三个基于1,5-二(2-乙基苯并咪唑基)-戊烷柔性配体的镉配合物
王岭 徐爽 孟伟*, 韩超 俞继康 俞书琪何章兴戴磊*,侯红卫
(1华北理工大学化学工程学院,唐山063009)
(2郑州大学化学与分子工程学院,郑州450052)
三个基于1,5-二(2-乙基苯并咪唑基)-戊烷柔性配体的镉配合物
王岭1徐爽1孟伟*,1韩超1俞继康1俞书琪1何章兴1戴磊*,1侯红卫2
(1华北理工大学化学工程学院,唐山063009)
(2郑州大学化学与分子工程学院,郑州450052)
通过设计柔性配体1,5-二(2-乙基苯并咪唑基)戊烷(bep),在二羧酸辅助配体的调控下成功制备了3个配位聚合物[Cd(bep) (sba)]n(1),[Cd(bep)(bda)]n(2),and{[Cd2(bep)(ada)2]·H2O}n(3)(H2sba=4,4′-磺酰基二苯甲酸,H2bda=4,4′-联苯二甲酸,H2ada=1,3-金刚烷二乙酸)。配合物均呈现二维层状结构。配合物1由交替的Cd(II)/bep/sba2-螺旋链构成。配合物2由Cd/bda2-单元构成二维结构,bep作为单齿配体与Cd(II)配位。配合物3的二维层通过Cd/ada2-/H2O氢键螺旋链拓展成三维超分子。此外,对配合物1~3的粉末X射线衍射、热稳定性以及荧光性质进行了研究。
柔性配体;晶体结构;螺旋;荧光性质
Coordination polymers(CPs)have developed rapidly recently duo to the fascinating structures and potential applications in adsorption,fluorescence, magnetism,chemical sensor,etc[1-5].The construction of CPs always beginswith the selection ofmetal nodes and organic building blocks.Among the two components,the selection of organic ligands is a key point tomanipulate the structures and properties[6].As we know,the conformationally rigid organic linkers are widely used to build targeted structures with certain topologies and applications[7-9].Compared with the coordination polymers based on rigid ligands,the CPs based on flexible ligands(denoted as FL-CPs) have attracted much interest[10-12].Such ligands bear the flexible spacers,whichmake the ligand rotate and bend freely to meet various coordination requirement ofmetal ions,and usually lead to intriguing structures such as helix,interpenetration and so on[13-15].
However,FL-CPs are more difficult to synthesis due to the flexible feature of the organic linkers.The synthesis process is strongly dependent on solvent, temperature,counter ions and other factors[16-18],which severely hinder the synthesis aswell as the prediction of extended network.To explore the effect of flexibility and conformation of the ligands on the self-assembly process,we designed a longer flexible ligand 1,5-bis(2-ethylbenzimidazole)pentane(bep).The distinct characteristics of bep ligand are as follows:(II)Five -CH2-spacers are introduced to the benzimidazole, which makes the ligand more flexible to suit the diverse coordinated environmentofmetal ions.(II)The 2-position substituentethyl could enhance the donated electrons ability of benzimidazole ring.(II)The long spacers of bep are in favor of the formation of coordination polymerswith helical features.
With the aforementioned consideration,three FL-CPs,[Cd(bep)(sba)]n(1),[Cd(bep)(bda)]n(2),and {[Cd2(bep)(ada)2]·H2O}n(3)(H2sda=4,4′-sulfonyldibenzoic acid,H2bda=biphenyl-4,4′-dicarboxylic acid, H2ada=1,3-adamantanediacetic acid)have been obtained based on the bep ligand and three assisted carboxylate ligands.Moreover,the thermal stability and luminescent behaviors of the complexes have also been studied.
1 Experimental
1.1 M aterials and methods
The ligand bep was synthesized according to the literature[19].All the other chemicals were purchased commercially.Infrared spectra(IR)were obtained on an Avatar 360(Nicolet)spectrophotometer.Elemental analyses(C,H,and N)were measured on a FLASH EA 1112 elemental analyzer.Thermogravimetric analysis(TGA)was performed on a NETZSZH TG 209 thermal analyzer in air ata heating rate of 10℃·min-1from room temperature to 800℃.Powder X-ray diffractions(XRD)weremade under a Rigaku D/Max-2500 PCT with Cu Kαradiation(λ=0.154 2 nm), using an operating voltage of 45 kV and an operating currentof 40 mA at room temperature in the 2θrange between 5°and 50°.Luminescence spectra for these crystallinematerials were carried out on a Hitachi F-7000 Fluorescence Spectrophotometer.
