Experimental study on combustion characteristics of Chinese RP-3 kerosene
M Hongn,Xie Mozho,Zeng Wen,Chen Bodong
aSchool of Energy and Power Engineering,Dalian University of Technology,Dalian 116024,China
bLiaoning Key Laboratory of Advanced Test Technology for Aerospace Propulsion System,Shenyang Aerospace University,Shenyang 110136,China
Experimental study on combustion characteristics of Chinese RP-3 kerosene
Ma Hongana,Xie Maozhaoa,Zeng Wenb,*,Chen Baodongb
aSchool of Energy and Power Engineering,Dalian University of Technology,Dalian 116024,China
bLiaoning Key Laboratory of Advanced Test Technology for Aerospace Propulsion System,Shenyang Aerospace University,Shenyang 110136,China
Combustion mechanism;Combustion stability;Laminar combustion speed;Markstein length;RP-3 kerosene
In order to illustrate the combustion characteristics of RP-3 kerosene which is widely used in Chinese aero-engines,the combustion characteristics of RP-3 kerosene were experimentally investigated in a constant volume combustion chamber.The experiments were performed at four different pressures of 0.1 MPa,0.3 MPa,0.5 MPa and 0.7 MPa,and three different temperatures of 390 K,420 K and 450 K,and over the equivalence ratio range of 0.6–1.6.Furthermore,the laminarcombustion speeds of a surrogate fuel for RP-3 kerosene were simulated under certain conditions.The results show that increasing the initial temperature or decreasing the initial pressure causes an increase in the laminar combustion speed of RP-3 kerosene.With the equivalence ratio increasing from 0.6 to 1.6,the laminar combustion speed increases initially and then decreases gradually.The highest laminar combustion speed is measured under fuel rich condition(the equivalence ratio is 1.2).At the same time,the Markstein length shows the same changing trend as the laminar combustion speed with modification of the initial pressure.Increasing the initial pressure will increase the instability of the flame front,which is established by decreased Markstein length.However,different from the effects of the initial temperature and equivalence ratio on the laminar combustion speed,increasing the equivalence ratio will lead to a decrease in the Markstein length and the stability of the flame front,and the effect of the initial temperature on the Markstein length is unclear.Furthermore,the simulated laminar combustion speeds of the surrogate fuel agree with the corresponding experimental datas of RP-3 kerosene within~10%deviation under certain conditions.
