原位水解沉积制备高效氮化钽微球太阳能分解水光阳极
杨立恒 罗文俊 李明雪 邹志刚
(1南京大学现代工程与应用科学学院,南京210093)
(2江苏省柔性电子重点实验室,先进材料研究院,江苏先进生物与化学制造协同创新中心,南京工业大学,南京211816)
(3南京大学环境材料与再生能源研究中心,固体微结构国家重点实验室,南京大学物理学院,南京210093)
(4中国矿业大学物理学院,徐州221116)
原位水解沉积制备高效氮化钽微球太阳能分解水光阳极
杨立恒1罗文俊*,2,3李明雪4邹志刚*,3
(1南京大学现代工程与应用科学学院,南京210093)
(2江苏省柔性电子重点实验室,先进材料研究院,江苏先进生物与化学制造协同创新中心,南京工业大学,南京211816)
(3南京大学环境材料与再生能源研究中心,固体微结构国家重点实验室,南京大学物理学院,南京210093)
(4中国矿业大学物理学院,徐州221116)
利用一种新的原位水解沉积方法,以在高湿度空气中老化的甲醇中作为溶剂,通过乙醇钽水解而成前驱体微球颗粒沉积,制备出了高效的Ta3N5微球光电极,其1.6 V(vs RHE)电极电位下的光电流值达到了6.6 mA·cm-2。相反地,在新鲜的甲醇溶液中没有钽前驱体微球颗粒沉积。这表明甲醇中水的含量对Ta3N5微球光电极的形成十分重要。另外,本制备方法也能方便地在其他透明导电衬底上制备出Ta3N5。
太阳能水分解;Ta3N5光阳极;微球;原位沉积;湿度
(3Eco-materials and Renewable Energy Research Center(ERERC),National Laboratory of Solid State(Microstructures,Colledge of Physics,Nanjing University,Nanjing 210093,China)
(4Department of Physics,China University of Mining and Technology,Xuzhou,Jiangsu 221116,China)
0 Introduction
Since a TiO2-based photoelectrochemical(PEC) cell was reported to split water into H2and O2under illumination,solar water splitting has been considered to be a promising technology to produce H2on a large scale[1].Ta3N5is regarded as one of themost promising candidates due to its high theoretical energy conversion efficiency(15.9%)and suitable band positions[2-3].Many preparation methods,such as thermal oxidation and nitridation of Ta foil,throughmask anodization,anodization combined with hydrothermalmethod,electrophoretic deposition,dropcasting andmagnetron sputtering,havebeen reported to prepare Ta3N5[4-9].However,p-n tandem photoelectrochemical cell requires efficient and translucent/ transparent Ta3N5photoanode,which still remains unfulfilled.Therefore,it isstilldesirable toexplorenew preparation methods of Ta3N5films.In addition, spherical structure can lead to efficient light absorption and improve performance of a photoelectrode[10-11]. However,current preparation methods for spheres are often in the assistance of additional reagents,which increasespreparation cost[12-13].
Herein,an efficient microsphere Ta3N5photoanode was prepared by a new in situ hydrolysis deposition method without any additional reagents. Microsphere precursor films were firstly deposited on substrates in tantalum ethoxide(Ta(OEt)5)solution of aged methanol.After oxidation and nitridation, microsphere Ta3N5films were obtained.A 6.6 mA· cm-2photocurrent was achieved at 1.6 V vs RHE.In this context,exploration of new preparation method and the synthesis mechanism of Ta3N5film are our research focus and we hope it can give some hints for preparation of efficient and translucent/transparent Ta3N5.
