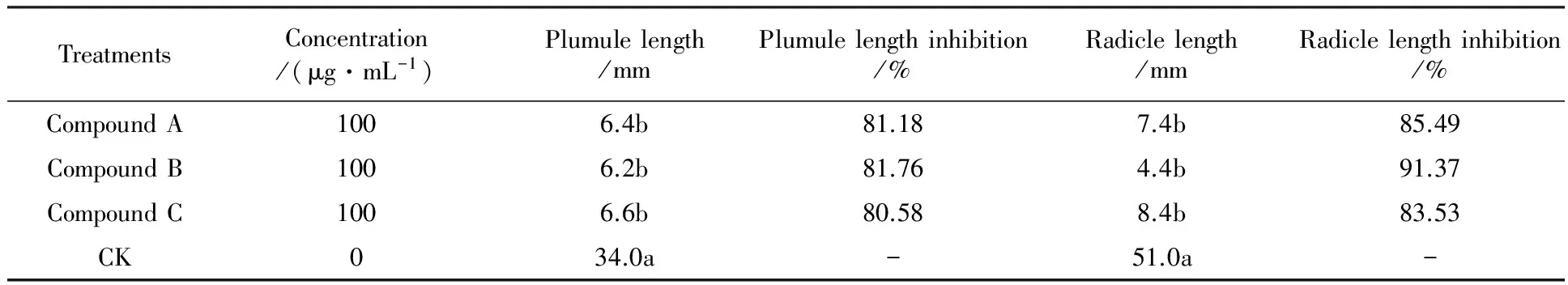
Table 5 Effect of compound A-C on seed seedling growth of A .fatua L.
UV absorption spectra of compounds A to C detected with the photodiode array detector on HPLC separation revealed that each compound showed three similar peaks (approximately 220, and 260 nm) of absorption (Fig.1), suggesting that the chemical structure of the compounds is similar.
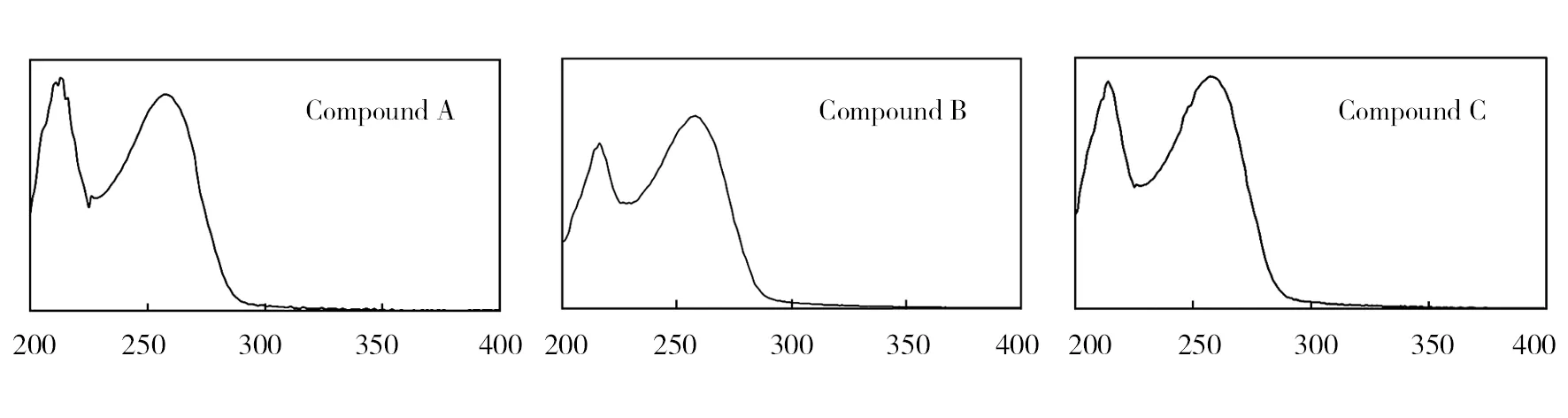
Wave length/nm
3 Discussion
In this study, it was observed thatF.avenaceumGD-2 produced phytotoxin in irregular lesions on weed leaves.Fusariumavenaceumis able to produce a wide range of chemical different bioactive secondary metabolites on artificial laboratory media, including deoxynivalenol (DON), zearalenone (ZEA), nivalenol (NIV), T-2 toxin, HT-2 toxins, moniliformin (MON), beauvericin (BEA) and enniatins (ENNs)[5,7,18-19]. The toxicity severalF.avenaceummetabolites has been thoroughly investigated. The earlier studies regarding the herbicidal activity ofFusariumspp. were restricted to the use ofF.oxysporum,F.proliferatum,F.semitectum,F.nygama,F.sambucinum,F.nivale,F.compactumandF.solani. These results are in agreement with reports of[20]for toxic metabolites ofF.avenaceumonRubusspecies weeds. However, this study reveals that the metabolites ofF.avenaceumspecies also exhibit herbicidal activity at low concentration.