1.2 Synthesis of[Cd(bep)(sba)]n(1)
Cd(OAc)2·2H2O(53.2 mg,0.2 mmol),bep(36 mg,0.1mmol)and H2sda(30.6mg,0.1 mmol)were mixed in 8 mL H2O.Themixture was sealed in a 25 mL Teflon-lined stainless steel container,and heated at130℃for 3 days.After themixture cooled to room temperature at a rate of 5℃·h-1,colorless block crystals were collected.Yield:60%,based on Cd. Anal.Calcd.for C37H36N4O6SCd(%):C,57.18;H,4.67; N,7.21,S,4.13.Found(%):C,57.23;H,4.82;N, 7.35,S,4.23.IR(KBr,cm-1):3137(s),2367(w),1597 (s),1552(s),1393(s),1327(w),1297(w),1161(m),1101 (m),1 067(w),1 012(w),858(m),750(s),620(m),580 (m),474(m).
1.3 Synthesis of[Cd(bep)(bda)]n(2)
The synthesismethod is similar to 1,except that H2bda(25.2mg,0.1mmol)was used instead of H2sba. Colorless prismatic crystals were collected.Yield: 35%,based on Cd.Anal.Calcd.for C51H44N4O8Cd2(%): C,57.48 H,4.16;N,5.26.Found(%):C,57.49;H, 4.36;N,5.15.IR(KBr,cm-1):3131(s),2374(w),1935 (w),1 607(s),1 548(m),1 393(s),1 173(m),1 072(m), 1011(m),851(s),761(s),691(m),445(m).
1.4 Synthesis of{[Cd2(bep)(ada)2]·H2O}n(3)
The synthesismethod is similar to 1,except that H2ada(25.2mg,0.1mmol)was used instead of H2sba. Colorless prismatic crystalswere collected.Yield:45% based on Cd.Anal.Calcd.for C37H48N4O5Cd(%):C, 59.96;H,6.53;N,7.56.Found(%):C,60.03;H,6.49; N,7.60.IR(KBr,cm-1):3 130(s),2 372(w),1 547(s), 1 397(s),1 252(w),1 156(m),1 068(m),935(w),751 (s),675(w),469(w).
