基于二吡唑配体的镉配合物:温度诱导自组装、荧光和光催化应用
李慧军 闫玲玲 王元*, 徐周庆 徐君 吴伟娜
(1河南理工大学化学化工学院,焦作454000)
(2河南理工大学物理与电子信息学院,焦作454000)
基于二吡唑配体的镉配合物:温度诱导自组装、荧光和光催化应用
李慧军1闫玲玲*,2王元*,1徐周庆1徐君1吴伟娜1
(1河南理工大学化学化工学院,焦作454000)
(2河南理工大学物理与电子信息学院,焦作454000)
通过改变溶剂热反应温度,合成了2个结构不同的镉配合物[Cd2(H2ppty)2(SO4)2(H2O)2]·3H2O(1)和[Cd2(ppty)(SO4)(H2O)2]n(2) (H2ppty=3,5-bis-(1H-pyrazol-3-yl)-[1,2,4]triazol-4-ylamine)。进一步的研究表明,1和2的结构分别包含有双核和四核镉簇单元,呈现出零维和二维的结构。并且在室温下对2个配合物的荧光性质也进行了测试。固体紫外漫反射表明1和2的光学带隙分别为1.87和2.32 eV,因此它们对亚甲基蓝的光降解反应表现出了良好的催化活性。
镉配合物;双核和四核镉簇单元;荧光;光催化
0 Introduction
Up to now,in view of the intense desire for“green life”,the treatment of wastewater is an increasing serious problem[1-3].Dyemolecule,as one of themain pollutants in wastewater not only reduce the water quality,threaten aquatic life,but also significantly impact the health of human beings due to their toxic,mutagenic,and carcinogenic characteristics[4-6].Therefore,removing the harm ful and poor
biodegradable dyes from wastewater has been attracting a great deal of attention and becoming a hot research topic[7-10].Although conventional contaminated water treatments such as adsorption,separation, chemical treatment are commonly used to deal with the organic dye,they do not completely eliminate the waste,involve operating costs and worse still,can generate toxic secondary pollutants[11-13].Comparably, photocatalyst could be ecologically and economically used in eliminating organic dyes and other noxious contaminants,which affords an effective and convenient approach[14-17].Thus,achievement of inexpensive and efficient materials with improved photocatalysis activities has always been our pursuit.
More recently,a number of studies on clusterbased coordination complexes have attracted enormous interest because of their catalytic and particularly effective photocatalytic degradation activities[18-19]. Based on their rich metal-containing nodes,organic bridging ligands,as well as the controllability of the synthesis,it iseasy to construct coordination complexes with tailorable capacity to improve light-harvesting, thereby act as hosts for other more photosensitive molecules[20-21].In the pursuit of required coordination complexes,multitopic nitrogen-donor ligands with rigid spacers like those triazolyl-based linkers can be exploited to assemble various cluster-based coordination polymers with interesting topological networks and physical properties.So in this paper,a nitrogendonor ligand with two pH-dependent protons and seven donor sites is used to synthesize cluster-based coordination complexes.Two temperature-controlled syntheses and structural characterizations of 0D binuclear cluster-based complex[Cd2(H2ppty)2(SO4)2(H2O)2]·3H2O(1)and 2D tetranuclear cluster-based [Cd2(ppty)(SO4)(H2O)2]n(2)(H2ppty=3,5-bis-(1H-pyrazol -3-yl)-[1,2,4]triazol-4-ylamine)have been successfully acquired to conduct the photocatalysis experiments on MB degradation.And they exhibit good photocatalytic activities.Photoluminescence studies are also done on the two complexes.It turned out that the two complexes show different photoluminescence properties.
1 Experimental
1.1 M aterials and measurements
All chemicals were obtained from commercial sources and used as received without purification.IR spectra were recorded on a BRUKER TENSOR 27 spectrophotometer with KBr pellets in the region of 400~4 000 cm-1.Elementalanalyses(C,H and N)were carried out on a Flash EA 1112 elemental analyzer. Powder X-ray diffraction(PXRD)patterns were recorded using Cu Kαradiation on a PANalytical X′Pert PRO diffractometer(λ=0.154 06 nm,40 kV,40 mA).The crushed single crystalline powder samples were prepared by crushing the crystals and scanned from 5°to 50°.Thermal analyseswere performed on a Netzsch STA 449C thermal analyzer at a heating rate of 10℃·min-1in air.The luminescence spectra for the powdered solid samples were measured at room temperature on a Hitachi F-7000 Fluorescence Spectrophotometer.The excitation slit,as well as the emission slitwere 2.5 nm.