InA.fatuaseed-germination bioassay, the radicle growth had a greater response to the fungal crude extracts with respect to plumule growth at the same concentration level. There are similar reports of effective inhibition of weed seeds germination by fungal species toxins, such asDrechslerarostrata,Drechsleraaustraliensis,Alternariaalternata,FusariumsolaniandFusariumoxysporumagainst the radicle growth ofPartheniumhysterophorusandMyrotheciumverrucaria,Fusariumcompactumagainst seed germination ofOrobancheramoseseeds have also been reported[21-23]. This could be due to the direct contact with toxic compounds and may be may be ascribed to the high rate of absorption of the toxic metabolites[24].
The occurrence of several different types of mycotoxins produced byF.avenaceumis also a risk factor due to possible synergistic effects. As data on the cytotoxicity and mode of action of many of the mycotoxins produced byF.avenaceumare almost completely lacking, more effort in this area is needed for proper risk assessment[25]. Enniatins are cyclic hexadepsipeptides, which are able to form cation selective channels in cellular membranes[26]. They are cytotoxic and toxic to insects, bacteria, and fungi. The toxic mode of action of moniliformin is suggested to be linked with inhibition of enzyme system and DNA synthesis, and also induce apoptosis in eukaryotic cells.
Despite high toxic activity was found in then-butanol extract of theF.avenaceumculture filtrate in the greenhouse experiment, the inhibitory activity of all foliar spray treatments was not significant on plumule length and biomass inA.fatuaplants. This may be because of the presence and variations in concentration of toxic principles in particular fractions.
The nonspecific toxins produced byF.avenaceumwere confirmed in this study by the ability to cause different degree disease symptoms in tested plants. Many toxins are produced byF.avenaceumare not selective, as they are able to cause the same toxic effects both on host and on non-host plants. For this reason, the toxicity to crop plants has to be ascertained. The isolate was able to weakly infect on pea, broad bean and mung bean. However, this fungus highly infected a large number of crop species in the Poaceae. These results suggest that toxins are host nonspecific, as in case of toxins produced by several otherFusariumspecies[27].
The toxins produced in cultural filtrate consisted of three active compounds. Yield of each compound was less than 0.1 mg per 5 L fermentation liquor, indicating that toxicity of the compounds toA.fatuaseed germination and detached leaves is very potent. Due to the low yields, the structure of these compounds could not be identified in this study. However, the characteristic information about their UV spectra, each showing the same two absorption peaks, may facilitate the identification of these compounds in further studies. Therefore, further research will expand ongoing chemical properties identification of these bioactive compounds.
Reference
[1] Tu H.L., Qiu X. L., Xin C.Y., et al. Study on key techniques of integrated control over wild oat on farmland[J]. Sci. Agric. Sin, 1993, 26 (4):49-56.
[2] Wei S.H., Zhang C.X., Zhu W.D., et al. Influence ofAvenafatuaon the yield characters of different wheat cultivars and its economic threshold[J]. J Triticeae Crops, 2008, 28(5):893-899.
