Improvement and application of Y2O3directional solidification crucible
M Limin,Zhng Jizhen,Yue Gungqun,Zhng Hurui,Zhou Li,Zhng Hu
aBeijing Aeronautical Science&Technology Research Institute of COMAC,Beijing 102211,China
bSchool of Materials Science and Engineering,Beihang University,Beijing 100191,China
Improvement and application of Y2O3directional solidification crucible
Ma Limina,*,Zhang Jiazhena,Yue Guangquana,Zhang Huaruib,Zhou Lib,Zhang Hub
aBeijing Aeronautical Science&Technology Research Institute of COMAC,Beijing 102211,China
bSchool of Materials Science and Engineering,Beihang University,Beijing 100191,China
Directional solidification;Erosion resistance;Microstructure;Nb–Si based alloy;Thermal shock resistance;Y2O3crucible
In order to satisfy the drastic temperature change and high chemical activity in directional solidification of Nb–Si based alloys,Y2O3crucible is demanded to possess high thermal shock resistance and erosion resistance.This paper improved the sintering degree and density of Y2O3crucible by optimizing the sintering temperature and time,and its practical application performance was investigated.Y2O3grains gathered with the increase of sintering temperature and time,and the contact area enlarged,resulting in the open pores being changed into closed pores.The higher density caused the improvement of erosion resistance of Y2O3crucibles.However,excessive density weakened the thermal shock resistance.Considering high-temperature strength,erosion resistance,thermal shock resistance and costs,optimum sintering temperature and time of Y2O3directional solidification crucible were 1800°C and 120 min,respectively,and the porosity was 20%.Improved Y2O3crucible has been successfully applied to directional solidification of Nb–Si based alloys,and significantly reduced the oxygen contamination.Slight interaction occurred between Hf and Y2O3,but no obvious dissolution,penetration or erosion was found,showing good erosion resistance and thermal shock resistance.
1.Introduction
The next-generation aeroengine blades need higher temperature-bearing structure material to substitute nickelbased superalloy which has reached the limit of operating temperature.With higher melting point,relatively lower density and good processability,Nb–Si based alloys show great promise for application in aero-engine blades operating at 1200–1400°C.1Directional solidification technology enables the alloy grains well-aligned in certain direction,so that the creep rupture life and thermal fatigue strength would be greatly improved.2At present,directional solidification is widely used in the preparation process of aeroengine blades.
However,directional solidification of Nb–Si based alloys excludes almost all of traditional crucible materials becauseof the high melting point(≥1800 °C)and high active elements,such as Ti,Hf and Y.3The directional solidification crucibles not only require sufficient refractoriness and hightemperature strength,but also should have excellent thermal shock resistancewhich could bearrapid coolingfrom~2000 °C to room temperature.Meanwhile,the crucible materials should present good erosion resistance and chemical stability,no reaction or only slight reaction occurring with the alloying elements.
Based on theoretical analysis,Y2O3is found more thermodynamically stable than traditional ceramic materials,such as CaO,ZrO2,MgO,Al2O3and SiO2.4Experimental results showed that relative stability of rare earth oxides follows an increasing sequence of CeO2–ZrO2–Gd2O3–didymium oxide–Sm2O3–Nd2O3–Y2O3.5Kuang et al.6found that pure Y2O3was very promising in application to melting and casting high activity alloys among several refractory materials.Our previous research has applied Y2O3to the vacuum induction melting process and investment casting process.7However,Y2O3suffers from two drawbacks:inherently poor thermal shock resistance and high cost.Thus,crucibles/moulds coated by a Y2O3protective coating seem to be effective and economical,but its operating temperature is completely limited by the basic crucible material.8,9Moreover,it seems inevitable that the molten alloys would interact with the crucible materials10–12and the intensity is closely related to the crucible material and its density.13Currently,sintering temperature of Y2O3crucible is 1550 °C14-1650 °C,15much lower than its melting point(≥2400 °C),which means that excessive porosity makes Y2O3crucible suffer more erosion and dissolution by the molten alloys.16
The directional solidification of Nb–Si based alloys needs an improvement of Y2O3crucible to increase density and erosion resistance against high active melt.Meanwhile,the crucible should have sufficient thermal shock resistance to meet the rapid temperature changes during the process.In this paper,optimized sintering degree and density of Y2O3crucible were studied by controlling the sintering temperature and time,and the practical application performance was investigated.