1 Experimental
1.1 Preparation of Ta3N5m icrosphere photoanodes
A typical preparation procedure of Ta3N5microsphere photoanode is as follows.Firstly, methanol(Purity≥99.5%,Nanjing Chemical Reagent Co.,Ltd.)was aged in airwith 7%relative humidity at 25℃for 4 h before use.Secondly,10 mmol·L-1precursor solution of tantalum ethoxide(Purity≥99.95%,Zhuzhou Cemented Carbide Group Corp., Ltd)was prepared with aged methanol.Then,Ta foils (Purity≥99.95%,Zhongnuo Advanced Material Technology Co.,Ltd)were immersed in Ta(OEt)5methanol solution and films were deposited at 7℃for 48 h.Next,the obtained films were rinsed with deionized water and dried in air at room temperature, followed by calcination in air at250℃for 30minutes. Finally,Ta3N5microsphere photoanodes were obtained by nitridation of oxidized samples in a horizontal tube furnace at 850℃for 500 min under 800 mL·min-1NH3flow(Referred as Ta3N5/aged and Ta3N5/aged/Co-Pi for pristine and Co-Pi loaded samples, respectively).In order to investigate the effect of aging methanol,a reference sample was prepared in fresh methanol as solvent under the same conditions (Referred as Ta3N5/fresh and Ta3N5/fresh/Co-Pi for pristine and Co-Pi loaded samples,respectively).
1.2 Photo-assisted electrodeposition of Co-Pi co-catalyst
Following previous studies,Co-Pi co-catalyst was electrodeposited on Ta3N5film by chronopotentiometry under illumination with constant current of 50μA for 4 min[5].The electrodeposition was conducted in a three-electrode cell,with the solution of 0.5 mmol·L-1Co(NO3)2·6H2O(Purity≥99.0%,Shanghai Zhenxin Reagent Factory)and 0.1 mol·L-1K2HPO4·3H2O (Purity≥99.0%,Shanghai Lingfeng Chemical Reagent Co.,Ltd.)buffer at pH=7 as electrolyte.Ta3N5was used as working electrode,Pt foil as counter electrode and saturated calomel electrode(SCE)as reference electrode.An AM 1.5G-simulated sunlight simulator (Oriel 92251A-1000,light intensity=100 mW·cm-2) wasused as light source.During the deposition process, Co2+was oxidized into Co3+[14-15].The total amount of charge was about20mC·cm-2.Assuming that Faradaic efficiency was 100%,the amount of deposited Co was calculated as follow:
Where NCois the amount of Co-Pi deposited on Ta3N5per square centimeter.96 485(C·mol-1)is the Faradaic constant.
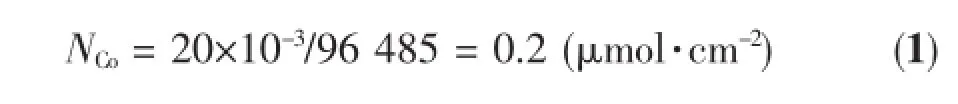
After Co-Pi deposition,the electrode was rinsed with deionized water and dried in air for use.
1.3 Characterization of sam p les
The crystal structures of samples were determined by an X-ray diffractometer(XRD,Rigaku UltimaⅢ)with Cu Kαray(λ=0.154 3 nm)at 40 kV and 40 mA.The range is from 10°to 80°. Morphologies of electrodes were observed on a field emission scanning electron microscope(SEM,Zeiss, Ultra 55-44-08)at an accelerating voltage of 15 kV. Water content of methanol was measured on a moisture analyzer(Metrohm,KF787 Titrino). Absorption spectra were investigated on a UV-vis spectrophotometer(Shimadzu,UV-2550).FTIR spectra were obtained on a Nexus870 spectrophotometer in the range of 4 000~400 cm-1.Thermogravimetric analysis was carried out in air with a Netzsch STA 449F3 instrument by increasing temperature from 30 to 600℃with 5℃·min-1.