[3] Jordan D.L., Lancaster S.H., Lanier J.E., et al. Weed management in peanut with herbicide combinations containing imazapic and other pesticides[J]. Weed Technol, 2009, 23(1):6-10.
[4] Travlos I.S., Giannopolitis C.N., Economou G. Diclofop resistance in sterile wild oat (AvenasterilisL.) in wheat fields in Greece and its management by other post-emergence herbicides [J]. Crop Prot, 2011, 30 (11): 1449-1454.
[5] Amalfitano C., Pengue R., Andolfi A., et al. HPLC analysis of FA, 9, 10-dehydrofusaric acid, their methyl esters, toxic metabolites from weed pathogenicFusariumspecies[J]. Phytochem. Analysis, 2002, 13(5):277-282.
[6] Dor E., Evidente A., Amalfitano C., et al. The influence of growth conditions on biomass, toxins and pathogenicity ofFusariumoxysporumf. sp.orthoceras, a potential agent for broomrape biocontrol[J]. Weed Res, 2007, 47(4):345-352.
[7] Idris A.E., Abouzeid M.A., Boari A., et al. Identification of phytotoxic metabolites of a newFusariumsp. inhibiting germination ofStrigahermonthicaseeds[J]. Phytopathol. Mediterr, 2003, 42(1):65-70.
[8] Souza A.P.S., Duarte M.L.R. Allelopathic activity of culture filtrate produced byFusariumsolani[J]. Planta daninha, 2007, 25(1):227-230.
[9] Kroschel J., Elzein A. Bioherbicidal effect of Fumonisin B1, a phytotoxic metabolite naturally produced byFusariumnygamai, on parasitic weeds of the Genus Striga[J]. Biocontrol Sci. Technol, 2004, 14(2):117-128.
[10]Abbas H.K., Boyette C. D., Hoagland R.E., et al. Bioherbicidal potential ofFusariummoniliformeand its phytotoxin, fumonisin[J]. Weed Sci, 1991,39(4): 673-677.
[11]Hershenhorn J., Park S.H., Stierle A., et al.Fusariumavenaceumas a novel pathogen of spotted knap-weed and its phytotoxins, acetamido-butenolide and enniatin B[J]. Plant Sci, 1992, 86(2): 155-160.
[12]Capasso R., Evidente A., Cutignano, A., et al. Fusaric and 9,10-dehydrofusaric acids and their methyl esters fromFusariumnygamai[J]. Phytochemistry, 1996, 41(4):1035-1039.
[13]Zonno M.C., Vurro M., Evidente A., et al. Phytotoxic metabolites produced byFusariumnygamaifromStrigahermonthica[A]. In: Proceedings 1996 IX International Symposium on Biological Control of Weeds. Stellenbosch, South Africa, 1996, 223-226.
[14]Saxena S., Pandey A.K. Microbial metabolites as ecofriendly agrochemicals for the next millenium[J]. Appl. Microbiol. Biotechnol, 2001, 55(4):395-403.
[15]Pandey A.K., Singh J., Shrivastava G.M., et al. Fungi as herbicides: Current status and future prospects[M]. In: Trivedi PC (ed), Plant Protection: A Biological Approach, Jaipur, India, Avishkar Publishers and Distributors, 2003:305-339.
[16]Mutsuo N, Kazuko I, Yasuyuki T, et al. Hydantocidin: a new compound with herbicidal activity fromStreptomyceshygroscopicus[J]. J Antibiot, 1991, 44(3):293-300.
[17]Zhu H. X., Cheng L., Guo Q. Y. Identification and virulence of threeFusariumstrain againstAvenafatuaand safety on 5 crops[J]. China Journal of Biological Control, 2010, 26(S):84-89.
[18]Evidente A., Amalfitano C., Agrelli D., et al. The influence of growth conditions on biomass, toxins and pathogenicity ofFusariumoxysporumf. sp.orthoceras, a potential agent for broomrape biocontrol[J]. Weed Res, 2007, 47(4):345-352.
[19]Lindblad M, Gidlund A., Sulyok M., et al. Deoxynivalenol and other selectedFusariumtoxins in Swedish oats-occurrence and correlation to specificFusariumspecies[J]. Int J Food Microbial, 2013, 167(2):284-291.