ⓒ2016 Chinese Society of Aeronautics and Astronautics.Published by Elsevier Ltd.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Laminar combustion speed reflects the reactivity,diffusivity and exothermicity characteristics of aviation fuels,and the accurate value of laminar combustion speed is important for designing the combustor of an aero-engine,simulating turbulent combustion process and verifying chemical reactionkinetics.At the same time,a turbulent flame is employed in the aero-engine combustor,which is affected by stretch.The response of a flame to stretch can be defined by Markstein length,which also describes the stability of flame front.
There are several methods for measuring the laminar combustion speed and Markstein length of combustible gas,such as the stagnation plane flame method1,the heat flux method2and the constant volume combustion chamber method.3Compared with the stagnation plane flame method and the heat flux method,a linear relationship between the flame speed and flame stretch can be defined and Markstein lengths can also be deduced easily from the experimental measurement simultaneously through the third method.4Therefore,spherically expanding flames which are used in the constant volume combustion chamber method are extensively utilized to determine the laminar combustion speed and Markstein length of kerosene and the surrogate fuelofkerosene in Refs.5–10Eisazadeh-Far et al.5,6experimentally studied the flame structures and laminar combustion speeds of JP-8/air and JP-8/O2/diluent premixed flames at high temperatures and pressures using two constant volume cylindrical and spherical chambers.Kelley et al.7experimentally studied laminar combustion speeds and Markstein lengths of C5–C8n-alkane mixtures with air at elevated pressures in a constant pressure chamber.Fuller et al.8experimentally studied the effects of vitiation on the flame speed ofn-decane using the spherical combustion chambertechnique.However,corresponding experimental data of RP-3 kerosene which is widely used in Chinese aero-engine are scarce.
As discussed above,the constant volume combustion chamber method has already been testified to be an appropriate method to measure the combustion characteristics of gaseous fuels.3Therefore,in the present work,this method is used to determine the combustion characteristics of RP-3 kerosene.Because the laminar combustion speed and Markstein length of a premixed flame depend on the initial temperature,initial pressure and composition of the gas mixture,experiments are performed at three different temperatures of 390 K,420 K and 450 K,four different pressures of 0.1 MPa,0.3 MPa,0.5 MPa and 0.7 MPa and a wide range of equivalence ratios from 0.6 to 1.6 in the present work.
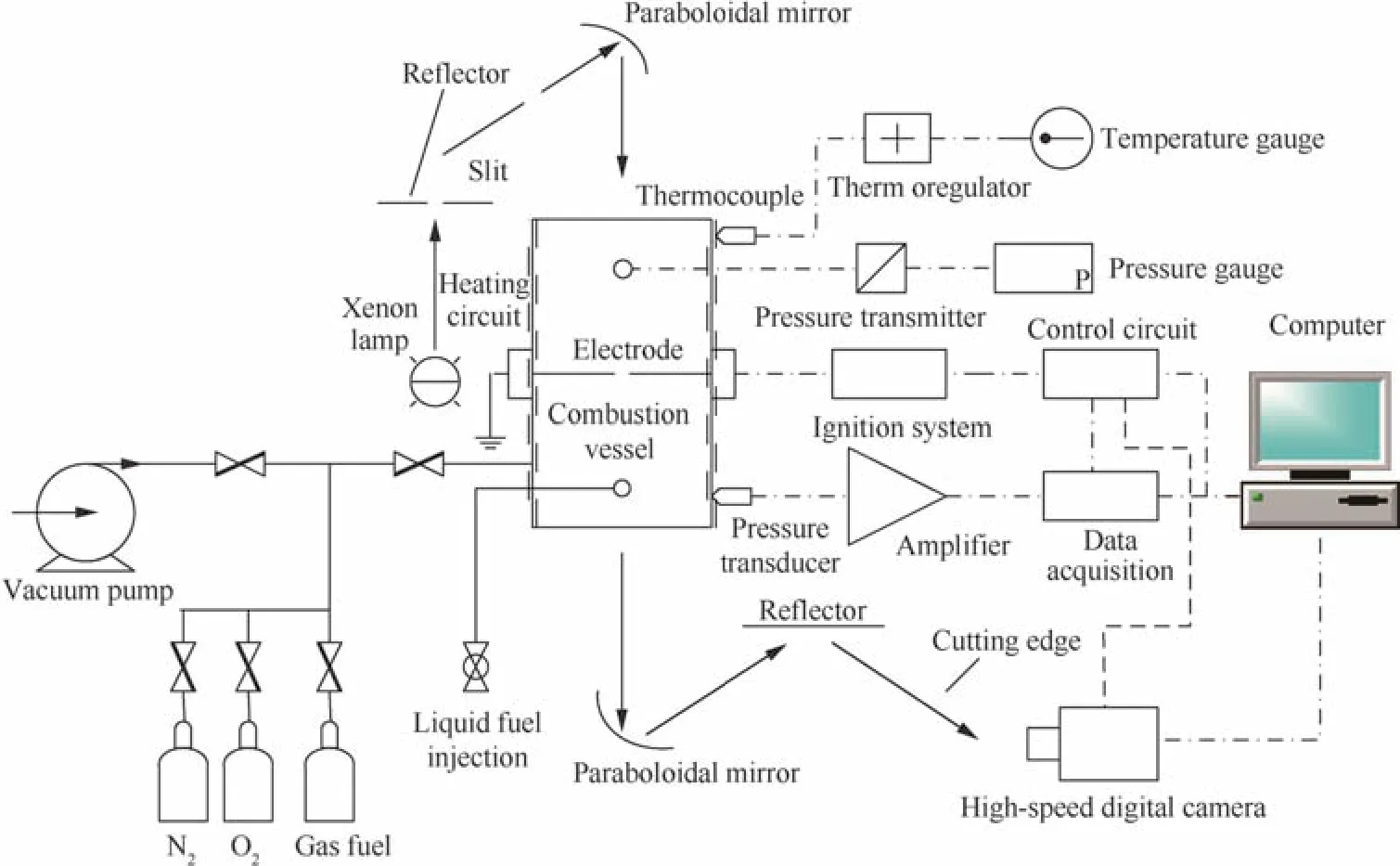
Fig.1 Schematic diagram of experimental facility.