1.4 Photoelectrochem icalmeasurements
Photoelectrochemical performance was measured in a three-electrode cell using an electrochemical analyzer(CHI-633C,Shanghai Chenhua).Ta3N5microsphere electrode was used as working electrode, Pt foil as counter electrode and saturated calomel electrode(SCE)as reference electrode.Aqueous solution of 1 mol·L-1NaOH was employed as electrolyte.A commercial AM 1.5G-simulated sunlight simulator(Oriel 92251A-1000,light intensity=100 mW·cm-2)was used as light source.Current-potential curveswere recorded at a scan rate of 10mV·s-1.The potential of working electrode versus SCE was converted into RHE(reversible hydrogen electrode) potential scale according to the following formula:
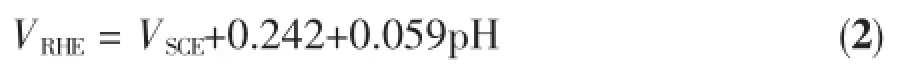
where VRHEis the potential versus RHE(V),VSCEis the potential versus SCE(V),and pH is the pH value of electrolyte.The incident photon-to-current efficiency (IPCE)was determined under the irradiation of differentwavelength light generated bymonochromatic filters according to the following formula:
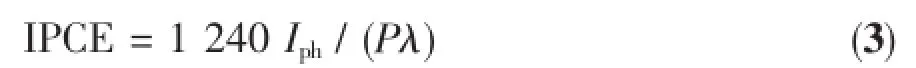
where Iphis the photocurrent density(μA·cm-2),P and λare the incident light intensity(μW·cm-2)and wavelength(nm),respectively.The incident light intensity wasmeasured by a photometer(Newport,84 0-C,USA).

Fig.1 Photographs of precursor solution of(a)fresh and(b)aged methanol before and after deposition
2 Results and discussion
Fig.1 shows photographs of Ta(OEt)5solution of fresh and aged methanol before and after deposition, respectively.Both solutions are transparent at the beginning.After depositing at 7℃for 48 h,solution of aged methanol became white(Fig.1(b)).However, solution of fresh methanolwas still transparent.White films were deposited on substrates in solution of aged methanol,whereas there were no samples on substrates in solution of fresh methanol.The results suggest that film deposition