Cu含量对Pd-Cu/铝土矿CO氧化催化剂结构和性能的影响
詹瑛瑛 徐聪波 陈崇启 刘 弦 马永德 江莉龙
(福州大学化肥催化剂国家工程研究中心,福州350002)
Cu含量对Pd-Cu/铝土矿CO氧化催化剂结构和性能的影响
詹瑛瑛 徐聪波 陈崇启 刘 弦 马永德 江莉龙*
(福州大学化肥催化剂国家工程研究中心,福州350002)
利用资源丰富的天然铝土矿经NaOH溶液水热处理后焙烧,获得比表面积达174m2·g-1铝土矿载体,制备了双金属Pd-Cu为活性组分的催化剂,金属Pd负载量为0.5%(质量百分数),以CO氧化反应为探针反应,详细考察了Cu含量的变化对催化剂物化性能的影响。研究发现,Cu的引入有利于提高金属Pd的分散度,同时随着Cu含量的变化,金属Pd与Cu之间以及金属与铝土矿载体之间的相互作用随之改变。催化剂的CO氧化反应性能评价结果表明,Pd和Cu负载量分别为0.5%和4%的样品(PdCu4/MB)催化反应性能最佳。结合表征结果认为,PdCu4/MB的高活性归因于良好的Pd和Cu分散度,金属Pd、Cu以及金属与载体之间较强的相互作用。此外,CO-TPD表征结果说明较强的CO吸附能力和从载体中获取氧的能力也有利于提高PdCu4/MB样品的CO氧化反应性能。
钯-铜双金属;碱溶液处理铝土矿;一氧化碳氧化;铜含量
0 Introduction
CO oxidation(CO-Ox)is considered as one of the most direct and cheap methods to achieve CO tolerance in various applications,i.e.hydrogen purification for polymer electrolyte membrane fuel cells(PEMFCs)[1],CO gas sensor[2-3],and CO removal in automotive application[4-5]etc.An ideal catalyst for CO-Ox should be cost effective and exhibit high activity,selectivity and thermal stability.
The noble metals(Pt[6-7],Pd[8-9],Au[10-11],etc.)and non-noble metal catalysts,such as Cu[12-13],Mn[14], Co[15-16],etc.,have been extensively studied for CO-Ox applications.In addition,noble metal and non-noble metal bimetallic catalysts were also developed due to their synergistic activity[17-19].A series of Au-based bimetallic catalysts were studied by Wang et al.[17],it was found that Au-M bimetallic catalysts showed greater tunability in structure and chemical composition than the single-metal analogues,thus providing more possibilities for improvement in activity,selectivity,and/or stability.Liu et al.[19]also reported that SBA-15-supported Au-Cu showed much better performance than monometallic(Au-or Cu-) particleswith the rich present of H2.Pd-M(M=Pt,Cu, Ni,etc.)bimetallic catalysts were also extensively investigated[20-23].A series of bimetallic Pd-Cu catalysts were synthesized from different copper precursors by Wang et al.[23],and distinguished dispersion of Cu was obtained.The Pd and Cu existed in alloy state can be reduced easily,thus showing excellent performance in CO-Ox.
The chemically synthesized metal oxides,such as CeO2[9,24],Al2O3[22],TiO2[10]etc,were selected as supports for CO-Ox catalysts.The natural abounded and costeffective mineral,such as bauxite,diatomite or rectorite,is rarely explored for catalyst support. Natural rectoritemineralwas used as raw material for synthesis ZSM-5 by Bao et al.,and it was found that hierarchical pore structure and enhanced catalytic performance can be obtained[25].Natural bauxite, composed of Al2O3,FeOx,TiO2,CaO,SiO2[26],is found to be an excellent support for the NOxreduction in our previous work[26-28].Yet,it has not been investigated as support for CO-Ox.Herein the natural bauxite was firstly treated by hydrothermal method without any additive,following by impregnated with active metal. It is well known that NaOH can react with the Al2O3, TiO2and SiO2,hence the composition and texture(i.e. pore volume,pore diameter and surface area)of bauxitemay bemodulated by NaOH treatment.To the best of our know ledge,the investigation of NaOH-treated bauxite as support for catalyst is still unexplored.
Therefore,herein a series of catalysts of Pd-Cu supported on bauxite,which is hydrothermal treated in NaOH aqueous solution,were fabricated.Their catalytic performances for CO-Ox were investigated. The chemical and physical properties of those catalysts were characterized by XRD,N2-physisorption,H2-TPR,CO-TPD and CO2-TPD techniques,and the relationship between their physicochemical properties and catalytic performances are discussed in detail.
1 Experimental
1.1 Catalysts preparation
1.1.1 Materials
Natural bauxite(technical grade)is from Zhangpu County,Fujian Provice,China;Palladium chloride(PdCl2),Copper(II)nitrate(Cu(NO3)2·3H2O) and Sodium hydroxide(NaOH)are all analytical grades and used without further purification.