1.2 Preparations of the ligand and comp lexes
The ligand H2ppty was synthesized according to the literature method[22].Yield:93.1%.Anal.Calcd. for C8H8N8(%):C,44.44;H,3.72;N,51.82.Found(%): C,44.65;H,3.61;N,51.78.FT-IR(KBr,cm-1):3 195 (s),1 632(s),1 377(m),1 246(m),1 101(s),957(s), 794(s),731(m),623(s).
Synthesis of[Cd2(H2ppty)2(SO4)2(H2O)2]·3H2O(1): amixture of CdSO4(0.1mmol),H2ppty(0.05mmol), H2O(2 mL)and CH3CH2OH(2 mL)were sealed in a Teflon-lined stainless steel container and heated at 80℃for 3 days.After slowly cooling to room temperature at a rate of 5℃·h-1,colorless crystals of 1 were acquired in 57%yield.Anal.Calcd.for Cd2C16N16S2O13H26(%):C 20.45 H 2.78 N 23.85.Found(%):C 20.27,H 2.87,N 24.18.IR:3 194(s),1 631(s),1 377 (m),1 245(m),1 107(s),954(s),797(s),733(m), 619(s).
Synthesis of[Cd2(ppty)(SO4)(H2O)2]n(2):the colorless block crystals of 2 were easily produced via the reaction of CdSO4(0.1 mmol),H2ppty(0.05 mmol)in the solution of H2O(5 mL)and CH3CH2OH(5 mL)at
160℃for 4 days.Yield:47%.Anal.calcd.for Cd2C8N8SO6H12(%):C 16.76 H 2.11 N 19.55.Found (%):C 16.74,H 2.21,N 19.69.IR:3 189(s),1 629 (m),1 378(m),1 112(s),1 065(s),969(s),804(m), 734(m),604(s).
1.3 Photocatalyticmeasurem ent
A photocatalytic experiment in aqueous solution was carried out in a typical process.In practice,30 mg powder of the two complexes and 2 mL of 30% H2O2were added into themethylene blue(MB)aqueous solutions(250mL,3×10-5mg·L-1).Subsequently,the mixture was stirred for half an hour in a dark environment to ensure a balance of adsorption and desorption.Then,the solution was stirred constantly under the radiation of a 300 W high pressure xenon lamp.Every 20 min,5 mL of samples were taken out of the reactor and then subjected to spectroscopic measurement on the UVvisible spectrometer.By contrast,the simple photolysis comparative experiment was completed under the same conditions without any catalyst.The concentration of organic dye was estimated by the absorbance at 665 nm,which directly relates to the structure change of its chromophore.
1.4 X-ray crystallography
Two single crystals with dimensions of 0.2 mm× 0.15 mm×0.11 mm(1)and 0.2 mm×0.17 mm×0.14 mm(2)were selected andmounted on a Bruker SMART APEXⅡCCD diffractometer equipped with a graphite monochromatized Mo Kαradiation(λ=0.071 073 nm) by usingφ-ωscan mode.The structures were solved by direct methods and refined by full matrix leastsquare on F2using the SHELXTL-97 program[23].All non-hydrogen atoms were refined anisotropically.All the H atomswere positioned geometrically and refined using a ridingmodel.Details of the crystal parameters, data collection and refinements for 1 and 2 are summarized in Table 1.Selected bond lengths and bond angles for complexes 1 and 2 are listed in Table S1.Classical hydrogen bonds for 1 and 2 are also listed in Table 2.
CCDC:1502061,1;1427971,2.