Fig.2 Schematic diagram of constant volume combustion chamber.
2.Experimental facility
Fig.1 shows the schematic diagram of the experimental facility11,which consists of a constant volume combustion chamber and the systems for ignition,heating,data collection and high-speed schlieren photography.Fig.2 provides the schematic diagram of constant volume combustion chamber.As shown in Fig.2,the cylindrical type combustion chamber is made of stainless steel,and the inner diameter is 180 mm and the volume is 5.5 L.Both sides of the chamber are transparent to make the inside observable and to offer optical access,and the diameter of visual field size is 80 mm.The gas entrance system consists of a valve connected to the gas cylinder for preparation of the mixtures,a vacuum pump for evacuating the system,and some temperature and pressure test facilities.The initial temperature is measured by thermocouples with the accuracy of 1 K and a high-precision pressure transducer is used to measure the initial pressure in the cylindrical chamber.A dynamic pressure sensor with the accuracy of 0.8%installed on the inner wall of the combustion chamber is used to measure the combustion pressure.
A conventional battery-coil system is applied to igniting the mixture at the center of the cylinder,and the data collection program synchronizes the ignition with the dynamic pressure recording and schlieren photography.The schlieren photography is installed within the cylindrical vessel to take optical recordings of the burning event.A high speed camera(Phantom V611)with the frequency of 10000 pictures per second is used to keep a record of the dimension and shape of the developing flame front.To make sure that the fresh mixture is quiescent and homogeneous in the chamber,at least ten minutes must remain after the chamber is filled with fuel and oxidizer.Each experimentaltestundereach condition mustbe performed at least three times.
3.Theoretical model
The stretched flame speedSnwhich reflects the flame propagation can be deduced from the flame radius versus time as demonstrated by Bradley et al.12Snis given as