1.1.2 Synthesis
Firstly,the natural bauxite was pre-treated with NaOH by hydrothermal method.Typically,10 g natural bauxite,which had been washed by deionized water and dried at100℃for 10 h,was dispersed in a certain amount of NaOH solution by ultrasonication. After that the suspension was transferred to a Teflonlined stainless steel autoclave of 100 mL capacity, sealed and heated at 120℃for 36 h.When the autoclave was cooled to room temperature naturally, the precipitate was centrifuged,dried at 110℃for 4 h,and calcined at 450℃for 2 h.The as-prepared support is named asmodified bauxite(MB).Secondly,
the Pd and/or Cu was loaded on MB by incipient impregnationmethod,and the loading amount of Pd is fixed to 0.5%(weight percentage,wt.)with different amounts of Cu loading,e.g.0%,2%,4%,and 6%.In detail,5g of the MB and a certain amount of PdCl2and/or Cu(NO3)2·3H2O aqueous solution were mixed and soaked for 12 h at room temperature; subsequently,the as-obtained slurry was dried at 120℃for 4 h,following by calcination at 500℃for 3 h. The as-prepared Pd-Cu/bauxite catalyst is denoted as PdCux/MB,where the X is the weight ratio of Cu.
For comparison,the monometallic Cu/MB catalysts were prepared by the same incipient impregnation method and calcined under air at 500℃for 3 h.The loading of Cu is 2%.
1.2 Characterizations
The powder X-ray diffraction(XRD)patterns of the samples were recorded by a PANalytical X Pert Pro diffractometer using Co Kαradiation(λ=0.179 nm)at 40 kV and 40 mA.To determine the textural properties of the as-prepared samples,nitrogen adsorption-desorption measurements were carried out at 77 K using a Micrometrics ASAP 2020 system after the sample was degassed at 200℃in a vacuum for 4 h.Temperature-programmed reduction of hydrogen (H2-TPR)measurement was carried out on an AutoChem 2920 instrument.The H2-TPR was performed by passing 10%H2/Ar(flowing rate=30 mL·min-1)on 50mg catalystata heating rate of 10℃·min-1.Prior to the measurement,the samples were pre-treated under He atmosphere at 250℃for 60 min,then cooled to ambient temperature purging with pure Argon gas.The hydrogen consumption was monitored using a Thermal Conductivity Detector (TCD).The temperature-programmed desorption of CO or CO2(CO-TPD or CO2-TPD)was also performed on the AutoChem 2920 instrument equipped with a mass spectrometer(Hiden,HPR 20).Firstly,50 mg of the sample was pre-treated with 10%H2-Ar(300℃for CO-TPD)or pure He(250℃for CO2-TPD)for 1 h. After cooling to the room temperature,the sample was purged with 5%CO-He(for CO-TPD)or pure CO2(for CO2-TPD)for 1 h.Subsequently,the sample was purging with He for 60 min until the baseline was stable,then heating to 900℃at a rate of 10℃·min-1with He.
1.3 Evaluation of catalytic perform ance
The catalytic activity of the catalyst for CO catalytic oxidation was tested in a fixed bed reactor at atmospheric pressure from 150 to 250℃at an interval of 25℃.Typically,1 mL of catalyst(20~40 mesh)was used and the space velocity was calculated to be 5 000 h-1;feed gas was 3%CO(volume percentage),3%O2and balance with He.Prior to the catalytic test,the catalyst was first reduced with 10% H2/N2at 250℃for 2 h.The inlet and outlet concentrations of CO were monitored by a gas chromatograph(Shimadzu GC-8A)equipped with a thermal conductivity detector(TCD).The activity was expressed by the conversion of CO,defined as:
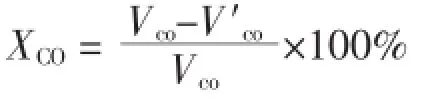
where VCOand VCOare the inlet and outlet content of CO,respectively.
2 Results and discussion
2.1 Characterizations
2.1.1 Crystal structure and texture of the Pd-Cu/MB catalyst
The XRD patterns of the as-prepared Pd-Cu/MB catalysts are shown in Fig.1.Those characteristic diffraction peaks of SiO2(JCPDSNo.77-1060,labeled with“#”)and Fe2O3(JCPDS No.84-0307,labeled with“*”)are ascribed to the MB support.According to the XRF characterization,the MB support ismainly composed of SiO2(13.0%,weight percentage),Fe2O3(19.7%)and Al2O3(57.6%,amorphous).When PdO and/or CuO are loaded,some diffraction peaks(2θ= 40.1°,50.2°and 65.2°)of PdO(JCPDSNo.88-2434, labeled with“‡”)and CuO(JCPDS No.05-0661, labelled with“†”)are observed.By carefully examining those characteristic peaks of PdO and CuO, it can be found that their intensities are distinguished from each other with the variation of Cu loading.For the Pd/MB sample with no CuO,the diffraction peaks are sharp and narrow(Fig.1a);with the increase of
CuO loading,the peak intensity becomes weaker. When 4%Cu was introduced,no characteristic peaks for PdO can be observed,suggesting that there must be interaction between PdO and CuO.It can be concluded that the introduction of CuO is favor for the dispersion of PdO on MB support and acquiring small crystal size of PdO.