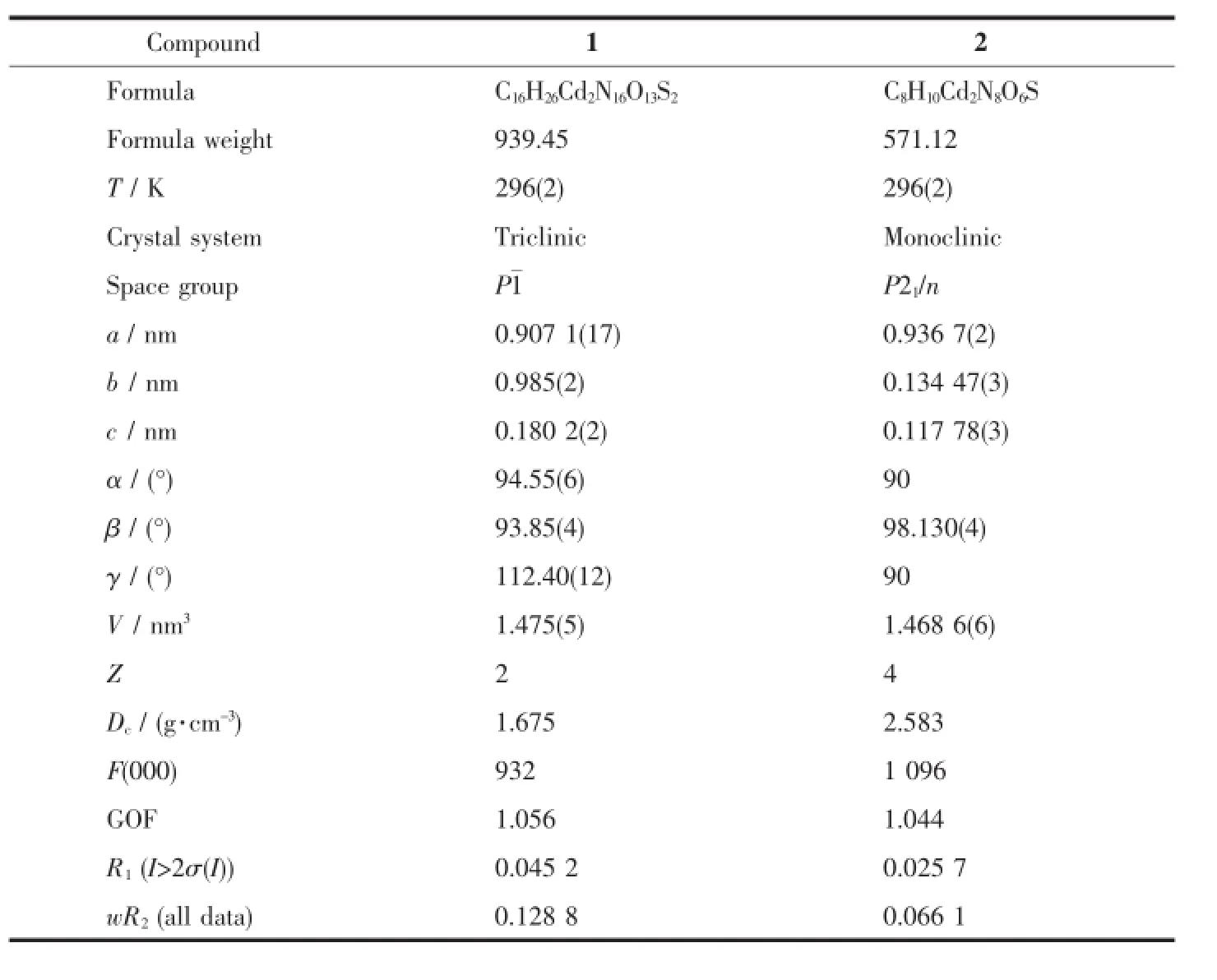
Table 1 Crystal data and structure refinement parameters for 1 and 2
2 Result and discussion
2.1 Crystal structures of complexes 1 and 2
Single crystal X-ray analysis has revealed that complex 1 crystallizes in the triclinic space group P1,andtheasymmet r icuni t compr isesof twoCdCd1 cations,two H2 ppty ligands and two SO42-anions.Cd1 displays a distorted octahedral geometry,being coordinated by four nitrogen atoms(N2,N3,N4 and N7)of two H2ppty ligands(Co-N 0.232 9(4)~0.236 6(5) nm)and two oxygen atoms(O9 and O1)from one water molecule and one SO42-(Co-O 0.231 1(5)and 0.225 7(55)nm)(Fig.1a).Adjacent Cd1 atoms are connected by N3 and N4 giving rise to an independentbinuclear unitwith the distance of Cd1…Cd1 0.474 7 nm.Cd2 cation is located in the samecoordination environment as that of Cd1,completed by N10,N11,N12 and N15 from two H2 ppty ligands and chelated by O6W and O10 atoms from one water molecule and one SO42-.The bond lengths of Cd-N and Cd-O are in thenormal range of 0.232 3(3)~0.245 5(6) nm.Two Cd2 atoms are linked by N11 and N12 forming a similar binuclear unit(the distance between Cd2 atoms is 0.507 2 nm)(Fig.1b).Interestingly,the binuclear units constructed by Cd1 atoms are connected to each other byπ…πinteraction between two planes(plane A:C6,C7,C8,N7,N8;plane B:
C1,C2,C3,N1,N2)generating a ring(the centroidto-centroid separation of about 0.354 05 nm).The centroid coordinates of planes A and B are(0.069, 0.335,0.662)and(0.703,0.142,0.551).While the binuclear units containing Cd2 form a layer under the influence ofπ…πinteraction between two planes (plane C:C12,C13,N11,N12,N13;plane D:C14, C15,C16,N15,N16,the centroid-to-centroid separation of about 0.368 36 nm).The centroid coordinates of planes C and D are(0.266,0.430,0.076)and (0.108,0.718,0.052).The dihedral angles between planes A and B,C and D are 7.75°and 6.43°, respectively(Fig.1c and 1d).