comes from hydrolysis of Ta(OEt)5in aged methanol.Since the only difference between the two kinds ofmethanol was the methanol whether exposed in moist air or not,little water in methanol was essential for the formation of films.The water content was measured about 0.15%(w/w)by a moisture analyzer.The detail effect of water will be
discussed below.
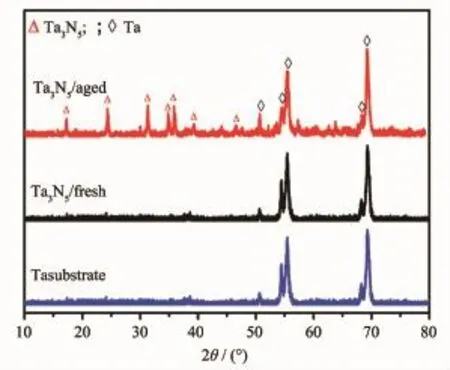
Fig.2 XRD patterns of Ta3N5/fresh and Ta3N5/aged
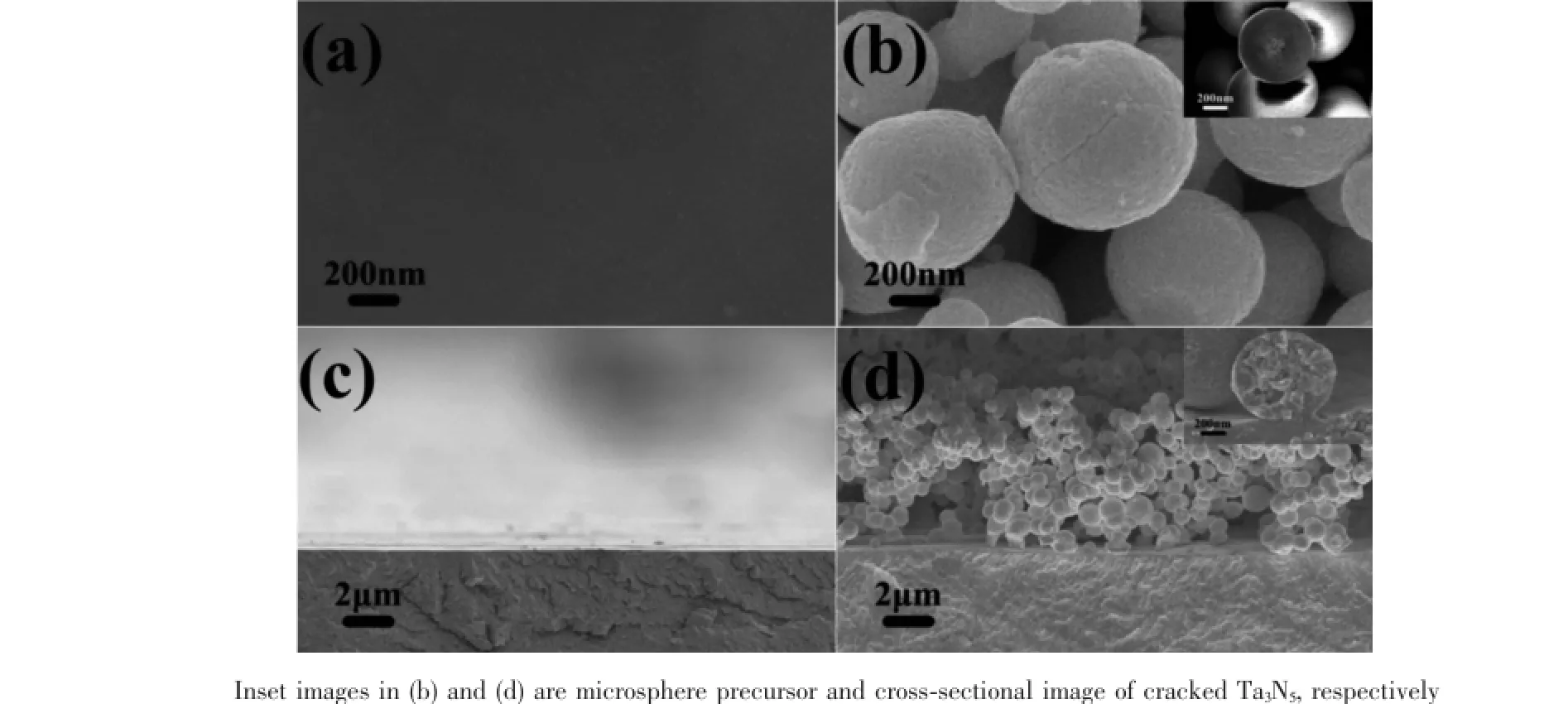
Fig.3 High magnification SEM images of Ta3N5/fresh(a)and Ta3N5/aged(b);Cross-sectional images of Ta3N5/fresh(d)and Ta3N5/aged(d)
XRD patterns were measured to determine phases and crystal structures of the two samples,as shown in Fig.2.Orthorhombic phase Ta3N5(PDF No. 19-1291)was obtained for Ta3N5/aged.In contrast, Ta3N5/fresh shows no peaks of Ta3N5.Fig.3 shows scanning electron microscopy(SEM)images of Ta3N5/ fresh and Ta3N5/aged.Surface and cross-sectional SEM images in Fig.3(a,c)indicate that Ta3N5/fresh shows only morphology of Ta substrate and no Ta3N5is observed.The result is in agreement with the XRD data.However,Fig.3(b)shows that Ta3N5/aged is composed of spherical particles,with the diameter around 1μm.Discernible roughness,many nanopores and cracks are observed on the surface,which come from volume shrinkage from transition of Ta2O5into Ta3N5and the decomposition of residual organics (Fig.4)during nitridation[12].High magnification SEM image of precursor is displayed in the inset picture of Fig.3(b).The result suggests that microspheres are formed during precipitation.Fig.3(d)is the crosssectional image of Ta3N5/aged.It shows that Ta3N5film electrode is composed of microsphere particles and the thickness is about 7.5μm.From the inset in Fig.3 (d),Ta3N5microsphere is solid and composed of smaller particles,which suggests that Ta3N5microsphere originates from the agglomeration of nanoparticles.