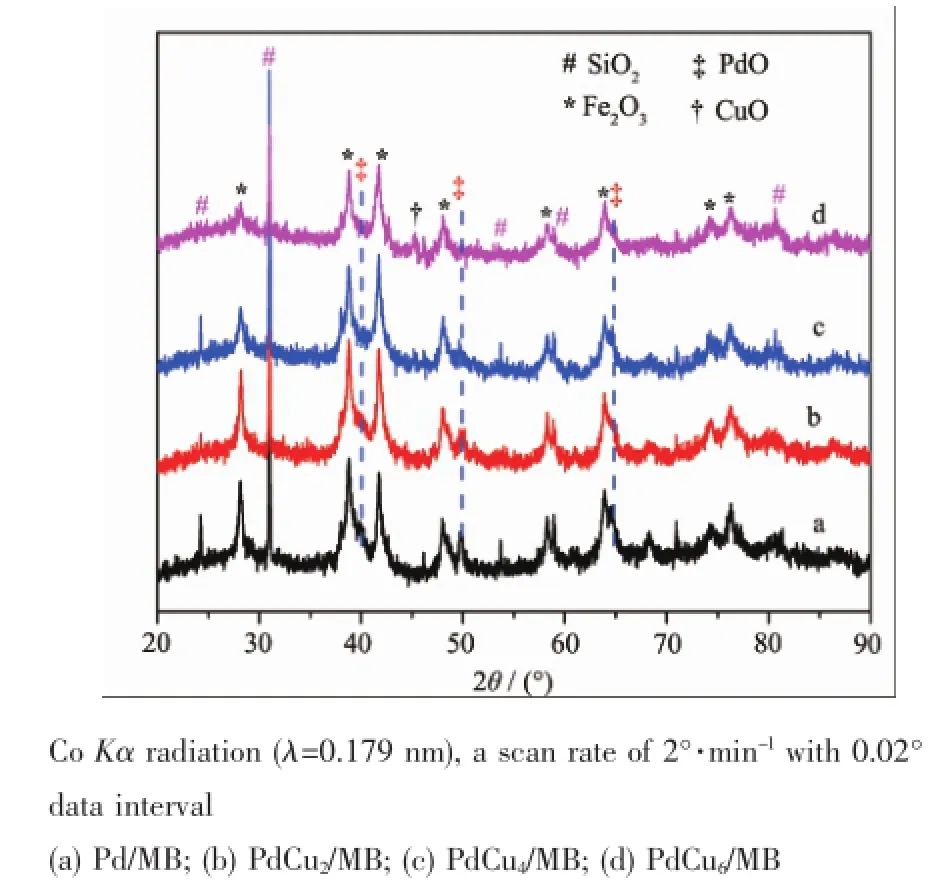
Fig.1 XRD patterns of the as-prepared Pd-Cu/MB catalysts
The BET surface area(SBET)of the natural bauxite is 40 m2·g-1with pore volume(Vp)of 0.09 cm3·g-1and average pore diameter(Dp)of 13.7 nm.After hydrothermal treated with NaOH aqueous solution (MB),the SBETand Vpincrease to 174 m2·g-1and 0.25 cm3·g-1,respectively,and the Dpis greatly decrease to 5.3 nm.It is suggested that the MB provides a possibility to serve as an ideal support for CO-Ox catalyst with good texture parameters after NaOH modification.After impregnated with Pd and/or Cu, the specific surface area of those Pd-Cu/MB catalysts decrease while their Dpincrease oppositely,suggesting that the PdO and/or CuO are loaded on the surface of MB,thus blocking the partialmicro-pore ofMB.
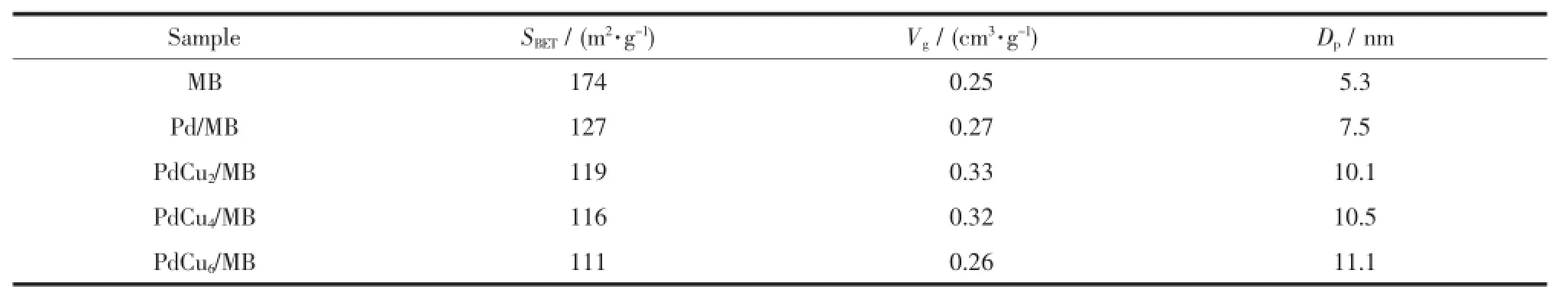
Table 1 Textural parameters of Pd-Cu/MB catalystwith different Cu loading
2.1.2 Reduction property of the Pd-Cu/MB catalyst
As seen in Fig.2,only two reduction peaks(460 and 689℃)can be observed for the MB support, which is ascribed to the reduction of Fe2O3→Fe3O4and Fe3O4→Fe,respectively;after the introduction of Pd and/or Cu,new reduction peaks are detected in the temperature range of 50~250℃.H2-TPR analysis for 5%PdO/Fe2O3catalyst was carried out by Kast et al.[8],and the PdO was started to be reduced at around 325 K.In our case,the small peak at ca.75℃for monometallic Pd/MB sample(Fig.2)can be assigned to the reduction of PdO→Pd,as is in agreement with the diffraction peaks of PdO are clearly observed in the XRD investigation(Fig.1a). Meanwhile,the reduction process of Fe2O3is greater enhanced when small amount of Pd was added, suggesting that theremust be interaction between MB support and impregnated Pd.It was reported that the formation of Pd would induce the H-spillover from Pd to support[8,29],thus lowing the reduction temperature of Fe2O3.It should be also noted that there is a broad signal in the temperature of 100~200℃(rectangle by dash line in Fig.2),suggesting the strong interaction between PdO and MB support[8].After loading with CuO(CuO/MB),a sharp peak locates at 208℃is detected to the reduction of CuO→Cu,and the shift of Fe2O3reduction temperature to lower also occurs, owing to the H-spillover effect of Cu,which originates from the interaction between CuO and MB support.As for bimetallic PdCu2/MB sample,a broad peak
centered at 132℃up to 276℃is detected,no separate peak for the reduction of PdO can be seen, indicating that the presence of Pd alongwith Cumakes the reduction of CuO and PdO as a single broad peak. This reveals that a Pd-Cu interaction also exists in the Pd-Cu/MB catalyst,as is in good agreement with the result of XRD characterization,and the synergistic interaction between Cu and Pd further improves the reducibility of Cu species.In order to carefully examine the effectof differentamountof CuO on the reducibility of the Pd-Cu/MB catalyst,whose H2-TPR characterizationwasalso carried out,and the resultsare shown in Fig.3.