1.5 Crystal structural determ ination
The data of complexes 1~3 were collected on a Rigaku 007HFSaturn 724 CCD diffractometer(Mo Kα, λ=0.071 073 nm)at(293±1)K.The structures were solved by directmethods with SHELXS-97 program[20]and refined with a full-matrix least-squares technique based on F2with the SHELXL-97 crystallographic software package[21].All non-hydrogen atoms were treated anisotropically.Hydrogen atoms were located at geometrically calculated positions and refined with isotropic thermal parameters.Crystallographic data and selected bond lengths and angles are presented in Table 1 and 2.
CCDC:1469166,1;1469167,2;1469168,3.
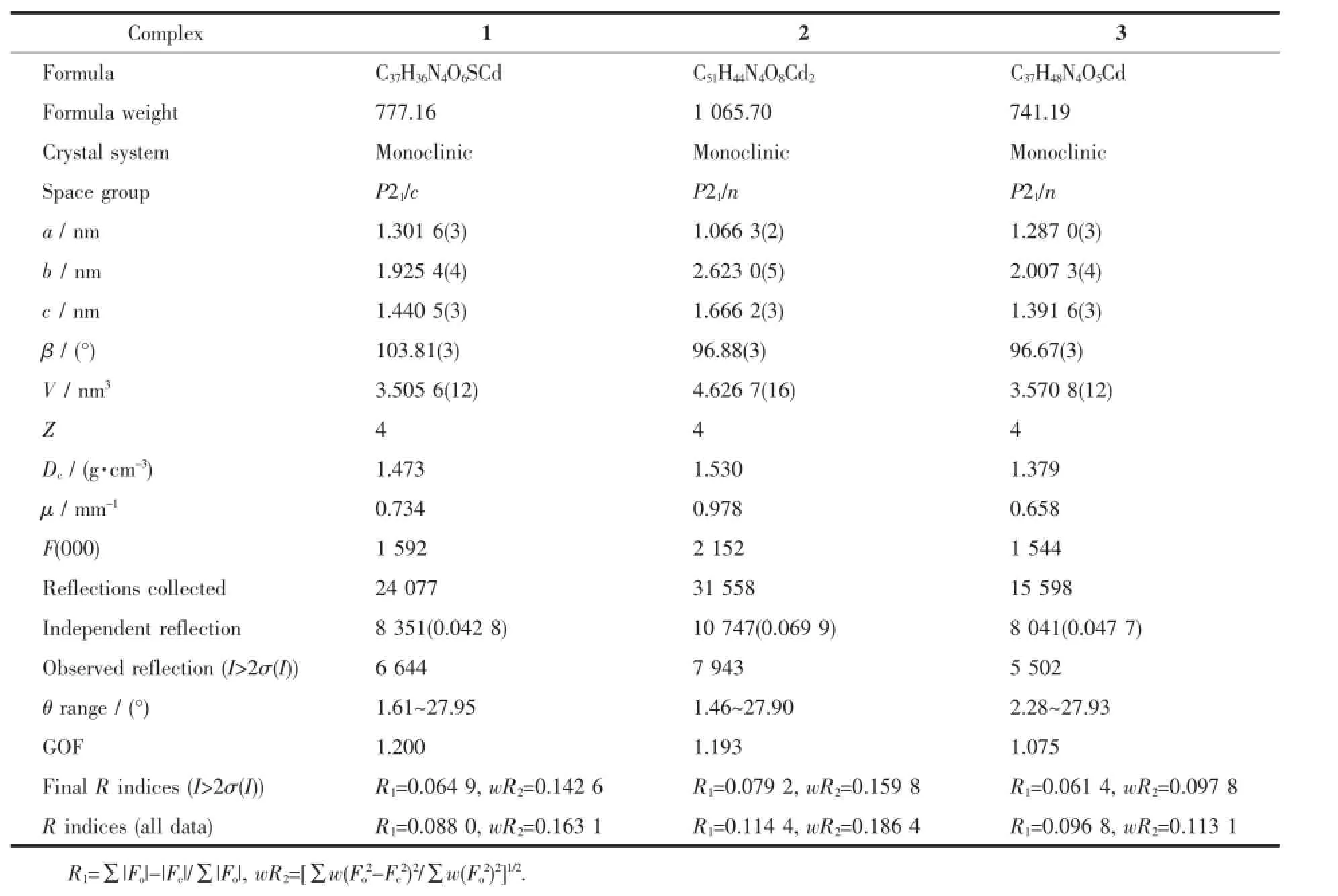
Table 1 Crystallographic data of com plexes 1~3
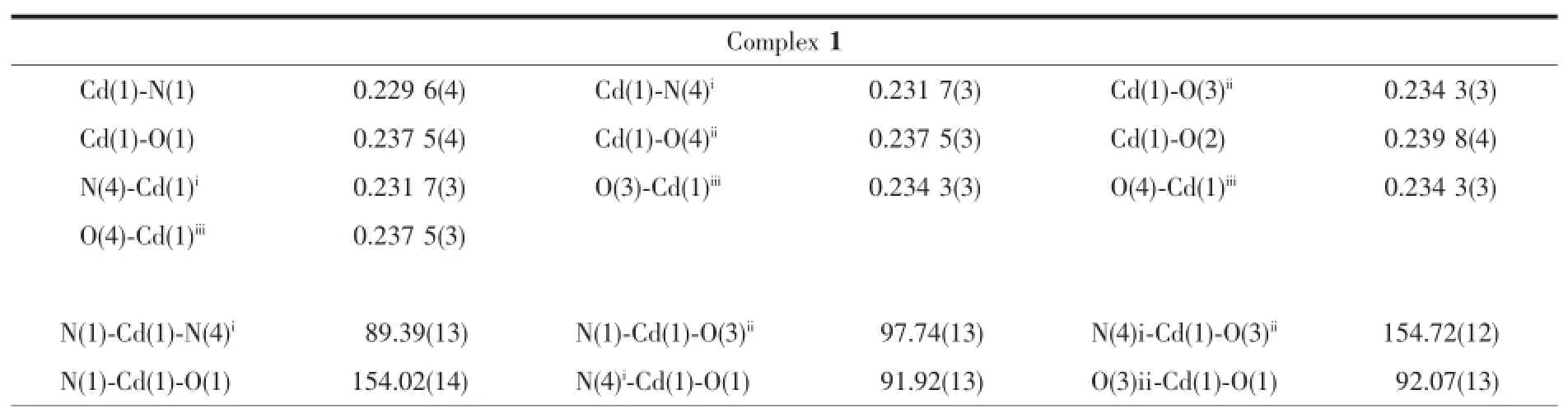
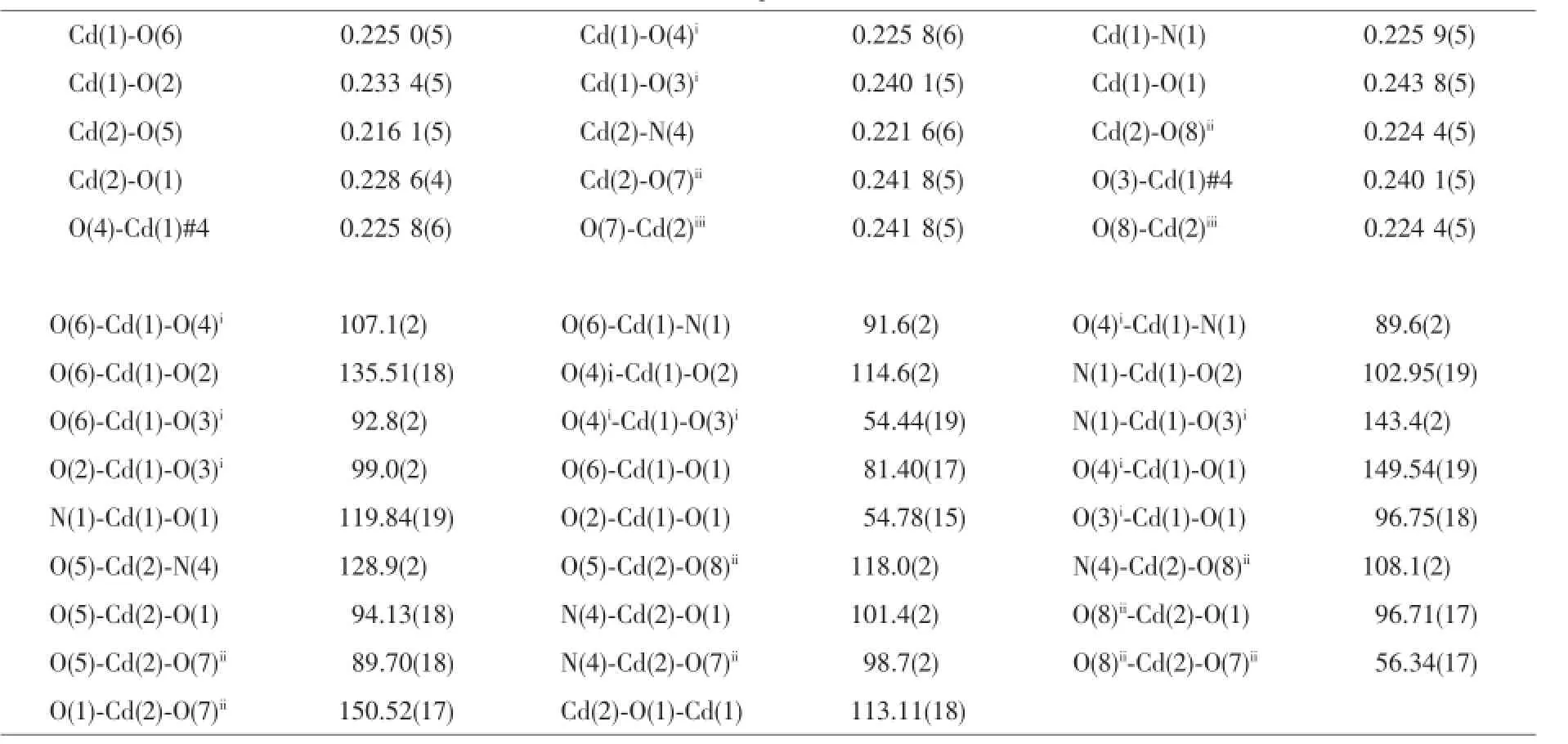
Table 2 Selected bond lengths(nm)and bond angles(°)for complexes 1~3

ContinuedTable2
Complex2
Complex3
2 Resultsanddiscussion
2.1 Structuredescriptionof[Cd(bep)(sba)]n(1)
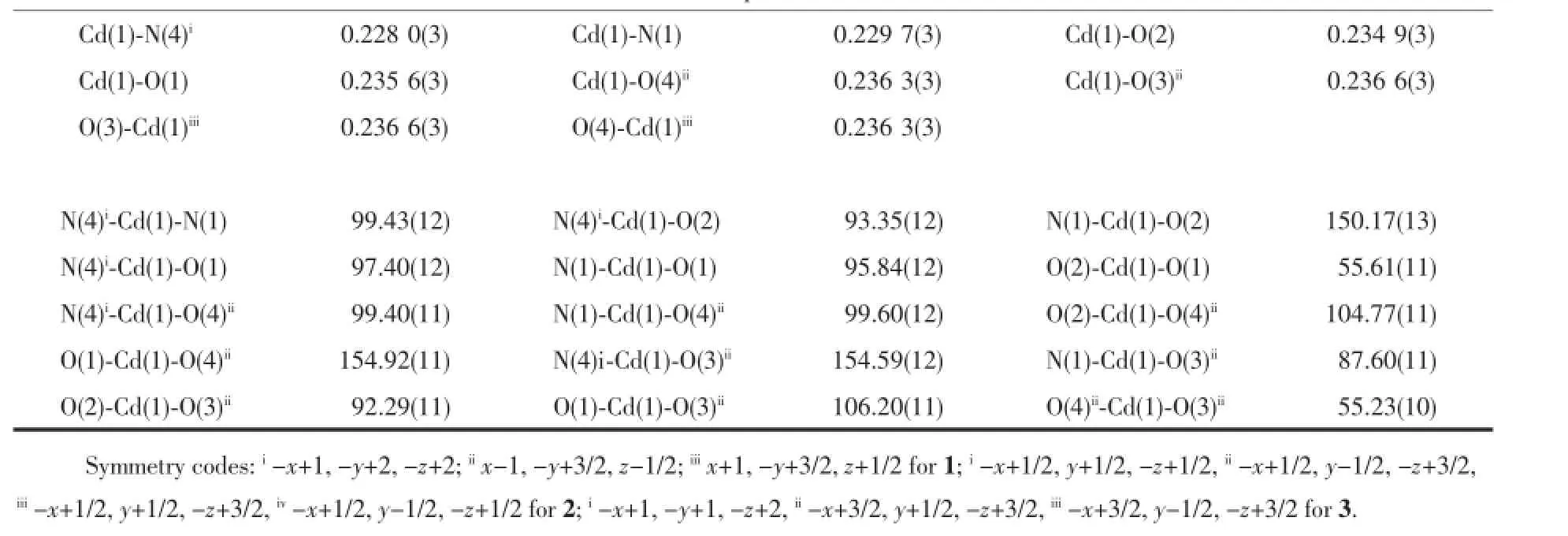