Spherical structure is one of favorable microstructures in both photoelectrochemical and solar cells[10-11].Usually,spherical Ta3N5particles obtained by solution methods are assisted with additional agents[12-13].Though the distribution size of Ta3N5spheres can be narrowed,introduction of additional reagents actually increases the possibility of inclusion of impurities,as well as experimental difficulties and preparation cost.In our study,however,Ta3N5microsphere was prepared in a more simple way, without any additional agents,and thus those shortcomings are avoided.
FTIR spectra were used to investigate formation process of microsphere,and the results are shown in Fig.4.Peaks below 1 000 cm-1are attributed to stretching,bending and torsion modes of Ta-O[16-17].
The broad absorption between 800 and 1 000 cm-1corresponds to the presence of Ta suboxides[18].A peak at~3 342 cm-1is assigned to OH stretching modes, and peak at~1 626 cm-1is associated with OH bending modes[17,19].Both of them are weakened after calcination at 250℃.The existence of-OH group confirms that microsphere is from the hydrolysis of tantalum ethoxide.
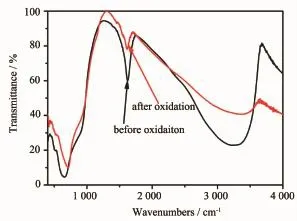
Fig.4 FTIR spectra ofmicrosphere precursor before and after calcined at250℃for 30min in air
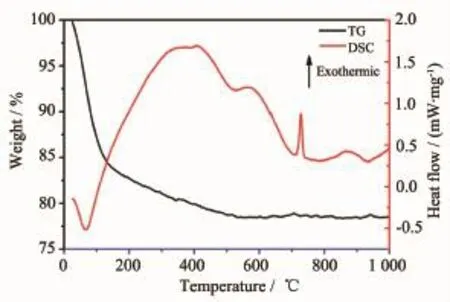
Fig.5 Thermogravimetric spectrum ofmicrosphere precursor
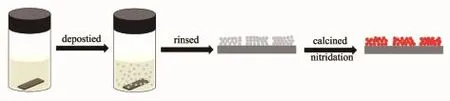
Fig.6 Schematic illustration of formation process of Ta3N5m icrosphere film
In order to further investigate composition of asdeposited microsphere precursor before calcination, thermogravimetric(TG)ismeasured and the result is shown in Fig.5.The endothermic peak under 100℃comes from evaporation of adsorbed water.Weight loss with exothermic peak ended at around 500℃arises from the decomposition of organics in microsphere, which comes from the organic group-CH2CH3of Ta (OEt)5[16-17].However,organic compounds cannot be removed completely when calcined at 250℃and thus lead to the formation of Ta suboxides.
According to the above discussion,formation process of Ta3N5microsphere can be concluded as followswith the simplified chemical reactions[20-21]:
Hydrolysis:
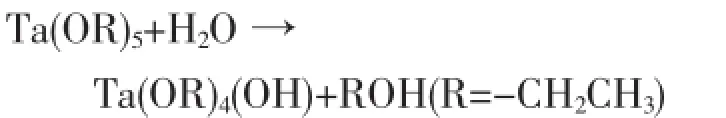
Polycondensation:
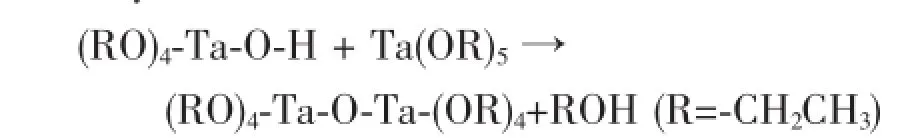
Water content in methanol is a key factor to trigger the whole reaction.Actually,the two reactions proceed simultaneously once the hydrolysiscondensation reaction is triggered.As long as a critical radius is reached,nucleation will take place. And nanocrystalline will agglomerate into spherical particle due to its lowest surface energy.Finally,when the spherical particles are big enough,sedimentation happens and a film is deposited on the substrate. After oxidation and nitridation,Ta3N5microsphere film is obtained.A schematic diagram of formation process of Ta3N5microsphere is illustrated in Fig.6.