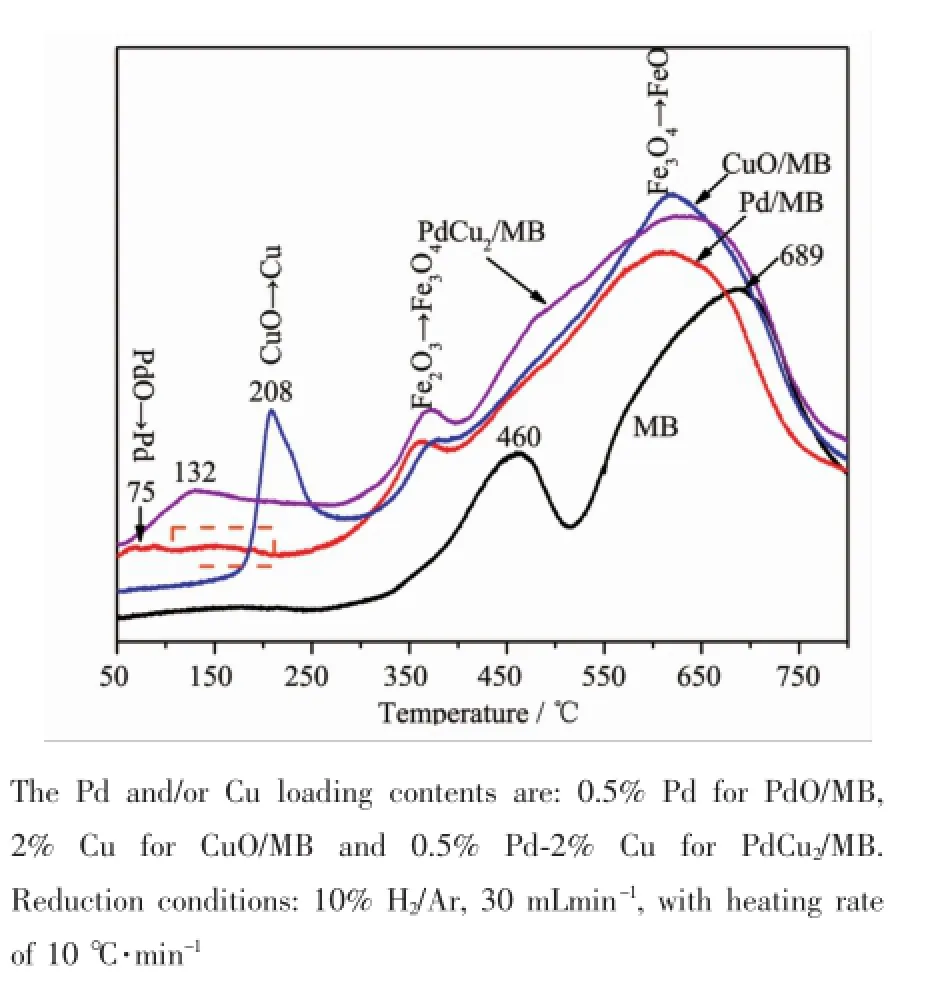
Fig.2 H2-TPR profiles of the MB supportand as-prepared catalysts
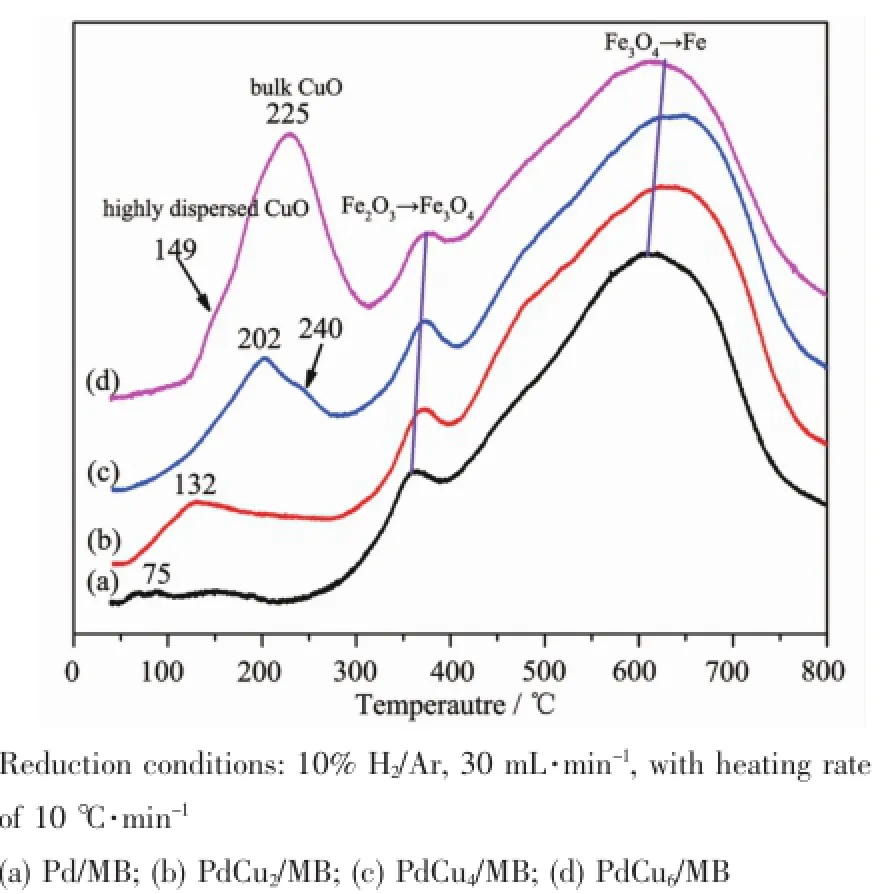
Fig.3 H2-TPR profiles of the as-prepared Pd-Cu/MB catalysts
From Fig.3,it can be seen that the lowest reduction temperature for Fe2O3→Fe3O4is found for the one loaded with monometallic Pd(Fig.3a), suggesting the interaction between Pd and MB ismore efficacious in promoting the reduction of Fe2O3than that of Cu species.With the increase of CuO loading, the reduction peaks of Fe2O3→Fe3O4and Fe3O4→Fe slightly shift to higher temperature.The differences in temperature shift could be ascribed to the blocking effect of bulk CuO.Themore the CuO is introduced, the stronger the blocking effectwill be.This view will be detailedly discussed subsequently.
In Fig.3,those peaks locate below the 300℃are assigned to the reduction of PdO and/or CuO species. H2-TPR characterization for bimetallic CuPd/CeO2catalyst was carried out by Fox et al.[30],and an intense peak centered around 160℃with a broad envelop up to 310℃was detected;after treated with HNO3,the CuO particles(similar to the bulk CuO) was removed,and only a sharp peak centering at 130℃was found,which can be attributed to the reduction of CuO clusters that highly dispersed on the CeO2surface and interacted closely with PdO.It is noteworthy in Fig.3 that all peaks of the bimetallic Pd-Cu/MB catalyst for PdO and/or CuO reduction are asymmetric,which can be decomposed to amain peak and a shoulder.Therefore there must be more than one CuOxspecies(highly dispersed and bulk CuO)in the catalysts.