Complex1crystallizesinthemonoclinicspace groupP21/candtheasymmetricunitcontainsone Cd(II),onebepligandandonesba2-(Fig.1a).Cd(II)is six-coordinatedinadistortedoctahedralgeometryby threeOatoms(O1,O2,O4i)oftwosba2-,oneN1atom ofonebepligandattheequatorialplane,andone carboxylateO3iatomandoneN4iiatomoccupyingthe axialsite.ThedistancesofCd-O/Nbondareinthe rangeof0.2296(4)~0.2397(4)nm,whicharesimilar withothercadmiumcomplexes[22-23].AsshowninFig. 1b,eachCd(II)isbridgedbythesba2-with(κ2)-(κ2)-μ2coordinationmodetoforma1Dchainstructure.The bepliganddisplaysanasymmetrictrans-conformation andtheNdonor…N-Csp3…Csp3torsionanglesare79.949° and139.146°,respectively.A20-memberedringmotif isformedbytwoCd(II)andtwobep(Fig.1c).Each ringisfurtherconnectedbythe1Dchainintoa2D undulatinglayer.Interestingly,thereexistfascinating left-andright-handedhelicalchainsproducedbythe sba2-andthe20-memberedringinthe2Dframework (Fig.1d).Thehelicalpitchis1.9254nmandthe neighboringCd…Cdseparationsalongbepandsba2-are1.41574nmand1.40813nm,respectively.From
the topological viewpoint,the network of complex 1 could be described as 63topology by treating Cd(II)as a 3-connector(Fig.1e).