whereris the flame radius in the image andtthe elapsing time after the mixture is ignited.
Flame stretch rate α in a quiescent mixture can be defined as

whereAis the area of flame front.For a spherically outward expanding flame,the flame stretch rate can be defined as

At the early stage of flame development,a linear relationship between the flame speed and the flame stretch rate exists,such as,

The unstretched flame speedSlis generally acquired as the intercept value at α =0 in the plot ofSnagainst α,and the Markstein lengthLbof burned gas is the negative value of the slope ofSnversus α curve.
A simple relationship linking the laminar combustion speedulto the unstretched flame speedSlis given as

where ρuand ρbare the densities of unburned and burned gas.From Eq.(5),the laminar combustion speed can be obtained as

4.Experimental results and discussions
In the present experiment,the investigations of the flame propagation characteristics,laminar combustion speed and Markstein length are carried outforRP-3 kerosene.The experimental facility and technique employed in the present work have been validated in the work of Hu et al.11In order to illustrate the effects of initial parameters(such as pressure,temperature and composition of the mixture)on the combustion properties,three different initial temperatures of 390 K,420 K and 450 K,four different pressures of 0.1 MPa,0.3 MPa,0.5 MPa and 0.7 MPa and a wide range of equivalence ratios from 0.6 to 1.6 are chosen for the investigations.
In order to determine the formula of RP-3 kerosene,which affects the determination of experimental equivalence ratio,the main components and the main physical properties of RP-3 kerosene are experimentally analyzed.RP-3 kerosene consists of saturated hydrocarbons(92.1%volume)and aromatic hydrocarbons(7.9%volume).Compositions of this type kerosene are listed in Table 1,including saturated straight chain alkanes,saturated naphthenes and aromatic compounds.
The molecular weight and the hydrogen-carbon of RP-3 kerosene are 149.1 g/mol and 2.04,respectively,so,in the present study,the formula of RP-3 kerosene is determined as C10.62H21.66.
4.1.Flame propagation characteristics of RP-3 kerosene
The characteristics of the igniter can affect the flame propagation characteristics as discussed by Liang et al.13Thus,when the spherically propagating flame method is used to determine the laminar combustion speed,the raw flame radius data should be carefully selected.Bradley et al.14and Huang et al.15showed that the flame speed became independent of the sparkenergy when the flame radius was bigger than 6 mm.In addition,the pressure in the whole chamber hardly varies when the flame radius is less than 25 mm,thus the burning process can be considered as a constant pressure one,just as presented by Burke et al.16In order to eliminate the effects of ignition energy and pressure rise in the combustion chamber,the flame radius in flame photos used in the present analysis is selected from 6 mm to 25 mm.