[20]Oleskevich C., Shamoun S.F., Vesonder R.F., et al. Evaluation ofFusariumavenaceumand other fungi for potential as biological control agents of invasiveRubusspecies in British Columbia[J]. Can. J Plant Pathol, 1998, 20(1):12-18.
[21]Adrees H., Javaid A. Screening of some pathogenic fungi for their herbicidal potential against parthenium weed[J]. Pak. J. Phytopathol,2008, 20(1): 150-155.
[22]Andolfi A., Boari A., Evidente A., et al. Metabolites inhibiting germination ofOrobancheramoseseeds produced byMyrotheciumverrucariaandFusariumcompactum[J]. J Agric. Food Chem, 2005, 53(5):1598-1603.
[23]Javaid A., Adrees H. Parthenium management by cultural filtrates of phytopathogenic fungi[J]. Nat. Prod. Res, 2009, 23(16):1541-1551.
[24]Javaid A., Shah M.B. Phytotoxic effects of aqueous leaf extracts of twoEucalyptusspp. againstPartheniumhysterophorusL[J]. Science International (Lahore), 2007, 19(4):303-306.
[25]Gutleb A. C., Morrison E., Murk A. J. Cytotoxicity assays for mycotoxins produced byFusariumstrains: a review[J]. Environ. Toxicol. Pharmacol, 2002, 11(3-4):309-320.
[26]Uhlig S., Jestoi M., Knutsen A. K., et al. Multiple regression analysis as a tool for the identification of relations between semi-quantitative LC-MS data and cytotoxicity of extracts of the fungusFusariumavenaceum(syn.F.arthrosporioides)[J].Toxicon, 2006,48(5):567-579.
[27]Bottalico A., Perrone G. ToxigenicFusariumspecies and mycotoxins associated with head blight in small-grain cereals in Europe[J].Eur. J. Plant Pathol, 2002, 108(7): 611-624.
国家自然科学基金项目(31160371,30860165);国家“十二五”科技支撑项目(2012BAD19B02);
程亮 男,博士研究生。研究领域为天然产物。Tel:0971-5313283,E-mail:liangcheng1979@163.com
燕麦镰刀菌GD-2毒素对野燕麦的除草活性研究
程 亮1,2, 郭青云1,2*
(1.青海省农林科学院植物保护研究所 青海省农业有害生物综合治理重点实验室,青海 西宁 810016;2.农业部西宁作物有害生物科学观测实验站,青海 西宁 810016)
评价了燕麦镰刀菌GD-2毒素对野燕麦的除草活性潜力。毒素对野燕麦种子萌发抑制试验表明,当毒素浓度达到5 mg/mL时,对野燕麦种子的萌发抑制效果达77.54%,在相同浓度下,对野燕麦种子胚根的抑制效果高于对胚芽的抑制效果。在温室条件下,毒素处理野燕麦植株后,各个不同处理野燕麦株高和地上部鲜重明显减少,然而,各处理间没有明显差异。用石油醚、氯仿、乙酸乙酯和正丁醇依次萃取燕麦镰刀菌发酵滤液并获得粗提物,其中正丁醇浸提物(5 mg/mL)在离体叶片实验中效果优于其他有机溶剂粗提物。此外,在毒素对10种植物的敏感性实验中,其中稗草和藜表现对燕麦镰刀菌毒素敏感。从燕麦镰刀菌中分离出3个除草活性化合物,且具有相同的紫外吸收峰。当前结果表明燕麦镰刀菌产生的毒素具有开发成为防除野燕麦生物源除草剂的潜力。
除草活性;植物毒素;燕麦镰刀菌;野燕麦;寄主范围
Q939.97
A
1005-7021(2016)02-0067-07
10.3969/j.issn.1005-7021.2016.02.012
国家高技术研究发展计划(国家“863”计划)(2011AA10A206)
* 通讯作者。女,硕士。研究领域为农田杂草综合治理。Tel:0971-5313283