2.2 Structure description of[Cd(bep)(bda)]n(2) Complex 2 crystallizes in the monoclinic space group P21/n.As depicted in Fig.2a,Cd1 is octahedrally coordinated by five carboxylate O atoms(O1,O2,O3i, O4i,O6)coming from three bda2-(Cd1-O/N 0.225 0(5)~0.243 8(5)nm)and one N1 atom from one bep ligand. Cd2 locates in a distorted square-pyramidal geometry with the structural index parameter(τ)of 0.36[24],and coordinated by four O atoms from three bda2-(Cd-O (O1,O5,O7ii,O8ii)0.224 4(5)~0.241 8(5)nm)and one N4 atom of one bep ligand(Cd-N(N4)0.221 6(6)nm). Interestingly,there exist two kinds of independent bda2-.One kind of bda2-adopts a(κ1-κ1)-(κ2)-μ3coordination mode,while the other adopts a(κ2-μ2)-(κ2)-μ3coordination mode.One carboxylate group links Cd1 and Cd2 in(κ2-μ2)mode to produce a binuclear Cd2unit with Cd…Cd separation of 0.394 28 nm.Then the binuclear unit is further connected by the carboxylate groups to produce a 2D layer(Fig.2b). The bep ligand takes an asymmetric trans-conformation with the Ndonor…N-Csp3…Csp3torsion angles of 110.4° and 105.4°,respectively.Under the help of one carboxylate group with(κ1-κ1)mode,Cd1,Cd2 and bep form a 14-membered ring.Different from complex 1, the bep ligand just decorate on the 2D layer from two sides,which looks like protruding flowers.If the dinuclear Cd2unit is considered as a 4-connected node,the 2D skeleton could be represented as a 44net(Fig.2c).