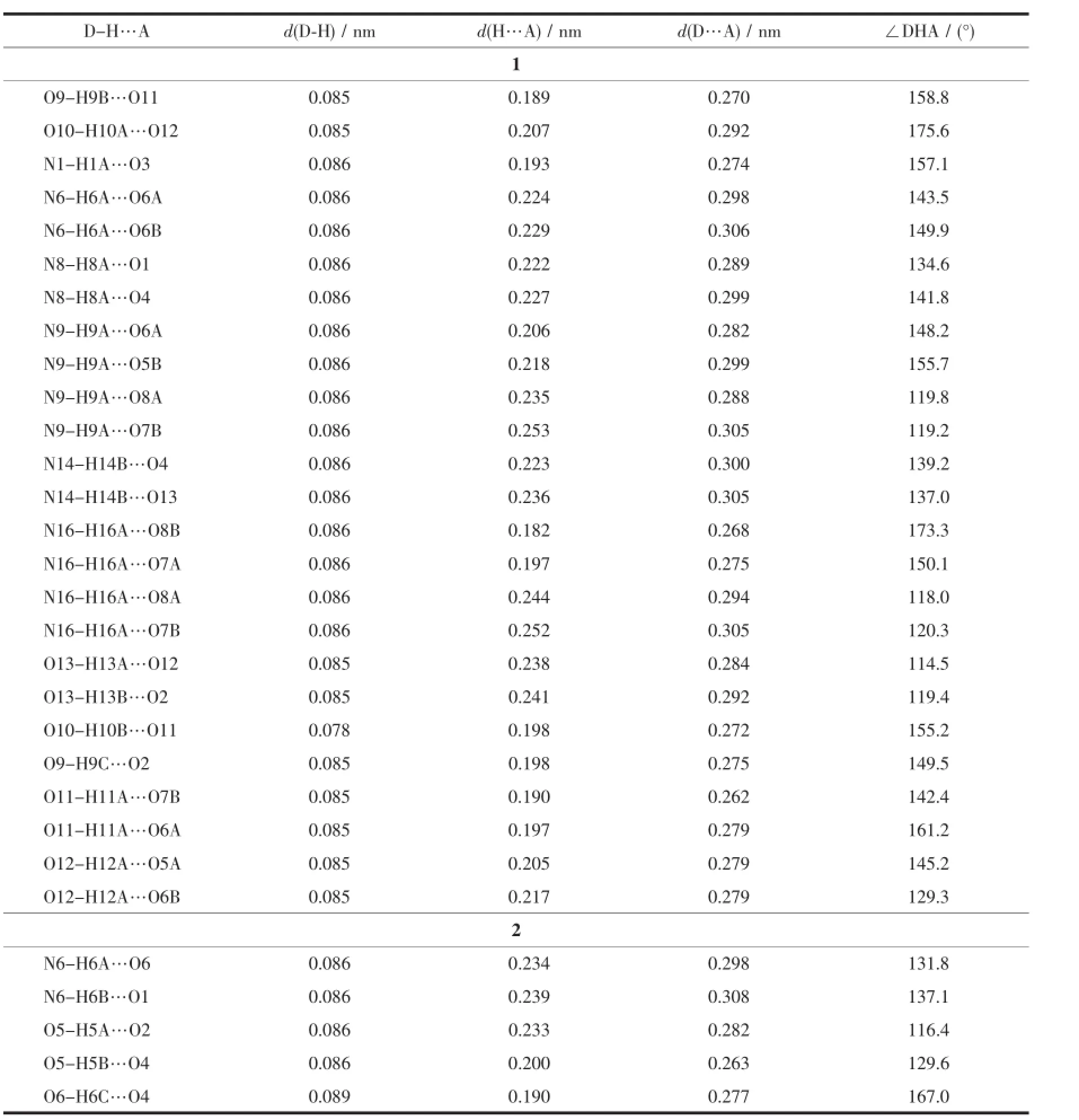
Table 2 C lassical hydrogen bonds for 1 and 2
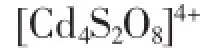
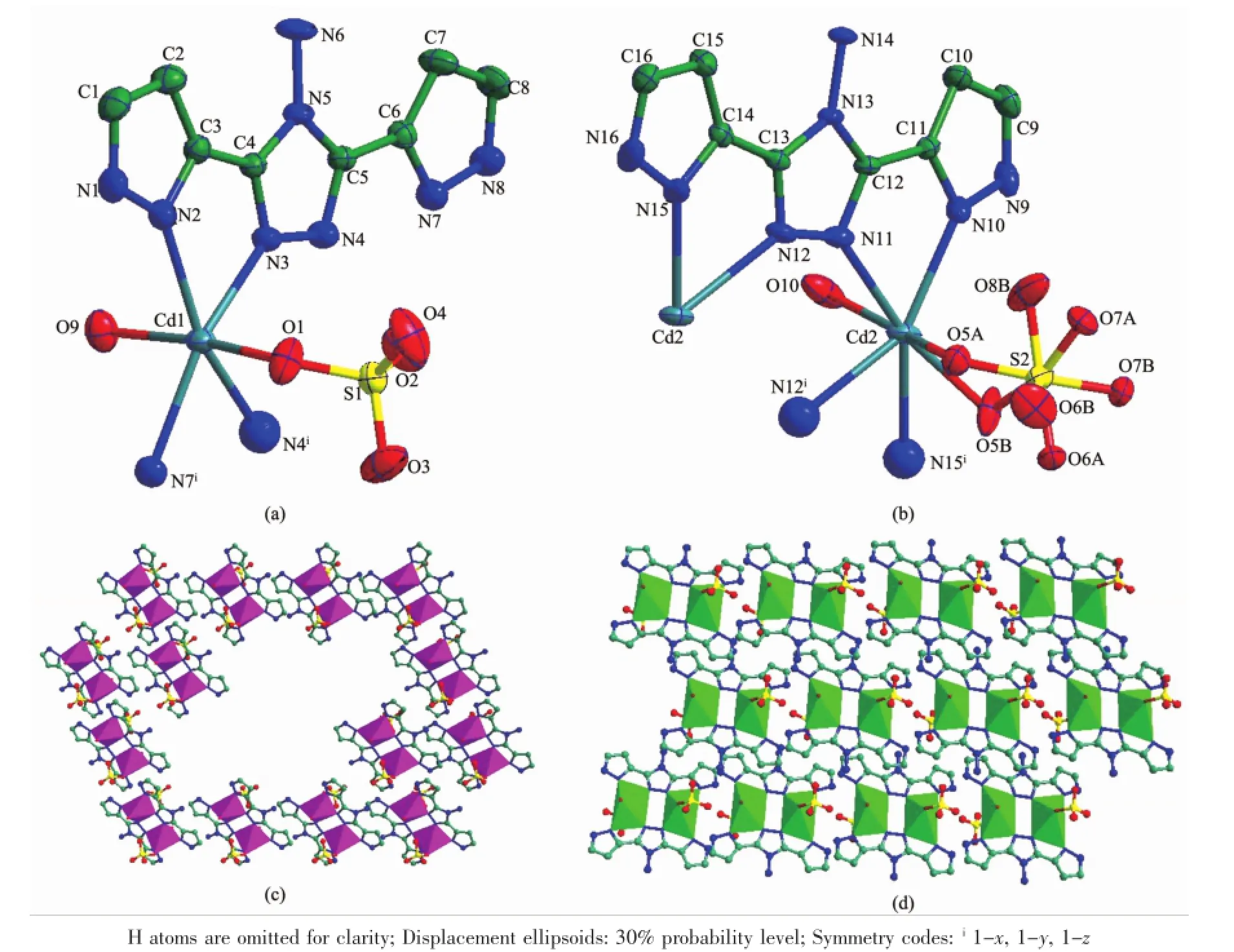
Fig.1(a and b)View of the coordination environments of Cd1 and Cd2 atoms in 1;(c)A ring generated by Cd1 atoms and H2ppty;(d)A layer formed by the binuclear units containing Cd2 atoms
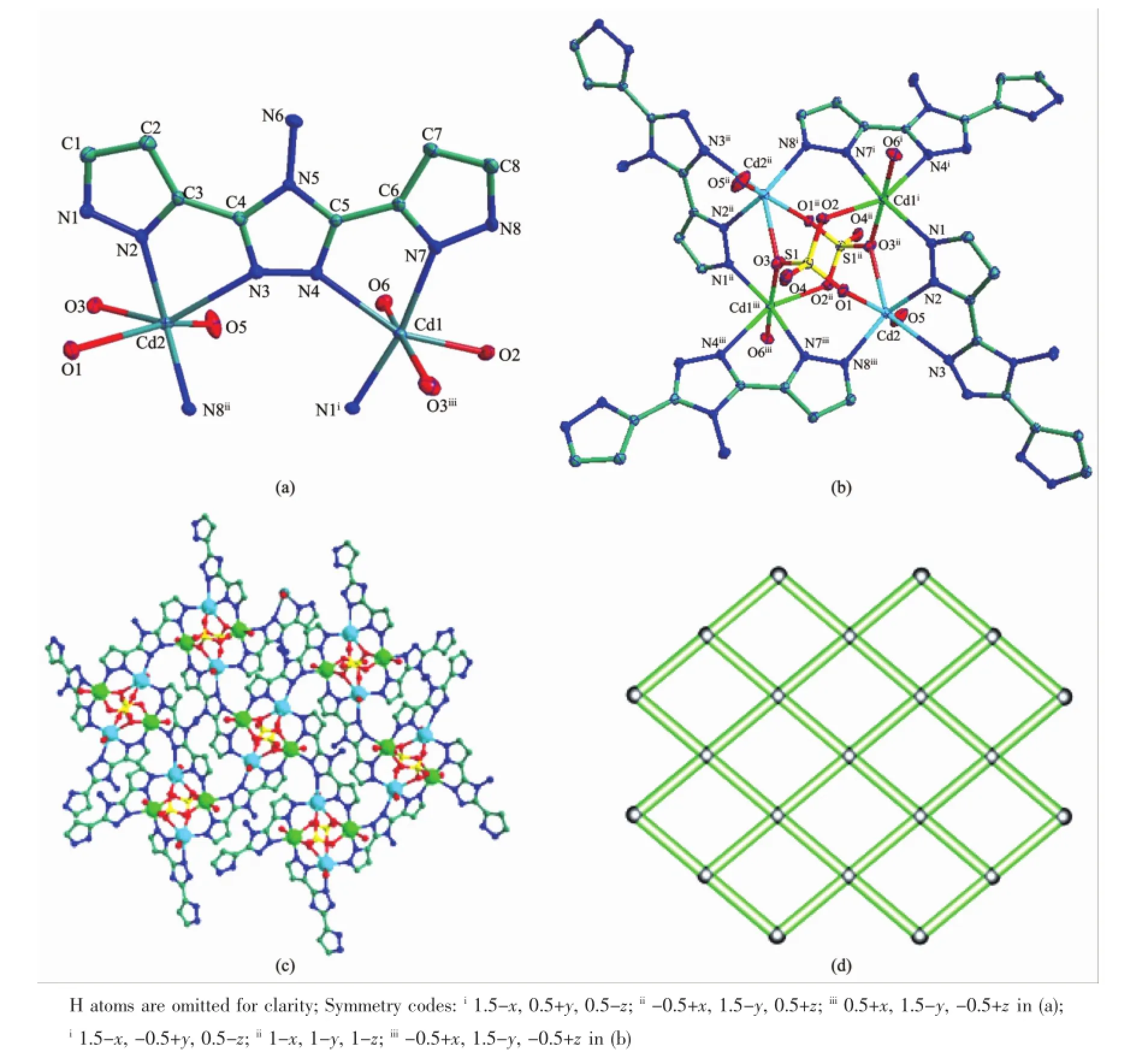
bridges giving rise to an interesting planar tetranuclear
cluster.The Cd…Cd distances between diagonal positions are 0.551 79(11)and 0.572 08(1)nm for Cd1…Cd1 and Cd2…Cd2,respectively.The neighboring Cd1…Cd2 and Cd1…Cd2A distances for 2 are 0.313 85(9)and 0.402 64(9)nm(Fig.2b). Adjacent