Based on the above analysis,it can be inferred that the added 2%Cu iswell dispersed on the surface of MB with extremely small amount of bulk;when more Cu is introduced,more CuO particle(bulk) appears,which is identified by the higher reduction temperature,i.e.240 and 225℃for PdCu4/MB and PdCu6/MB,respectively.As seen in Fig.3,the areas of the reduction peaks of bulk CuO increase with the increase of CuO addition,resulting in enhancing the blocking effect between Pd(and/or Cu)and MB support.The largest amount of highly dispersed CuO
is found for the PdCu4/MB,which is thought to be favor for the catalytic CO oxidation.
2.1.3 Surface basicity of the Pd-Cu/MB catalyst
CO2isextensively used asa probemolecule for the investigation ofbasic property of catalyst[31-32].Different species can be formed on basic sites with diverse strengths when CO2is chemisorbed on the surface of solid bases[33].The formation of bicarbonate species involves surface hydroxyl groups(OH-,weak basic sites),whereas unidentate and bidentate carbonates are formed on surface oxygen atoms(medium-strength basic sites).CO2adsorbed as unidentate species on lowcoordinated oxygen ions shows strong basic strength (isolated O2-ions).Asaproductof catalytic CO oxidation reaction,CO2can strongly affect catalyst performance through its adsorption properties[34].Hence,the CO2-TPD analysis was carried out for the Pd-Cu/MB catalysts,and the results are shown in Fig.4.