2.Experimental
The crucibles were made by gelcasting with the dimensions of 20 mm×180 mm.For gelcasting,acrylamide[C2H3CONH2](AM)was used as a monomer,N,N′-methylenebisacrylamide[(C2H3CONH)2CH2](MBAM)as a coupling agent,N,N,N′,N′-tetramethylethylenediamine (TEMED)as a catalyst,ammonium persulphate as an initiator and ammonium polyacrylate as a dispersant.The Y2O3powders were mixed in a weight proportion of 1:1:1 with particle sizes of <10 μm,30–60 μm and 100–150 μm,respectively.Binder burnout was operated at 200–600 °C for 6 h.Sintering was performed in a self-made vacuum sintering furnace.The porosity was measured by Archimedes’principle.
Directional solidifications were performed in a Bridgman furnace.Before heating,the furnace chamber was evacuated to 6×10-3Pa and then backfilled with high purity argon up to 0.05 MPa.The heating temperature was 1900°C and kept isothermally for 20 min.Then the bars were directionally solidified with a withdrawal rate of 1×10-4m/s.
Thermal shock resistance was evaluated by the times of thermal cycle.The samples were placed in a resistance furnace at 1400°C for 30 min,and then cooled immediately in roomtemperature water for 3 min.After drying in a thermostatic oven at 150°C for 60 min,the samples were repeated with this process until the cracks appeared.
Scanning electron microscopy(SEM,FEI Quanta600,USA)was used to investigate the microstructure.Energy dispersive spectrometry(EDS,Oxford INCAPentaFET-x3)was used to analyze the chemical composition.The oxygen contents were measured by the inert gas infrared–thermal conductivity technique(IGI,LECO TC-436).
3.Results and discussion
3.1.Effect of sintering temperature on microstructure
Fig.1 shows the microstructures of Y2O3crucible at sintering temperature(T)of 1650 °C,1800 °C and 1900 °C for 120 min,and the porosities are 38%,20%and 17%,respectively.At the sintering temperature of 1650°C,only fine Y2O3powders are preliminarily sintered.Apparent gaps between fine powders and coarse powders demonstrate that they are not well combined yet.Once the crucible cracked during the directional solidification,the cracks will propagate along the gaps and fracture the crucible.Atthe sintering temperature of 1800°C,fine powders and coarse powders are integrally sintered,and more contact areas are formed between them,suggesting that the strength of crucible improves correspondingly.17However,it could be found that there are still a certain number of pores existing in the inner of crucible.At the sintering temperature of 1900°C,fine powders and coarse powders are entirely sintered.The interconnected pores in Fig.1(b)become closed ones in Fig.1(c)and its number declines.Besides,as shown in Fig.2,the increasing sintering time(t)makes the microstructure of Y2O3crucible present similar variation trend,and its porosities are 25%,21%and 18%for 90 min,120 min and 150 min,respectively.The above evolutional results suggest that the increased sintering temperature and time cause the powders gradually to gather,the particle contact area to expand and the binding force to enhance,resulting in the strength improvement of Y2O3crucible.
Density closely relates to the erosion resistance of Y2O3crucible.18During the melting,the melt will penetrate along the pores or cracks into the ceramic structure,and then interact with Y2O3.As a result,a metamorphic layer with different properties from original material is formed.When directional solidification proceeds,the dramatic temperature change leads to the metamorphic layer splintering and flaking into the alloy melt,and the deeper penetration implies thicker metamorphic layer and more contamination.The melt penetration depth(x)into the Y2O3crucible can be estimated by18:

where σ is the surface tension of the melt,θ the wetting angle,η the viscosity of the melt,rthe radius of pore,and τ the time.Under the conditions of the other parameters fixed,reduced radius of pore(r)could decrease the depth of penetration,thereby enhancing the erosion resistance of Y2O3crucible against Nb–Si based alloys.Result in Figs.1 and 2 shows that higher sintering temperature and time effectively reduce the radius of pore.Therefore,in the view of erosion resistance,the microstructure of Y2O3crucible sintered at higher temperature and time presents a better performance.On the other hand,higher sintering temperature and time can also improve the binding force between ceramic powders,and Y2O3particles cannot be peeled off easily.