clusters are linked by-N3-N4-bridges of ppty2-ligands generating a layer(Fig.2c).From the topology point,each cluster are considered as a 4-connected node,the topology of complex 2 are shown in Fig.2d with the Schläflisymbol of(44·62).
2.2 Lum inescent properties
For complexes containing cadmium as metal centers and conjugated organic linkers,they are promising candidates for luminescent materials with good applications,such as chemical sensor,photochemistry and electroluminescent display[24-25].Thus, the solid-state photoluminescent spectra of complexes 1 and 2,together with the free H2ppty ligand have been investigated at room temperature under the same experimental conditions.As depicted in Fig.3,ligand H2ppty displays an intense emission band at about 453 nm upon excitation at 224 nm.As to 1,the emission peak exhibits a small blue-shift(about 8 nm) with respect to the free H2ppty ligand(λem=445 nm, λex=344 nm),which may be assigned to the solvent molecules and packing interactions in the polymer,
which could increase the HOMO-LUMO energy gap of the polymer[26-27].For 2,the emission band is slightly red-shift(λem=478 nm,λex=380 nm)compared to the free H2ppty ligand.It is possible that a mixture characteristic of intraligand and ligand-to-ligand charge transition(LLCT),as reported for other d10metal complexeswith N-donor ligands[28].