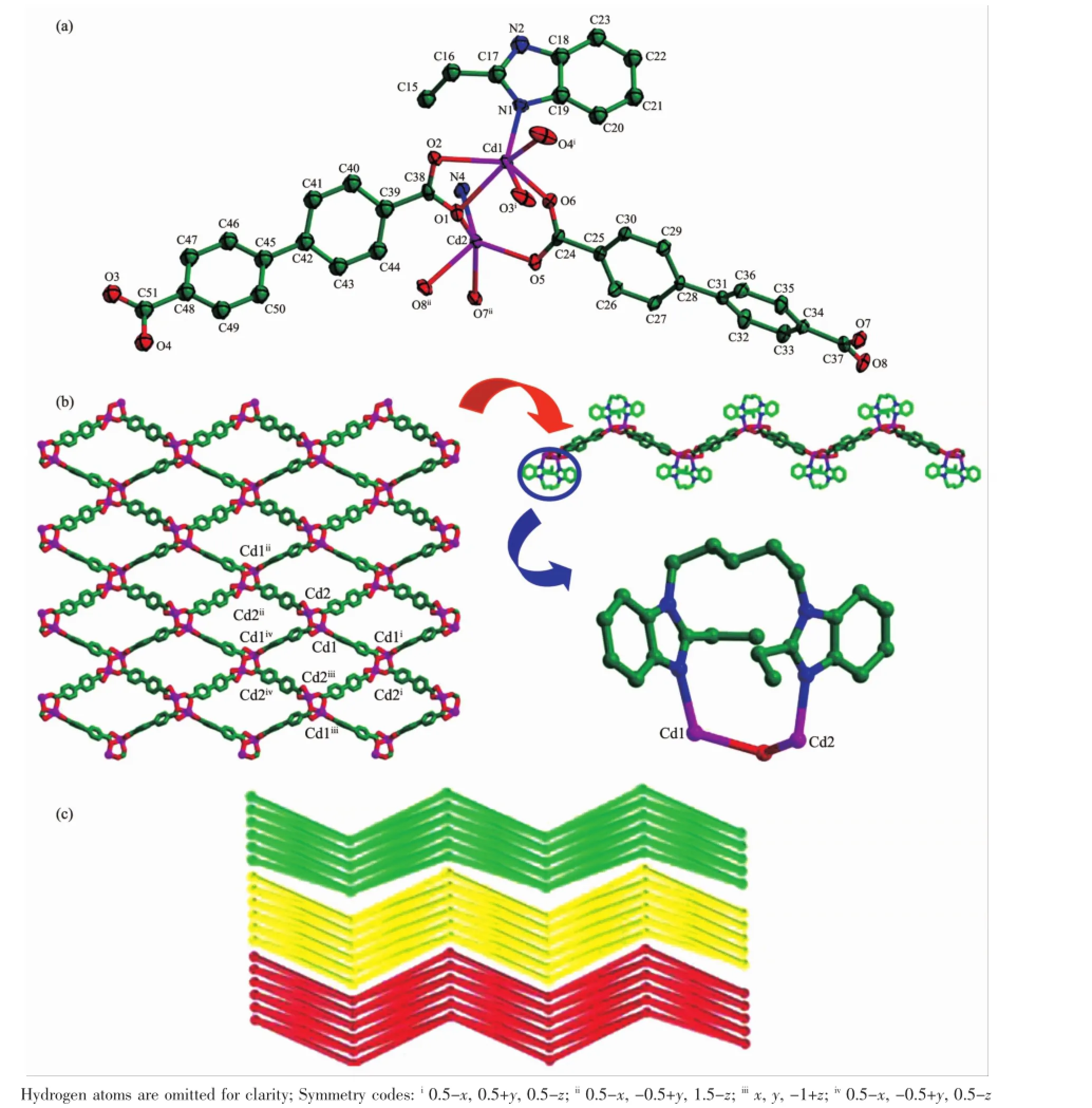
Fig.2(a)Coordination environmentof Cd(II)in 2 with thermal ellipsoid at30%probability levels; (b)2D layer constructed by Cd(II)and bda2-;(c)Undulating 2D network with 44topology
2.3 Structure description of{[Cd2(bep)(ada)2]· H2O}n(3)
Complex 3 crystallizes in monoclinic crystal system with a space group P21/n.Cd(II)is octahedrally coordinated with four O atoms(O1,O2,O3i,O4i)of twoada2-and two N atoms(N1,N4ii)of two bep ligands (Fig.3a).The Cd-N/O bond distances are in the range of0.2301(4)~0.236 5(3)nm.Theada2-takesabidentate chelated(κ2)-(κ2)-μ2coordination mode,linking each Cd(II)to form a 1D chain structure,in which the Cd…Cd distance is 1.019 68 nm.Then each 1D chain is linked further to produce a 2D framework by the asymmetric trans-conformational bep ligand with Ndonor…N-Csp3…Csp3torsion angles of 87.021°and 46.759°, respectively(Fig.3b).Moreover,two Cd(II)and two bep ligands also constructa 20-membered ringswith Cd…
Cd separation of 1.128 97 nm.Interestingly,under the help of hydrogen-bond interactions,Cd(II),the ada2-and the guest H2O molecules are connected together to form an extraordinary helical chain with a pitch of 2.007 30 nm.The helical chains finally ensure the assembling of the 2D layers into a 3D supramolecular (Fig.3c).Topological analysis reveals that,the framework of complex 3 could be described as 63topology, by treating Cd(II)ion as a 3-connector(Fig.3d).
Fig.3(a)Coordination environment of Cd(II)ion in 3 with thermal ellipsoid at30%probability levels;(b)2D layer constructed by Cd/ada2-chains and 20-membered rings;(c)3D supramolecular directed by Cd/ada2-/H2O under the help of coordination bond and hydrogen-bond;(d)2D network with 63topology
2.4 Influenceof thebep ligand and dicarboxylates
Based on the flexible features of bep,the ethylbenzimidazole could twist freely around the-CH2-spacers with different Ndonor…N-Csp3…C sp3torsion angles.In complex 1,the Ndonor…N-Csp3…Csp3torsion angles are 79.949°and 139.146°,respectively,and two bep connect two Cd(II)to construct a 20-membered ring motif.A similar 20-membered ring is also constructed by two bep and two Cd(II)in complex 3, where the torsion angles of bep are 87.021°and 46.759°,respectively.When both of the torsion angles are relative large,a smaller 14-membered ring is constructed with the help of bda2-in complex 2. Meanwhile,the bep just decorates on the 2D layer and plays no role in the control of dimensionality due to the large torsion angles.