Fig.1 Microstructure of Y2O3crucible at different sintering temperatures for 120 min.

Fig.2 Microstructure of Y2O3crucible for different sintering time at 1800°C.
3.2.Effect of sintering temperature on thermal shock resistance
During the directional solidification,the lower part of Y2O3crucible is rapidly cooled and shrunk.However,the upper part keeps hyperthermia and hinders contraction.Thus,the transition zone of Y2O3crucible suffers dramatic temperature change,which requires the crucible with good thermal shock resistance.Table 1 shows the thermal cycle times(N)of Y2O3crucible at different sintering temperatures and time.It is interesting to note that at the sintering temperature of 1900°C and for the sintering time of 150 min,both of the thermal cycles decrease to 4 times.
During the thermal cycle,the formation of thermal shock cracks is controlled by the porosities:as pre-existing cracks,the pores in crucible matrix could disperse the thermo-elastic strain energy and stop the crack propagation.As illustrated in Figs.1 and 2,the microstructure of crucible sintered at 1800°C for 120 min has higher porosity than the crucibles sintered at 1900 °C for 120 min and sintered at 1800 °C for 150 min.The abundant pores help to release the thermal stress and reduce the thermal shock damage.Therefore,from the viewpoint of thermal shock resistance,the sintering degree of Y2O3crucible is not the higher the better,but should ensure a certain amount of porosities.Usually,the best porosity of crucible is 10%–20%.19Comprehensively considering hightemperaturestrength,erosion resistance,thermalshock resistance and costs,the optimal sintering temperature of Y2O3directionalsolidification crucible is 1800°C for 120 min,with porosity of~20%.

Table 1 Thermal cycle times of Y2O3crucible with different sintering temperatures and time.
3.3.Applications of Y2O3directional solidification crucibles
Common alloying elements of Nb–Si based alloys include Ti,Al,Cr,Hf and Y.20–22In this paper,Nb–22Ti–16Si–2Al–2Cr alloy,Nb–22Ti–16Si–2Al–2Cr–2Hf alloy and Nb–22Ti–16Si–2Al–2Cr–2Hf–0.1Y alloy are selected to investigate the application performance of Y2O3directional solidification crucible.
Fig.3(a)shows the backscattered electron micrograph of interface between Nb–22Ti–16Si–2Al–2Cr alloy and Y2O3crucible.It is clear that no chemical reaction or significant erosion occurs.Fig.3(b)–(e)show the element distributions of Nb,Ti,Si and Y at the alloy-crucible interfaces.The clean-cut interfaces of elements distribution suggest that both the permeation of melt and the diffusion of ceramic are quite limited.
Fig.4(a)shows the micrograph of interface between Nb–22Ti–16Si–2Al–2Cr–2Hf alloy and Y2O3crucible.A continuous reaction layer with varied thickness is obviously found on the alloy-crucible interface.Alloying elements’distribution in Nb–22Ti–16Si–2Al–2Cr–2Hf is similar with Nb–22Ti–16Si–2Al–2Cr alloy,except Hf,as shown in Fig.4(b).It could be found that Hf is concentrated in the reaction layer.The EDS analysis suggests that the compositions of the reaction layer are Hf(13.49 at%),Y(22.95 at%)and O(63.56 at%).XRD analysis confirms that the reaction layer consists of HfO2,as shown in Fig.4(c).These results demonstrate that Y2O3crucible is not absolutely inert against Hf,and a mild chemical reaction occurs.
Fig.5 shows the backscattered electron micrograph of interface between Nb–22Ti–16Si–2Al–2Cr–2Hf–0.1Y alloy and Y2O3crucible.Because of the small content of Y,its interface presents similar microstructure of Nb–22Ti–16Si–2Al–2Cr–2Hf alloy:HfO2reaction layer is formed but no previous erosion is found.