Table 1 Compositions of Chinese RP-3 kerosene.
Fig.3 shows the selected snapshots of stoichiometric RP-3 kerosene/air flames with four different initial pressures(p)at the initial temperature(T)of 390 K.Eisazadeh-Far et al.6and Oran et al.17stated that flame thickness decreased at higher initial pressures,which reduced its resistance to perturbations.Thus,the flame instability would be enhanced,the flame front surfaces were not smooth,and cells appeared.However,in the present work,the effect of the initial pressure on the flame stability is not obvious at the initial temperature of 390 K and the equivalence ratio of 1.0.As shown in Fig.3,the spherically expanding flame is smooth and propagates outwardly from the core of the constant chamber under the condition that the initial pressure is 0.1 MPa.With the initial pressure increasing to 0.7 MPa,there is no evidence of wrinkles or cracks on the flame front surface and the flame remain stable.
Fig.4 shows the selected images of this combustible mixture for four different equivalence ratios at the initial pressure of 0.3 MPa and the initial temperature of 390 K.It can be seen that for RP-3 kerosene/air mixture,the flame instability is enhanced with the equivalence ratio increasing.The flame front surface is maintained smooth throughout the whole flame propagation process at the low equivalence ratio.However,with the equivalence ratio increasing,there are some cracks or small cells on the flame around the electrodes.With the equivalence ratio increasing to 1.5,the flame front will turn into the cellular structure,and large flaws or protruding cells occur.
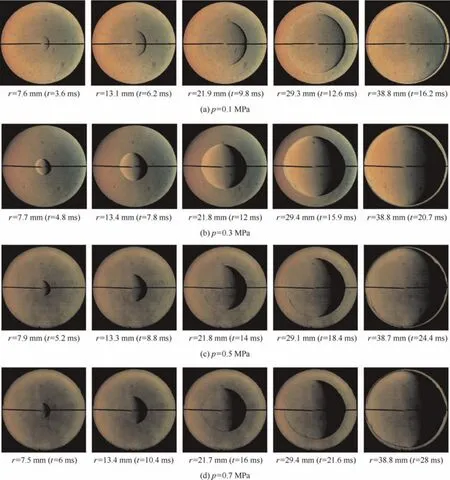
Fig.3 Effect of initial pressure on flame propagation characteristics(φ=1.0,T=390 K).
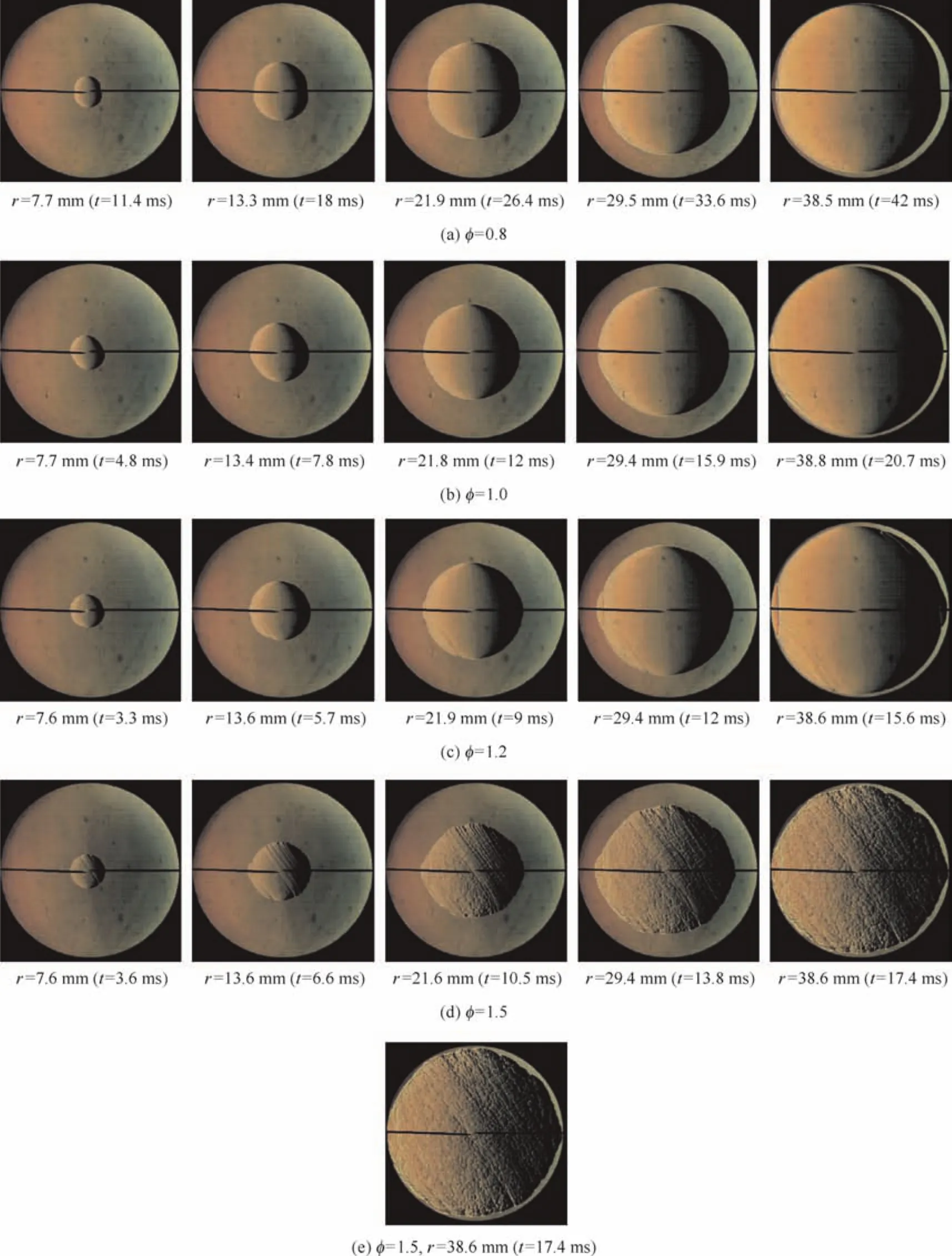
Fig.4 Effect of equivalence ratio on flame propagation characteristics(p=0.3 MPa,T=390 K).
To identify the effect of initial temperature on flame stability,the schlieren images of stoichiometric RP-3 kerosene/air mixtures for three different initial temperatures at the initial pressure of 0.1 MPa are provided in Fig.5.
The results show that flame stability is insensitive to the initial temperature.The reason maybe is that,with the initial temperature increasing,both thermal expansion and flame thickness decrease.The hydrodynamic instability18decreases with the thermal expansion decreasing or the flame thickness increasing,and the comprehensive effects of these two factors cause unclear variation in flame front stability at different initial temperatures.
Fig.6 shows the evolution of flame radius versus time at various initial pressures,temperatures and equivalence ratios.The evolution of flame radius versus time is linear under all conditions.Increasing speed of flame radius versus time is lower with the initial pressure increasing,which indicates that the flame propagation tends to be retarded.At the same time,the increasing speed of flame radius versus time under the condition that the initial temperature is 390 K is the same as that for 420 K.However,with the initial temperature increasing from 420 K to 450 K,the increasing speed of flame radius versus time is enhanced.Furthermore,with the equivalence ratio increasing from 0.9 to 1.5,the increasing speed of flame radius versus time increases initially and then decreases gradually.The highest increasing speed of flame radius is measured under the condition that the equivalence ratio is 1.2.
The stretched flame speeds versus the flame radius at various initial pressures,temperatures and equivalence ratios are shown in Fig.7.With the flame expanding,the stretched flame speeds show different trends at different initial pressures,temperatures and equivalence ratios.At the high initial pressures(such as 0.3 MPa,0.5 MPa and 0.7 MPa)and the equivalence ratios of 0.9 and 1.4,little variation of stretched flame speed versus flame radius is presented.However,with the flame radius increasing,the stretched flame speed decreases at the early stage of flame propagation and increases at the subsequent stage of flame propagation under the conditions that the initial pressure is 0.1 MPa and the equivalence ratios are 1.2 and 1.3.In addition,the stretched flame speed increases slightly with the increase of flame radius at the equivalence ratios of 1.0 and 1.1.