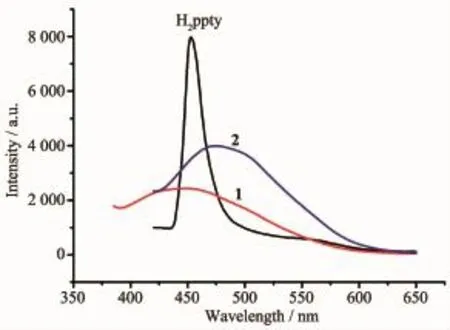
Fig.3 Solid-state emission spectra of ligand H2ppty and complexes 1 and 2 at room temperature
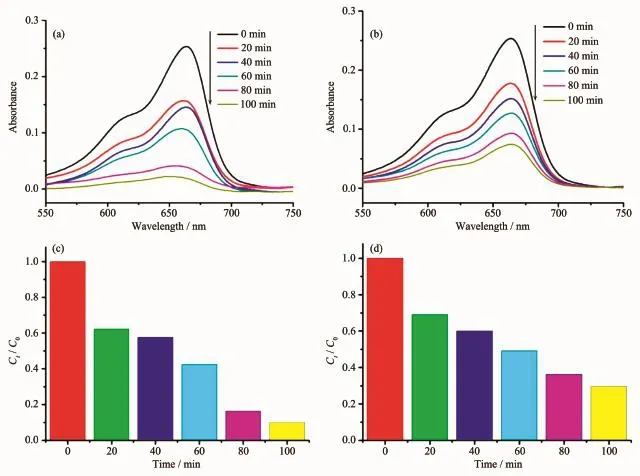
Fig.4 Absorption spectra of the MB solution during the decomposition reaction at the presence of complexes 1(a)and 2(b); Changes in the Ct/C0of MB solutions versus irradiation time at the presence of comp lexes 1(c)and 2(d)
2.3 Photocatalytic property
Photocatalysts have attracted much attention due to their potential applications in purifying water and air by thoroughly decomposing organic compounds[29-30]. The UV-Vis diffuse reflectance measurements reveal that the two compounds possess semiconductor behaviorswith smaller band gaps of 1.87 and 2.32 eV(Fig. S3).Therefore,narrow band gaps of the two complexes encourage us to investigate their visible-light photocatalytic activities,which are evaluated by the photodegradation of organic contaminants.Herein,the photocatalytic decomposition of MB was selected as a model of dye contaminant.Excitingly,complexes 1 and 2 exhibitgood photocatalytic activities under highpressure xenon lamp irradiation.
As can be seen in Fig.4a and 4b,the characteristic absorption peak of MB(665 nm)significantly decreases as the photocatalytic reaction proceeds. Besides,the degradation effects as a function of irradiation time were measured(Fig.4c and 4d)to clarify the photocatalytic results(wherein Ctis the concentration of the MB solutions at time t and C0is
the initial concentration of the MB solutions).The degradation rate increases from 23%for no photocatalyst to 70.4%for 1 and 90%for 2 when complexes 1 and 2 are added to the MB solution for 100 min.In addition,the stabilities of the two complexes are investigated by measuring the powder PXRD patterns after photocatalytic reactions(Fig.S2).The PXRD patterns are consistentwith those of the original ones, which confirm that the two complexes keep their skeleton well after the photocatalytic process.The photocatalytic research results demonstrate that complexes 1 and 2 are vigorous for the decomposition of MB and may have possible application in decomposing other dyestuff.
3 Conclusions
In summary,two new complexes have been obtained through the same reaction conditions except for the different temperatures.Further investigation indicates that the final structure of 1 and 2 containing binuclear and tetranuclear clusters,respectively,are obviously different.The cluster-based complexes including different clusters exert different influences on the structural differences and then produce different photocatalytic and luminescent properties. The photocatalytic results demonstrate that complexes 1 and 2 are vigorous for the decomposition of MB. Further research is underway to synthesize other cluster-based materials with better application in decomposing other dyestuff.