In this paper,three dicarboxylates are introduced into the synthetic process to increase the structural diversity.In complex 1,the sba2-exhibits chelating coordination modes and links each Cd(II)to construct 1D wave-like chains.Similarly,a 1D wave-like chain structure is also constructed by the ada2-with the same coordination fashion in complex 3.Then each chain is further bridged by the bep,forming a 2D layered structure.As to complex 2,the connecting modesofbda2-aremore complicated than those of sba2-and ada2-.Different from the simple chelating mode, the bda2-connects two binuclear Cd units by adopting the(κ2-μ2)-(κ2)-μ3fashion to directly produce a 2D puckered layer without the help of bep ligand.
2.5 XRD patterns and thermal analyses
The phase purity of these crystalline materials was recorded.As shown in Fig.4,the experimental XRD patterns were comparable to the simulated ones, which indicated a pure phase of each bulky sample.
To examine the thermal stability,thermogravimetric analysis of complex 1~3 were carried out(Fig. 5).For anhydrous complex 1 and 2,no obviousweight loss was observed before 350℃,and then the
frameworks begin to decompose.A white residue of CdO(Obsd.15.38%,Calcd.16.52%for 1;Obsd. 23.33%,Calcd.24.10%for 2)was obtained at 660 and 490℃,respectively.For complex 3,there was a weight loss of 2.73%before 137℃attributed to the release ofuncoordinated H2Omolecule(Calcd.2.43%). The overall framework began to collapse at 293℃and the residue was CdO(Obsd.17.15%,Calcd. 17.32%).
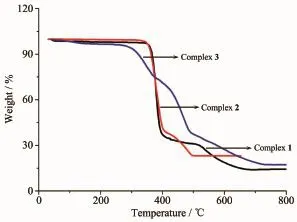
Fig.5 TGA curves of comp lexes 1~3
2.6 Lum inescent property
Given that d10coordination complexes have potential applications in photoactive materials,the luminescent properties of complexes 1~3 in the solid state aswellas freebep ligand and three dicarboxylates were investigated(Fig.6).The emission peak for bep was observed at 310 nm(λex=280 nm),while the emission peaks for dicarboxlates were observed at 370,390 and 420 nm(λex=340 nm)for H2sba,400 nm (λex=276 nm)for H2bda,and 390 nm(λex=210 nm)for H2ada,respectively.Complex 1 exhibited a wide emission band from 300~500 nm with the emission peaks at 320,370,and 427 nm(λex=277 nm).The peak at 320 nm(red shift of10 nm compared with the bep)was attributed to the ligand-to-metal charge transfer(LMCT),and the emission peak at 370 nm was attributed to the intraligand transition of H2sba, whereas the peak at 427 nm(red shift of 7 nm compared with H2sba)was also due to the LMCT[25]. Complex 2 showed an obvious emission peak at 372
nm(λex=277 nm),which was blue shift of 28 nm compared with that of H2bda,and it could be deduced as intraligand transition of H2bda[26-27].For complex 3, it displayed the emission peak at 304 nm(λex=286 nm),which could also be attributed to the intraligand transitions of bep ligand.
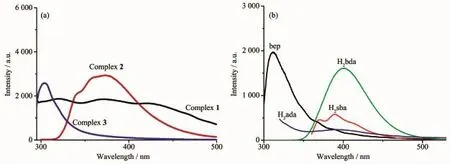
Fig.6 Emission spectra of complexes 1~3(a)and ligands bep,H2sba,H2bda and H2ada(b)in the solid state
3 Conclusions
By introducing multiple-CH2-spacers to the 2-ethylbenzimidazole,we designed a flexible N-containing ligand,and three FL-CPs were synthesized successfully based on the flexible ligand and three carboxylate ligands.These complexes all exhibited 2D layered structures.The structural diversity revealed that the design of flexible ligand was very important to construct diverse FL-CPs,especially with helical structures.In addition,the selection of assisted carboxylate ligands also provided an effective way to increase the structural diversities.