Fig.7 indicates UV-Vis absorption spectrum of Ta3N5microsphere photoanode.The Ta3N5microsphere film shows a high absorption,which comes from light scattering ofmicrospheres.Contribution from substrate is excluded through the absorption spectrum of Ta3N5/ fresh.Ambiguous ERERC can be identified through the Ta3N5microsphere film on quartz substrate in the inset(II)of Fig.7,which suggests that in situ hydrolysis deposition method can be used to prepare a translucent Ta3N5microsphere electrode.
Photoelectrochemical properties of Ta3N5micro-
sphere photonodes weremeasured and the results are shown in Fig.8.In order to exclude contribution of Ta substrate on photocurrent,Ta3N5/fresh was also measured as a reference.Dark currents of both electrodes are negligible.The photocurrent of Ta3N5/ fresh and Ta3N5/fresh/Co-Pi ismuch lower than that of a Ta3N5microsphere photonode.Therefore, photocurrents of Ta3N5/aged and Ta3N5/aged/Co-Pi entirely come from Ta3N5microsphere,rather than from substrate.Generally,a bare Ta3N5photoanode suffers from severe photo-corrosion in aqueous solution and surface combination,which can be remarkably suspressed by depositing a co-catalyst. Among different co-catalysts,Co-Pi is low-cost and operable undermild conditions[14,22].Therefore,in this study,Co-Pi(2μmol·cm-2)was electrodeposited on the Ta3N5film to improve the performance of the Ta3N5microsphere electrode.After deposition of Co-Pi,the photocurrent of Ta3N5/aged/Co-Pi is about 3 times as high as that of Ta3N5/aged.Current density of Ta3N5microsphere electrode by in situ hydrolysis deposition method is~2.34 mA·cm-2at 1.23 V vs RHE,and~6.6 mA·cm-2at 1.6 V vs RHE.A Ta3N5photoanode prepared by EPD indicated 3.18 mA·cm-2photocurrent at 1.23 V vs RHE and about 6 mA·cm-2at 1.6 V vs RHE[23].High photocurrents of 5.5 mA· cm-2and 6.7 mA·cm-2at 1.23 V vs RHE have been achieved by direct oxidation and nitridation of Ta foil[4-5].The photocurrent in this study is comparable to samples by EPD and oxidation and nitridation of Ta foil,butmuch lower than 12.1 mA·cm-2obtained by Ta3N5with integration of hole-storage layer,coupled molecular catalysts and TiOxblocking layer[6]. However,in this study,preparation conditions and cocatalysts have not yet been optimized.And thus it is promising to further improve Ta3N5microsphere photoanode by in situ hydrolysis deposition method in future work.