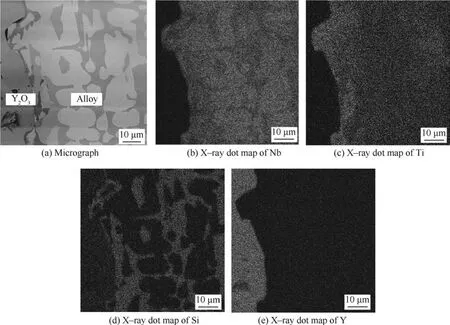
Fig.3 Analysis of interface between Nb–22Ti–16Si–2Al–2Cr alloy and Y2O3crucible.

Fig.4 Analysis of interface between Nb–22Ti–16Si–2Al–2Cr–2Hf alloy and Y2O3crucible.
Oxygen contamination seems inevitable while alloys are melted in oxide ceramics.23Y2O3will be dissociated at high temperature as follows6:


Fig.5 Backscattered electron micrograph of interface between Nb–22Ti–16Si–2Al–2Cr–2Hf–0.1Y alloy and Y2O3crucible.
The Gibbs free energy of reaction(ΔGr)is useful for comparison of the relative stability of oxides.Based on the data from Ref.24,Y2O3,followed by HfO2,has the lowest free energy of formation among the alloy constituents,as illustrated in Table 2.Therefore,in Nb–22T i–16Si–2Al–2Cr–2Hf alloy,Hf is the most reactive element and HfO2is formed as a result.In the case of Nb–22Ti–16Si–2Al–2Cr–2Hf–0.1Y alloy,Y is expected to react with O preferentially,but actually Y2O3and HfO2are formed simultaneously.The evidence for preferential formation of Y2O3is insufficient due to the extremely low content of Y.Fig.6 illustrates the increments of oxygen,in whichais oxygen increment in Ref.14,b,canddare oxygen increments of Nb–22Ti–16Si–2Al–2Cr–2Hf alloy,Nb–22T i–16Si–2Al–2Cr–2Hf–0.1Y alloy and Nb–22Ti–16Si–2Al–2 Cr alloy,respectively.The results present significant reduction of oxygen contamination compared with the previous study.
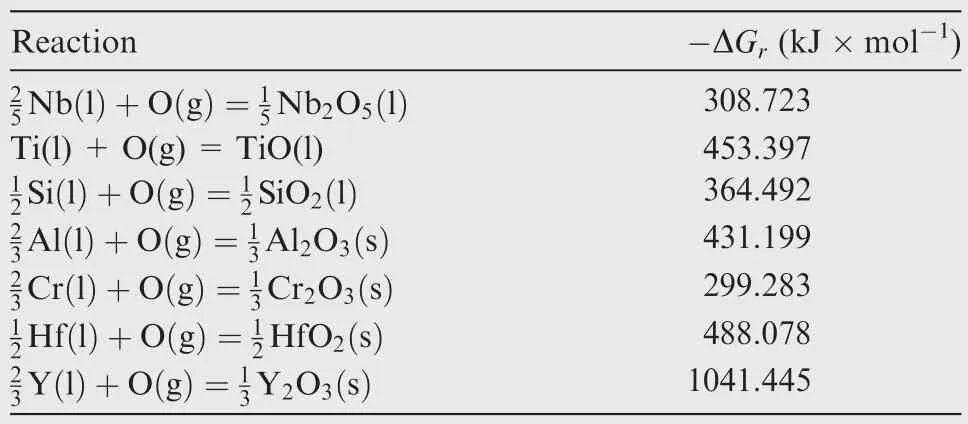
Table 2 Gibbs free energy of formation of metallic oxides at 2200 K.