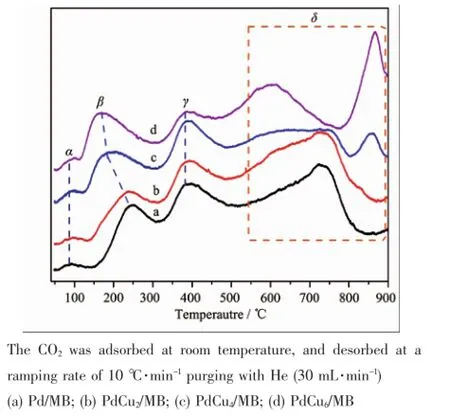
Fig.4 TCD signals of CO2-TPD characterizations Pd-Cu/ MB catalysts:
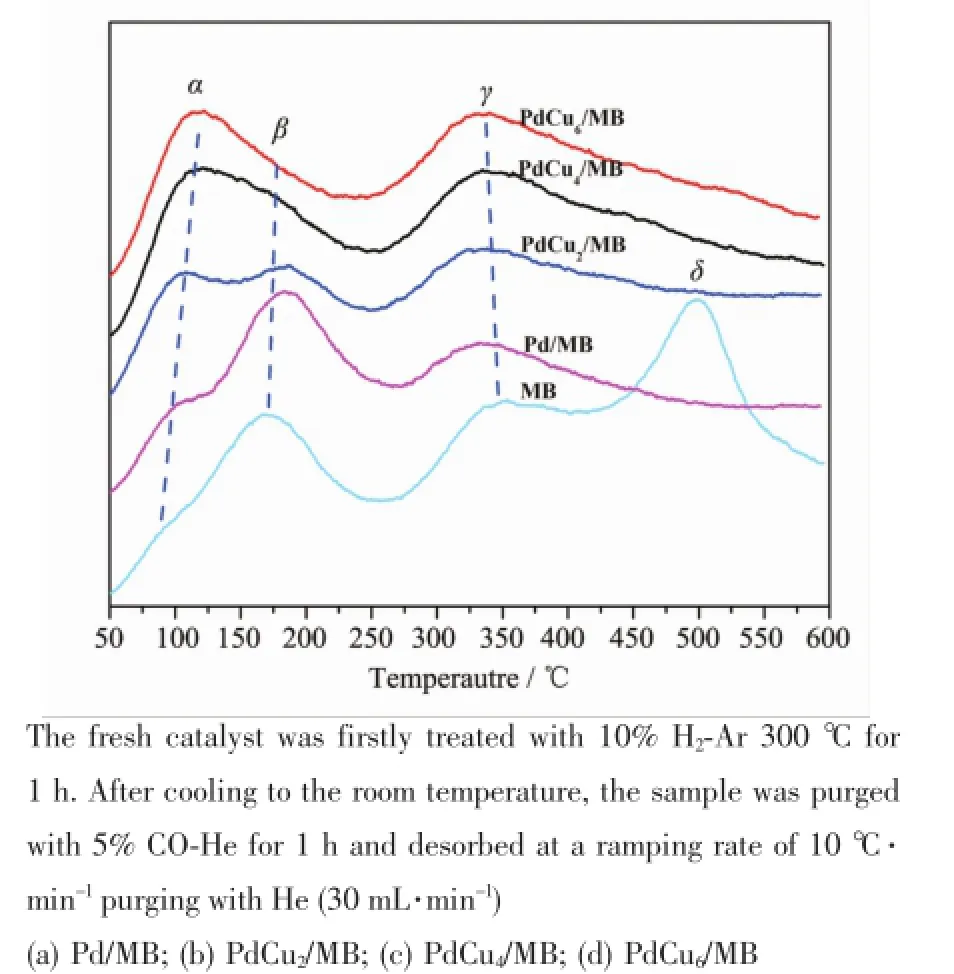
Fig.5 CO2signals of CO-TPD characterizations for MBsupport and Pd-Cu/MB catalysts
It is evident from Fig.4 that the basic properties of the as-prepared Pd-Cu/MB catalysts are influenced by the introduction of CuO.Four types of CO2-desorption peaks are collected:a weak peak(<100℃, peakα)is detected for each sample with similar intensity;a peak(peakβ)is evidence in the medium temperature range of 150~300℃,and the peak slightly increases in intensity and shifts to lower temperature with increased Cu loading;two hightemperature peaks(>300℃),peakγ(300~500℃) and peakδ(500~900℃)appear for all four catalysts, and the peak dissolves into two peaks with the increasing addition of Cu.The peakαis assigned to the weak basic sites that is associated with the bicarbonate absorbed on surface OH groups.The peak βis ascribed to the medium-strength basic sites absorbed on the M2+-O2-pairs(M=Pd or Cu),and the increase of peak intensity might be attributed to the increase of M2+-O2-pairs with rising Cu loading.The high-temperature peaks may be unidentate species strongly absorbed on low-coordinated O2-ions,and the addition of Cu results in the formation of new basic site,thus leading to the peakδdissolved.
2.1.4 CO-TPD characterization of the Pd-Cu/MB catalyst
Furthermore,to obtain a highly active catalyst, enough sites for catalytic adsorption of CO and providing oxygen from support are essential. Thereafter,the CO-TPD characterization for the asprepared Pd-Cu/MB was carried out,and the results are shown in Fig.5.
The effluents of CO-TPD analysiswere traced by amass spectrometer,only CO2(m/e=44)was detected, indicating that the absorbed CO is chemical bonded to the catalyst.The chemical bonded CO interacts with
the surface hydroxyl groups and/or oxygen species (including surface oxygen,lattice oxygen,interfacial oxygen,etc.),and desorbed as CO2during the heating process[31,35].Three desorption peaks are collected for the supported catalysts.With the increase of Cu loading,the intensity of peakαincreases accordingly, while the intensity of peakβdecreases linearly.The increase in the amount of CO2desorbs at low temperature(peakα)reflects that the ability to extract oxygen from the support by the adsorbed CO is enhanced.The largest intensity of peakαis found for the PdCu4/MB sample.
2.2 Catalytic activity for CO oxidation of the Pd-Cu/MB catalyst The catalytic performances of the series Pd-Cu/ MB catalysts modified with different content of CuO for CO oxidation are presented in Fig.6.The catalytic activity of CuO/MB and MB support are also present for comparison.Compared to the monometallic CuO/ MB and Pd/MB,the catalytic activity is enhanced for Cu modified bimetallic Pd-Cu/MB catalysts.It also can be seen that the amount of Cu loading strongly affects their catalytic performance.When small amount of Cu was introduced(PdCu2/MB and PdCu4/ MB),the added Cu has greatly positive effect on their catalytic performances for CO oxidation,and the optimized Cu loading is found to be 4%.However, further increase in Cu loading(PdCu6/MB)leads to lower CO conversion,which is possibly related to the blocking effect of bulk CuO on the interaction between Pd and MB support and/or excessively strong adsorption of surface carbonates on the new basic sites.