Fig.5 Effect of initial temperature on flame propagation characteristics(φ=1.0,p=0.1 MPa).
Fig.8 shows a selection of experimental data displaying the variations of stretched flame speed with total stretch rate at various initial pressures,temperatures and equivalence ratios.The evolution of the stretched flame speed versus the stretch rate is about linear under the conditions that the initial pressures are high and the equivalence ratios are 0.9,1.0,1.1 and 1.4.However,the trends are not linear under the conditions that the initial pressure is 0.1 MPa and the equivalence ratios are 1.2 and 1.3.Extrapolations of the datas in Fig.8 to a zero stretch rate yield the unstretched flame speed and the gradient gives the negative value of Markstein length.With the stretch rate increasing,the stretched flame speeds decrease,and the gradients of the lines are negative values,which represent the positive values of Markstein length at the initial pressures and temperatures and over the equivalence ratio range considered.
4.2.Combustion stability of RP-3 kerosene
Markstein length can be used to quantify the sensitivity of a flame on stretch.14A positive value ofLbindicates thatthe flametendsto restrain protuberancesin the flame front and this tendency causes a stable flame.Furthermore,greater positive value ofLbindicates that the flame is more stable.However,a negative value ofLbmeans that the stretched flame speed increases with the stretch rate increasing,and this tendency increases the instability of flame.