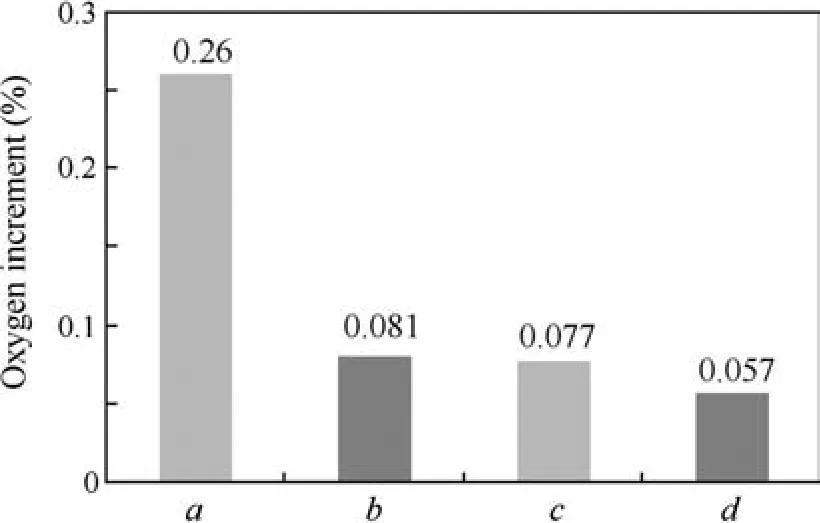
Fig. 6 Oxygen increments in alloys after directional solidification.
4.Conclusions
This paper studied the effects of sintering temperature and time on the microstructure and thermal shock resistance of Y2O3crucibles,and investigated the application performance in directional solidification of Nb–Si based alloys.Some conclusions can be listed as follows:
(1)The increase of sintering temperature and time made Y2O3grains gathered,the contact area between ceramic particles enlarged,the open pores changed into closed pores and the density increased.These results led to the improvement of erosion resistance of Y2O3crucibles.
(2)Excessive density weakened the thermal shock resistance of Y2O3crucibles.Comprehensively considering hightemperature strength,erosion resistance,thermal shock resistance and costs,the optimal sintering temperature of Y2O3directional solidification crucible was 1800°C for 120 min,with porosity of~20%.
(3)Improved Y2O3crucibles presented good performance in directional solidification of Nb–Si based alloys and significantly reduced the oxygen contamination.Slight interaction between Hf and Y2O3occurred,but no obvious dissolution,penetration or erosion was found.
Acknowledgement
This study was co-supported by the National Science&Technology Pillar Program of China(No.2013BAB11B04).
1.Bewlay BP,Jackson MR,Lipsitt HA.The balance of mechanical and environmental properties of a multielement niobium–niobium silicide-based in situ composite.Metall Mater Trans A1996;27(12):3801–8.
2.Bewlay BP,Lipsitt A,Jackson MR,Reeder WJ,Sutliff JA.Solidification processing of high temperature intermetallic eutectic-based alloys.Mater Sci Eng A1995;192–193:534–43.
3.Bewlay BP,Jackson MR,Zhao JC,Subramanian PR.A review of very-high-temperature Nb–silicide-based composites.Metall Mater Trans A2003;34(10):2043–52.
4.Kostov A,Friedrich B.Predicting thermodynamic stability of crucible oxides in molten titanium and titanium alloys.Comp Mater Sci2006;38(2):374–85.
5.Saha RL,Nandy TK,Misra RDK,Jacob KT.On the evaluation of stability of rare earth oxides as face coats for investment casting of titanium.Metall Trans B1990;21(3):559–66.
6.Kuang JP,Harding RA,Campbell J.Investigation into refractories as crucible and mould materials for melting and casting γ-TiAl alloys.Mater Sci Technol2000;16(9):1007–16.
7.Zhang H,Yuan SN,Zhou CG,Sha JB,Zhao XQ,Jia LN.Research progress on ultra-high-temperature Nb-silicide-based alloys.ActaAeronautAstronautSin2014;35(10):2756–66[Chinese].
8.Smeacetto F,Salvo M,Ferraris M.Protective coatings for induction casting of titanium.Surf Coat Technol2007;201(24):9541–8.
9.Holcombe CE,Serandos TR.Consideration of yttria for vacuum induction melting titanium.Metall Trans B1983;14(3):497–9.
10.Ma LM,Tang XX,Wang B,Jia LN,Yuan SN,Zhang H.Purification in interaction between yttria mould and Nb–silicide based alloy during directional solidification:a novel effect of yttrium.Scr Mater2012;67(3):233–6.