[1]Liu JW,Chen L F,Cui H,et al.Chem.Soc.Rev.,2014,43: 6011-6061
[2]Li SZ,Huo FW.Nanoscale,2015,7:7482-7501
[3]Kreno LE,Leong K,Farha O K,etal.Chem.Rev.,2012,112 (2):1105-1125
[4]Cui Y J,Yue Y F,Qian G D,et al.Chem.Rev.,2012,112 (2):1126-1162
[5]Cui Y J,Li B,He H J,et al.Acc.Chem.Res.,2016,49(3): 483-493
[6]Lin Z J,LüJ,Hong M C,et al.Chem.Soc.Rev.,2014,43: 5867-5895
[7]LIJin-Ping(李金萍),FAN Jian-Zhong(范建中),WANGDuo-Zhi(王多志).Chinese J.Inorg.Chem.(无机化学学报),2016, 32(5):753-761
[8]ZHANG Chun-Li(张春丽),WANG Hong-Yan(王红艳), ZHENG He-Gen(郑和根).Chinese J.Inorg.Chem.(无机化学学报),2016,32(5):859-863
[9]Chen J,Zhang Q,Liu Z F,et al.Dalton Trans.,2015,44: 10089-10096
[10]Nijem N,Thissen P,Yao Y P,et al.J.Am.Chem.Soc., 2011,133(32):12849-12857
[11]ZHANG Ya-Nan(张亚男),YIN Hai-Ju(殷海菊),DANG Bei-Jun(党蓓君).Chinese J.Inorg.Chem.(无机化学学报), 2016,32(5):846-852
[12]YUAN Hou-Qun(袁厚群),XIAOWei(肖伟),LIYan-Xia(李艳霞),etal.Chinese J.Inorg.Chem.(无机化学学报),2016, 32(5):899-905
[13]Kong G Q,Wu C D.Cryst.Growth Des.,2010,10(10):4590-4595
[14]Ding B,Liu Y Y,Huang Y Q,et al.Cryst.Growth Des., 2009,9(1):593-601
[15]Cheng PC,Kuo P T,Xie M Y,et al.CrystEngComm,2013, 15:6264-6272
[16]Lee E,Ju H,Kim S,et al.Cryst.Growth Des.,2015,15(11): 5427-5436
[17]Murdock C R,Lu Z,Jenkins D M.Inorg.Chem.,2013,52 (4):2182-2187
[18]Zhu L Z,Wang C Z,Mei L,et al.CrystEngComm,2015,17: 3031-3040
[19]Aakero C B,Desper J,Leonard B,etal.Cryst.Growth Des., 2005,5(3):865-873
[20]Sheldrick G M.SHELXS-97,Program for the Solution of Crystal Structures,University of Göttingen,Germany,1997.
[21]Sheldrick G M.SHELXL-97,Program for the Refinement of Crystal Structures,University of Göttingen,Germany,1997.
[22]Xu C Y,Li L K,Wang Y P,et al.Cryst.Growth Des.,2011, 11(10):4667-4675
[23]Li M T,Wang C G,Yu L Y,et al.Z.Anorg.A llg.Chem., 2012,638:466-472
[24]Liu L,Liu Y H,Han G,et al.Inorg.Chim.Acta,2013,403: 25-34
[25]Zheng SR,Zhang L,He JE,et al.Inorg.Chem.Commun., 2016,66:19-23
[26]MengW,Xu ZQ,Ding J,et al.Cryst.Growth Des.,2014,14 (2):730-738
[27]Xu C Y,Guo Q Q,Wang X J,et al.Cryst.Growth Des., 2011,11(5):1869-1879
Three Cd(II)Coordination Polymers Based on Flexible Ligand 1,5-Bis(2-ethylbenzim idazole)pentane
WANG Ling1XU Shuang1MENGWei*,1HAN Chao1YU Ji-Kang1YU Shu-Qi1HE Zhang-Xing1DAILei*,1HOU Hong-Wei2
(1College of Chemical Engineering,North China University of Science and Technology,Tangshan,Hebei 063009,China)
(2College of Chemistry and Molecular Engineering,Zhengzhou University,Zhengzhou 450052,China)
Three coordination polymers,[Cd(bep)(sba)]n(1),[Cd(bep)(bda)]n(2),and{[Cd2(bep)(ada)2]·H2O}n(3) have been prepared based on a newly designed flexible ligand 1,5-bis(2-ethylbenzimidazole)pentane(bep)and three dicarboxylates(H2sba=4,4′-sulfonyldibenzoic acid,H2bda=biphenyl-4,4′-dicarboxylic acid,H2ada=1,3-adamantanediacetic acid).The complexes all exhibit 2D layer frameworks.Complex 1 is constructed by the alternant Cd(II)/bep/sba2-helical chains.In complex 2,the 2D layer is composed of Cd/bda2-units and the bep ligand just coordinates to Cd(II)asmonodentate ligand.Complex 3 is connected by the hydrogen-bonding helical chains Cd/ada2-/H2O to form a 3D supramolecule network.Solid-state properties of X-ray powder diffractions, thermal stability and luminescent properties for these crystallinematerials are also investigated.CCDC:1469166, 1;1469167,2;1469168,3.
flexible ligand;crystal structure;helix;luminescent property
O614.24+2
A
1001-4861(2016)10-1847-10
10.11862/CJIC.2016.219
2016-05-30。收修改稿日期:2016-08-09。
河北省自然科学基金青年科学基金(No.B2016209266)、河北省高等学校科学技术研究指导项目(No.ZC2016035)和河北省教育厅青年基金(No.QN2016183)资助。
*通信联系人。E-mail:2007mengwei@163.com,dailei_b@163.com;会员登记号:S06N0600M1607(孟伟),S06N4647M1607(戴磊)。