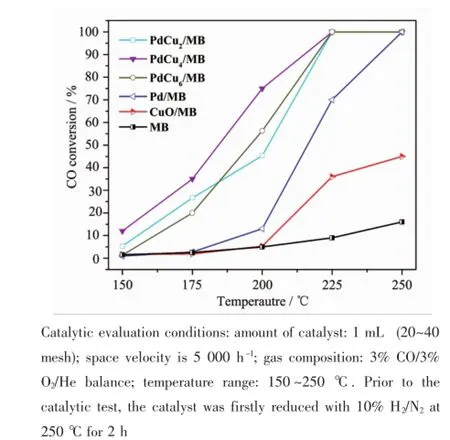
Fig.6 Catalytic activities of the as-prepared Pd-Cu/MB catalysts
2.3 Discussions
In the Pd-rich bimetallic Pd-Cu catalysts,the beneficial effect of copper specie is related to the formation of an active Pd-rich Pd-Cu alloy,which is more reactive than either of the monometallic(Cu orPd)clusters[36].Whereas for the Cu-rich samples, effects of copper specie are complicated,it is highly depended on the amountof Cu loading.
Based on the above analysis,the addition of Cu is favor for the Pd dispersion,and lowering the particle size of Pd,no evident diffraction peaks for PdO can be observed when the Cu loading is higher than 4%.The good dispersion of PdO and small size of PdO particles can also be confirmed by the CO2-TPD characterization:the medium-strength basic sites increase with the increasing of Cu loading,suggesting thatmore M2+-O2-pairs(M=Pd or Cu)were generated due to the highly dispersion of Pd.Moreover,the strong interaction between metal and support or bimetallic components plays an important role in modulating the catalytic performance of supported catalyst[6,37].In Fig.2,the reduction of Fe2O3is promoted by the addition of Pd or Cu,owing to the metal-support interaction;besides,the Pd-Cu interaction can also be evidenced by the better dispersion of Pd after Cu introduction as well as asymmetric reduction peaks for the reduction of PdO and CuO shown in Fig.3.Therefore,it is reasonable to infer that the increase of Cu loading leads to better catalytic activity of the Pd-Cu/MB for CO oxidation. Actually,as seen in Fig.5,the sequitur is only fitted for the Cu loading lower than 4%,the reason is that the negative effect of the addition of excessive Cu should be taken into account.Supported Cu catalyst is reported to be also effective in catalytic CO oxidation[24,38],those Cu nanoparticles with highly
dispersion and strongly interacts with the support are supposed to be the active sites.As clearly seen in Fig.3,the reduction peak of highly dispersed and bulk CuO can be found for the Pd-Cu/MB,and the PdCu4/ MB shows the largest amount of highly dispersion CuO nanoparticles(peak at 202℃in Fig.3c),which is thought to be favor for the catalytic CO oxidation; meanwhile,blocking effect of CuO is validated by the shift of reduction temperature of Fe2O3.Furthermore, the largest intensity of peakα(in Fig.5)is found for the PdCu4/MB sample,suggesting that for PdCu4/MB catalyst,the CO is themost easily reacts with oxygen species generating CO2.Hence,the PdCu4/MB is reasonable to show the highest catalytic activity due to the loading ofmoderate Cu.
3 Conclusions
A series of bim etallic Pd-Cu/MB catalysts were successfully fabricated by impregnation method using earth-abundant bauxite materials as supports.It is demonstrated that the dispersion of Pd can be enhanced by the introduction of Cu;the interaction between metal(Pd or Cu)and MB support as well as Pd with Cu results in lowering the reduction temperature of Fe2O3in MB and supported CuO.In addition,the introduction of extremely high content of Cu leads to the formation of bulk CuO and shows blocking effect on Pd-MB interaction.The highest catalytic activity for PdCu4/MB sample is ascribed to the best dispersion of Pd and Cu,strongest interaction between metal(Pd or Cu)and support as well as Pd and Cu.