11.Ma LM,Tang XX,Jia LN,Yuan SN,Ge JR,Zhang H.Influence of Hf contents on interactions between Nb–silicide based alloys and yttria moulds during directional solidification.Int J Refract Met Hard Mater2012;33:87–92.
12.Ma LM,Yuan SN,Cui RJ,Tang XX,Li YL,Gao M,et al.Interactions between Nb–silicide based alloys and yttria moulds during directional solidification.Int J Refract Met Hard Mater2012;30(1):96–101.
13.Wu MB.Selection of oxide crucibles used for melting Nb-based ultrahigh temperature alloys and the preparation of inert coatings on graphite crucibles[dissertation].Xi’an:Northwestern Polytechnical University;2007[Chinese].
14.Lapin J,Gabalcova Z,Pelachova T.Effect of Y2O3crucible on contamination of directionally solidified intermetallic Ti–46Al–8Nb alloy.Intermetallics2011;19(3):396–403.
15.Cui RJ,Gao M,Zhang H,Gong SK.Interactions between TiAl alloys and yttria refractory material in casting process.J Mater Process Technol2010;210(9):1190–6.
16.Gao M,Jia LN,Tang XX,Li XJ,Cui RJ,Zhang H.Interaction mechanism between niobium–silicide-based alloy melt and Y2O3refractory crucible in vacuum induction melting process.China Foundry2011;8(2):190–6.
17.Ge QL,Lei TQ,Zhou Y.Microstructure and mechanical properties of Al2O3–ZrO2–SiCw ceramic composite.Acta Aeronaut Astronaut Sin1992;13(7):381–7[Chinese].
18.Li HX.Refractories manual.Beijing:Metallurgical Industry Press;2007,p.8–9 in Chinese.
19.Wang CX,Zhang YX.Refractoriesforrefiningfurnace.Beijing:Metallurgical Industry Press;2007.p.18–9[Chinese].
20.Bao J,Huang Q,Tang L,Geng T,Zhao XQ,Ma CL.Liquid-solid phase equilibria of Nb–Si–Ti ternary alloys.Chin J Aeronaut2008;21(3):275–80.
21.Ding F,Jia LN,Yuan SN,Su LF,Weng JF,Zhang H.Microstructure evolution of a hypereutectic Nb–Ti–Si–Cr–Al–Hf alloy processed by directional solidification.Chin J Aeronaut2014;27(2):438–44.
22.Wang LG,Jia LN,Cui RJ,Zheng LJ,Zhang H.Microstructure,mechanical properties and oxidation resistance of Nb–22Ti–14Si–2Hf–2Al–xCr alloys.Chin J Aeronaut2012;25(2):292–6.
23.Barbosa J,Ribeiro CS,Monteiro AC.Influence of superheating on casting of γ-TiAl.Intermetallics2007;15(7):945–55.
24.Barin I.Thermochemical data of pure substances.3rd ed.Germany:Wiley-VCH;1997.p.589–97.
21 April 2015;revised 4 June 2015;accepted 27 June 2015
Available online 19 October 2015
ⓒ2015 The Authors.Production and hosting by Elsevier Ltd.on behalf of CSAA&BUAA.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
*Corresponding author.Tel.:+86 10 57808757.
E-mail address:malimin@comac.cc(L.Ma).
Peer review under responsibility of Editorial Committee of CJA.
MA Liminis an engineer in Beijing Aeronautical Science&Technology Research Institute of COMAC.He received his Ph.D.degree from School of Materials Science and Engineering,Beihang University.His area of research includes new material and new structure of civil aircraft.
 CHINESE JOURNAL OF AERONAUTICS2016年2期
CHINESE JOURNAL OF AERONAUTICS2016年2期
- CHINESE JOURNAL OF AERONAUTICS的其它文章
- Hypersonic starting flow at high angle of attack
- Advances and trends in plastic forming technologies for welded tubes
- Instability and sensitivity analysis of flows using OpenFOAM®
- Numerical simulations of high enthalpy flows around entry bodies
- Modeling and simulation of a time-varying inertia aircraft in aerial refueling
- Experimental investigations for parametric effects of dual synthetic jets on delaying stall of a thick airfoil