Supporting information isavailableathttp://www.wjhxxb.cn
[1]LüJ,Lin J X,Zhao X L,et al.Chem.Commun.,2012,48: 669-671
[2]Waranusantigul P,Pokethitiyook P,Kruatrachue M,et al. Environ.Pollut.,2003,125:385-392
[3]Dias EM,Petit C.J.Mater.Chem A,2015,45:22484-22506
[4]DuráG,Carrión M C,Jalón F A,et al.Cryst.Growth Des., 2015,15:5174-5182
[5]Guo J,Ma J F,Li J J,et al.Cryst.Growth Des.,2012,12: 6074-6082
[6]Guo J,Yang J,Liu Y Y,et al.CrystEngComm,2012,20: 6609-6617
[7]Kan W Q,Liu B,Yang J,et al.Cryst.Growth Des.,2012,5: 2288-2298
[8]Kang G,Jeon Y,Le K Y,et al.Cryst.Growth Des.,2015,11: 5183-5187
[9]Chen Y Q,Liu S J,Li YW,et al.Cryst.Growth Des.,2012, 12:5426-5431
[10]Meng Q,Xin X,Zhang L,et al.J.Mater.Chem.A,2015, 47:24016-24021
[11]Paul A K,MadrasG,Natarajan S.Phys.Chem.Chem.Phys., 2009,11:11285-11296
[12]Zhuang X,Wan Y,Feng C,et al.Chem.Mater.,2009,21: 706-716
[13]Yan Y,Zhang M,Gong K,et al.Chem.Mater.,2005,17: 3457-3463
[14]Wen L L,Wang F,Feng J,et al.Cryst.Growth Des.,2009, 9:3581-3589
[15]Tang J,Liu Y,Li H,et al.Chem.Commun.,2013,48:5498-5500
[16]Wang C C,Li JR,LüX L,et al.Energy Environ.Sci., 2014,9:2831-2867
[17]Wardana F Y,Ng SW,Wibowo A C.Cryst.Growth Des., 2015,12:5930-5938
[18]Meng W,Xu Z Q,Ding J,et al.Cryst.Growth Des.,2014, 14:730-738
[19]Liu L,Ding J,Huang C,et al.Cryst.Growth Des.,2014,14: 3035-3043
[20]Peng JB,Zhang Q C,Kong X J,et al.Angew.Chem.Int. Ed.,2011,50:10649-10652
[21]Cui J,Li Y,Guo Z,et al.Chem.Commun.,2013,49:555-557
[22]Browne W R,O′Connor C M,Hughes H P,et al.Dalton Trans.,2002:4048-4054
[23]Sheldrick G M.SHELXTL-97,University of Göttingen, Germany,1997.
[24]Yao X Q,Zhang M D,Hu J S,et al.Cryst.Growth Des., 2011,11:3039-3044
[25]Mu Y J,Song Y J,Wang C,et al.Inorg.Chim.Acta,2011, 365:167-176
[26]Borkowski L A,Cahill C L.Cryst.Growth Des.,2006,6: 2248-2259
[27]SchmittelM H,LinW.Inorg.Chem.,2007,46:9139-9145
[28]Liu H Y,Wu H,Ma JF,etal.Cryst.Growth Des.,2010,10: 4795-4805
[29]Zhang C,Ye F,Shen S,et al.RSC Adv.,2015,11:8228-8235
[30]Zhang T,LinW.Chem.Soc.Rev.,2014,16:5982-5993
Cd Com p lexes Based on Dipyrazolyl Ligand:Temperature-Driven Assembly,Lum inescent and App lication in Photocatalysis
LIHui-Jun1YAN Ling-Ling*,2WANG Yuan*,1XU Zhou-Qing1XU Jun1WUWei-Na1
(1College of Chemistry and Chemical Engineering,Henan Polytechnic University,Jiaozuo,Henan 454000,China)
(2School of Physics and Electronic Information Engineering,Henan Polytechnic University,Jiaozuo,Henan 454000,China)
By changing solvothermal temperature,two new Cd(II))-based coordination complexes[Cd2(H2ppty)2(SO4)2(H2O)2]·3H2O(1)and[Cd2(ppty)(SO4)(H2O)2]n(2),(H2ppty=3,5-bis-(1H-pyrazol-3-yl)-[1,2,4]triazol-4-ylamine)have been synthesized and structurally characterized.Further investigation indicates that the final structures of 1 and 2 containing binuclear and tetranuclear clusters show zero-(0D)and two-dimensional(2D)structures,respectively. Moreover,luminescent properties of the two complexes are examined in the solid state at room temperature.The UV-Vis diffuse reflectance measurements reveal that the two compounds possess semiconductor behaviors with smaller band gaps of 1.87 and 2.32 eV,and show effective photocatalytic degradation activities over organic pollutantunder visible-light irradiation.CCDC:1502061,1;1427971,2.
Cd(II)complex;binuclear and tetranuclear cluster;luminescent;photocatalysis
O614.24+2
A
1001-4861(2016)10-1831-08
10.11862/CJIC.2016.229
2016-05-13。收修改稿日期:2016-09-07。
河南理工大学博士基金(No.20971110,91022013)资助项目。
*通信联系人。E-mail:yll@hpu.edu.cn,wangyuan08@hpu.edu.cn