[1]Zhang Q,Liu X,Fan W,et al.Appl.Catal.B:Environ., 2011,102(1/2):207-214
[2]Yamaura H,Moriya K,Miura N,et al.Sens.Actuators B: Chem.,2000,65(1/2/3):39-41
[3]NishiboriM,Shin W,Izu N,et al.Catal.Today,2013,201: 85-91
[4]Heo I,Wiebenga M H,Gaudet JR,et al.Appl.Catal.B: Environ.,2014,160-161:365-373
[5]Seyfi B,Baghalha M,Kazemian H.Chem.Eng.J.,2009,148 (2/3):306-311
[6]Ivanova A S,Slavinskaya E M,Gulyaev R V,et al.Appl. Catal.B:Environ.,2010,97(1/2):57-71
[7]Li N,Chen Q,Luo L,et al.Appl.Catal.B:Environ.,2013,1 42-143:523-532
[8]Kast P,Friedrich M,Teschner D,et al.Appl.Catal.A:Gen., 2015,502:8-17
[9]Slavinskaya EM,Gulyaev R V,Zadesenets A V,et al.Appl. Catal.B:Environ.,2015,166-167:91-103
[10]Kast P,KuerováG,Behm R J.Catal.Today,2015,244: 146-160
[11]Laguna O H,Pérez A,Centeno M A,et al.Appl.Catal. B:Environ.,2015,176-177:385-395
[12]Li J,Han Y,Zhu Y,et al.Appl.Catal.B:Environ.,2011, 108-109:72-80
[13]de Oliveira Jardim E,Rico-Francés S,Abdelouahab-Reddam Z,etal.Appl.Catal.A:Gen.,2015,502:129-137
[14]Zhu J,Gao Q.Microporous Mesopor Mater.,2009,124(1/2/ 3):144-152
[15]Yu Y,Takei T,Ohashi H,et al.J.Catal.,2009,267(2):121-128
[16]Sun S,Gao Q,Wang H,et al.Appl.Catal.B:Environ., 2010,97(1/2):284-291
[17]Wang A,Liu X Y,Mou C Y,et al.J.Catal.,2013,308:258-271
[18]Nikolaev S A,Golubina E V,Krotova I N,et al.Appl. Catal.B:Environ.,2015,168-169:303-312
[19]Liu X,Wang A,Wang X,etal.Chem.Commun.,2008,(27): 3187-3189
[20]Rosseler O,Ulhaq-Bouillet C,Bonnefont A,et al.Appl. Catal.B:Environ.,2015,166-167:381-392
[21]Hilli Y,Kinnunen N M,Suvanto M,et al.App l.Catal.A: Gen.,2015,497:85-95
[22]Estifaee P,Haghighi M,Mohammadi N,et al.Ultrason. Sonochem.,2014,21(3):1155-1165
[23]Wang F,Lu G.Int.J.Hydrogen Energy,2010,35(13):7253-7260
[24]Barbato P S,Di Benedetto A,Landi G,et al.Chem.Eng.J., 2015,279:983-993
[25]Yue Y,Liu H,Yuan P,etal.J.Catal.,2014,319:200-210
[26]Wang X,Chen Z,Luo Y,etal.Sci.Rep.,2013,3:1559
[27]Wang X,Jiang L,Wang J,et al.Appl.Catal.B:Environ., 2015,165:700-705
[28]Wang X,Wu W,Chen Z,etal.Sci.Rep.,2015,5:9766
[29]Xu G,Zhu Y,Ma J,et al.Stud.Surf.Sci.Catal.,1997,112: 333-338
[30]Fox E B,Velu S,Engelhard M H,et al.J.Catal.,2008,260 (2):358-370
[31]Lin X,Chen C,Ma J,et al.Int.J.Hydrogen Energy, 2013,38(27):11847-11852
[32]Zhu X,Shen M,Lobban L L,et al.J.Catal.,2011,278(1): 123-132
[33]Liu Q,Wang L,Wang C,et al.Appl.Catal.B:Environ., 2013,136-137:210-217
[34]Di Cosimo J I,Díez V K,Xu M,et al.J.Catal.,1998,178 (2):499-510
[35]Martínez-Arias A,Fernández-Garca M,Gálvez O,et al.J. Catal.,2000,195(1):207-216
[36]Vinod C P,Harikumar K R,Kulkarni G U,et al.Top. Catal.,2000,11-12(1/2/3/4):293-298
[37]Li L,Song L,Wang H,et al.Int.J.Hydrogen Energy, 2011,36(15):8839-8849
[38]Cecilia J A,Arango-Díaz A,Rico-Pérez V,et al.Catal. Today,2015,253:115-125
Effect of Cu Content on Structure and Catalytic Performance of Pd-Cu/Bauxite for CO Oxidation Reaction
ZHAN Ying-Ying XU Cong-Bo CHEN Chong-Qi LIU Xian MA Yong-De JIANG Li-Long*
(National Engineering Research Center of Chemical Fertilizer Catalyst,Fuzhou University,Fuzhou 350002,China)
We develop a bimetallic Pd-Cu catalyst that takes the advantage of Pd-Cu bimetal as active species and earth-abundant bauxite as support.The bauxite support with high surface area was obtained by hydrothermal NaOH aqueous solution treatment,and the amount of Pd is fixed to a low concentration of 0.5%(weight percentage)by incipient-wetness impregnation method.Their catalytic activity for CO oxidation reaction has also been studied.The effect of different Cu loading content on the chemical-physical properties of the as-prepared catalysts is investigated in detail.It is found that the dispersion of Pd species is promoted by the introduction of Cu species;and both Pd-Cu interaction andmetal(Pd-Cu bimetals)-support(modified bauxite(MB)interaction are modulated by varying the Cu content.Furthermore,the catalytic activities with respect to the catalytic CO oxidation are performed,and the catalyst loaded with 0.5%Pd and 4%Cu(PdCu4/MB)shows the highest catalytic activity.It is believed that the well dispersion of Pd and Cu,the strong interactions between themetals and the support as well as between Pd and Cu are responsible for the highest catalytic activity of PdCu4/MB.Moreover,it can be concluded from the CO-TPD investigation that superior abilities in adsorption of CO and extraction of oxygen from the support are favor for high catalytic activity of PdCu4/MB catalyst in CO oxidation.
Pd-Cu bimetallic;alkali-modified bauxite;CO oxidation;Cu loading
O643.3;O614.121
A
1001-4861(2016)10-1867-09
10.11862/CJIC.2016.248
2016-04-17。收修改稿日期:2016-09-08。
国家高技术研究发展计划(No.2015AA03A402)资助项目。
*通信联系人。E-mail:jll@fzu.edu.cn