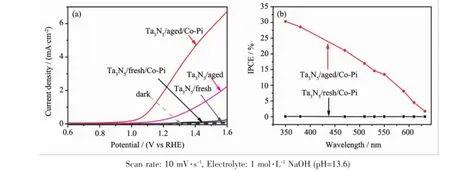
Fig.8(a)Current-potential curves of Ta3N5/fresh,Ta3N5/fresh/Co-Pi,Ta3N5/aged and Ta3N5/aged/Co-Pi in the dark(dash lines) and under AM 1.5G simulated sunlight irradiation(100mW·cm-2)(solid lines),respectively;(b)IPCE curves of Ta3N5/ fresh/Co-Piand Ta3N5/aged/Co-Piat 1.23 V vs RHE
Fig.8(b)is the incident photon-to-current efficiency(IPCE)of Ta3N5/fresh/Co-Pi and Ta3N5/aged/ Co-Pi.The IPCE of Ta3N5/fresh/Co-Pi is nearly zero in the spectrum range from 350 to 610 nm,which further excludes contribution of substrate on photocurrent. The IPCE of Ta3N5/aged/Co-Pi is~26%at 400 nm, but decreases at longer wavelength[24].The integrated
photocurrent(~2.35 mA·cm-2)shown in Fig.9 is very close to themeasured value(~2.34 mA·cm-2),which suggests that the measured photocurrent is reliable. The photocurrent response of Ta3N5/aged/Co-Pi in IPCE also agrees well with the absorption edge, suggesting that the photocurrent originates from the band gap transition of Ta3N5.The stability of Ta3N5/ aged and Ta3N5/aged/Co-Piwas alsomeasured and the result is shown in Fig.10.As we can see,the photocurrent of Ta3N5/aged declines over 50%after only 3~4 s under illumination,but the time was extended to about 2 000 s for Ta3N5/aged/Co-Pi. Though photocurrent of Ta3N5/aged/Co-Pi decreases obviously,nonzero photocurrent can still be observed. The stability of Ta3N5microsphere electrode should be further improved in future.
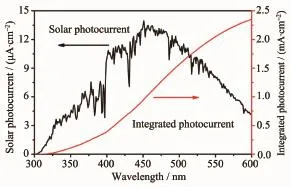
Fig.9 Integrated solar photocurrent at 1.23 V vs RHE from the standard solar spectrum
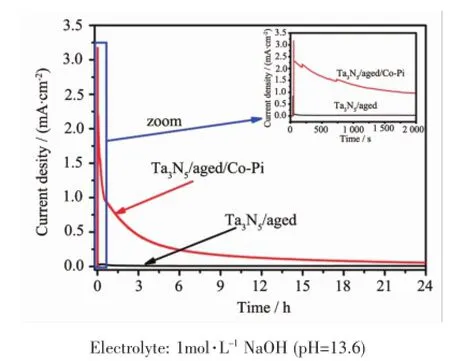
Fig.10 Current-time curves of Ta3N5/aged and Ta3N5/ aged/Co-Pimeasured at 1.23 V vs RHE
3 Conclusions
In summary,we synthesized an efficient Ta3N5microsphere photoanode by a new and facile in situ hydrolysis deposition method.A Ta3N5microsphere film was formed on Ta substrate in Ta(OEt)5solution of aged methanol.The microsphere is formed by hydrolysis of Ta(OEt)5and subsequentagglomeration of nanoparticles.Water content in solvent was indispensable to in situ deposition of Ta3N5film.High photocurrent density was obtained on the Ta3N5microsphere electrode,~2.34 mA·cm-2at 1.23 V vs RHE and~6.6 mA·cm-2at 1.6 V vs RHE under AM 1.5G simulated sunlight irradiation(100 mW·cm-2).In addition,in situ hydrolysis deposition method is a promising method to prepare efficient Ta3N5photoanodes on other transparent conducting substr ates.
[1]Fujishima A,Honda K.Nature,1972,238:37-38
[2]Wang L,Zhou X,Nguyen N T,et al.Adv.Mater.,2016,28 (12):2432-2438
[3]Fu G,Yan S,Yu T,et al.Appl.Phys.Lett.,2015,107(17): 171902
[4]LiM,LuoW,Cao D,etal.Angew.Chem.Int.Ed.,2013,52 (42):11016-11020
[5]Li Y,Zhang L,Torres-Pardo A,et al.Nat.Commun.,2013, 4:2566
[6]Liu G,Ye S,Yan P,et al.Energy Environ.Sci.,2016,9: 1327-1334
[7]Khan S,Zapata M JM,Pereira M B,et al.Phys.Chem. Chem.Phys.,2015,17:23952-23962
[8]Wang Z,Qi Y,Ding C,et al.Chem.Sci.,2016,7(7):4391-4399
[9]Cong Y,Park H S,Dang H X,et al.Chem.Mater.,2012,24 (3):579-586
[10]Pan JH,Wang Q,Bahnemann D W.Catal.Today,2014, 230:197-204
[11]Deepak T G,Anjusree G S,Thomas S,et al.RSC Adv., 2014,4(34):17615-17638
[12]Cao J,Ren L,Li N,et al.Chem.Eur.J.,2013,19(38): 12619-12623
[13]Liu X,Zhao L,Domen K,et al.Mater.Res.Bull.,2014,49: 58-65
[14]Kanan M W,Nocera D G.Science,2008,321(5892):1072-1075
[15]Lutterman D A,Surendranath Y,Nocera D G.J.Am.Chem. Soc.,2009,131(11):3838-3839
[16]Ndiege N,Subramanian TW V,Shannon M A,et al.Chem. Mater.,2007,19(13):3155-3161
[17]Zhao D,Jiang H,Gong H,et al.Transition Met.Chem., 2010,36(1):119-123
[18]Fang Q,Zhang J Y,Wang Z,et al.Thin Solid Films, 2003,428(1):248-252
[19]Antonelli D M,Ying JY.Chem.Mater.,1996,8(4):874-881
[20]Sun Y,Sermon P,Vong M.Thin Solid Films,1996,278(1): 135-139
[21]Wolf C,Rüssel C.J.Mater.Sci.,1992,27(14):3749-3755
[22]Pramanik M,LiC,Imura M,etal.Small,2016,12(13):1709-1715
[23]Liao M,Feng J,Luo W,et al.Adv.Funct.Mater.,2012,22 (14):3066-3074
[24]Hisatomi T,Kubota J,Domen K.Chem.Soc.Rev.,2014,43 (22):7520-7535
In Situ Hydrolysis Deposition of an Efficient Ta3N5M icrosphere Photoanode for Solar W ater Sp litting
YANG Li-Heng1LUOWen-Jun*,2,3LIMing-Xue4ZOU Zhi-Gang*,3
(1College of Engineering and Applied Science,Nanjing University,Nanjing 210093,China)
(2Key Laboratory of Flexible Electronics&Institute of Advanced Materials,Jiangsu National Synergetic Innovation
Center for Advanced Materials,Nanjing Tech University,Nanjing 211816,China)
A new in situ hydrolysis deposition method was used to prepare a Ta3N5microsphere photoanode, which indicates a high photocurrent of 6.6 mA·cm-2at1.6 V vs RHE.Microsphere precursor films are formed by hydrolysis of Ta(OEt)5and subsequent deposition on substrates,which is achieved by aging methanol solvent in air with high humidity.In contrast,no precursor films were obtained on substrates with fresh methanol.The results suggest thatwater in solvent is very essential to in situ depositing Ta3N5photoanode.In addition,the facile method can be used to deposit Ta3N5on other transparent conducting substrates.
solarwater splitting;Ta3N5photoanodes;microsphere;in situ deposition;humidity
O614.51+3
A
1001-4861(2016)10-1839-08
10.11862/CJIC.2016.330
2016-04-26。收修改稿日期:2016-08-18。
国家重点基础研究发展计划(973计划,No.2013CB632404,2014CB239303)、江苏省自然科学基金(No.15KJB150010,BK20140197)、南京大学纳米技术江苏省重点实验室开放研究基金资助项目。
*通信联系人。E-mail:iamwjluo@njtech.edu.cn,zgzou@nju.edu.cn