Fig.6 Evolution of flame radius versus time.
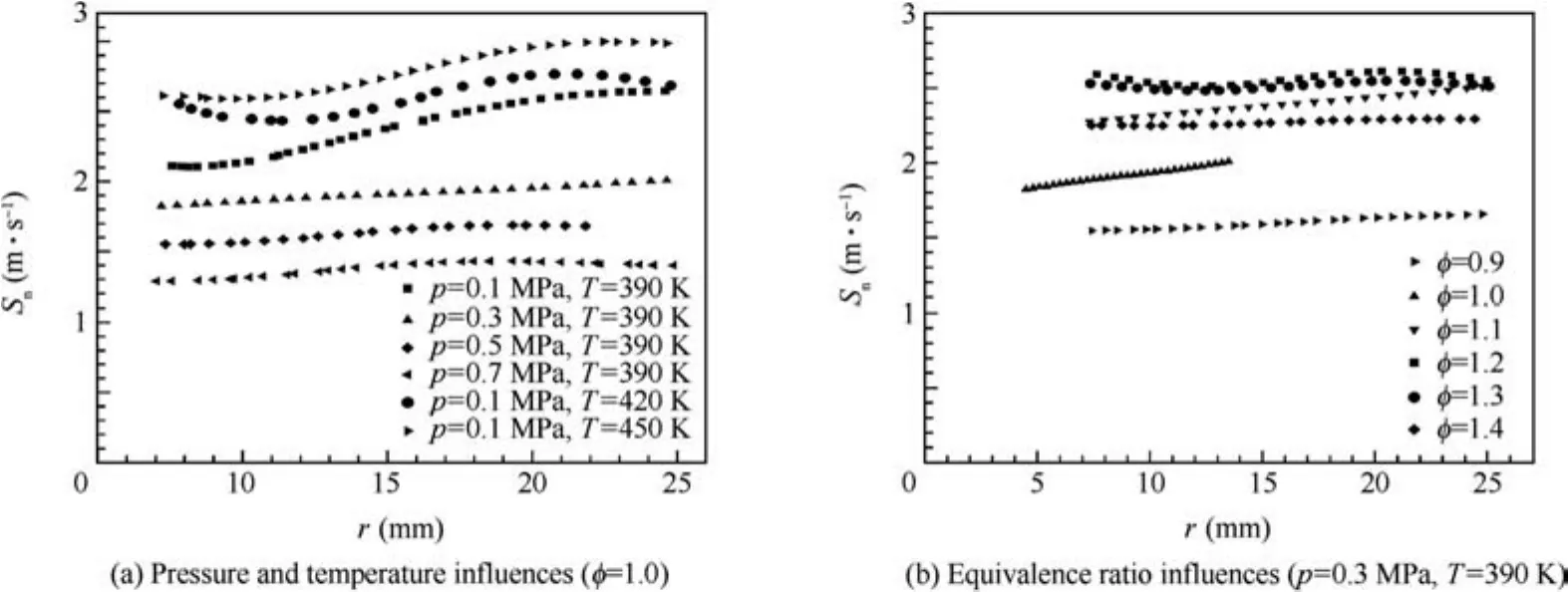
Fig.7 Variations of stretched flame speed with flame radius.

Fig.8 Variations of stretched flame speed with flame stretch.

Fig.9 Variations of Markstein length with equivalence ratio.

Fig.10 Variations of unstretched flame speed and laminar combustion speed with equivalence ratio.
Fig.9 shows the evolution of Markstein length versus the equivalence ratio at various initial pressures and temperatures.The Markstein length shows a decrease trend with the increase of equivalence ratio at all initial pressure and temperature conditions.This suggests that the flame front instability is enhanced with the increase of equivalence ratio.With the equivalence ratio increasing to 1.4 or 1.5 at the initial pressure of 0.5 MPa or 0.1 MPa,the negative value ofLbindicates that the flame front is unstable,which is consistent with the results presented in Fig.4.

Fig.11 Experimental and simulated laminar combustion speeds of Chinese RP-3 kerosene.

Fig.12 Experimental laminar combustion speeds of Chinese RP-3 kerosene,Jet A and Jet A-1.
Furthermore,with the initial pressure increasing,the flame instability is enhanced,which can be reflected through decreasing values ofLb.As discussed by Vukadinovic et al.10,the flame thickness was a crucial parameter which can explain a decrease in flame front stability with pressure rising.Also in others’experimental works,19,20the flame thickness was described as basic parameter for the flame front stability in the case of pressure change.Therefore,with the initial pressure increasing,the flame thickness decreases,which causes the flame front more sensitive to disturbances.It can also be observed that there is more obvious difference between Markstein lengths at 0.1 MPa and 0.3 MPa than that at higher pressures of 0.3 MPa and 0.5 MPa,which indicates that the dependency of Markstein length on the initial pressure decreases.The reason of that maybe is that the changes in the flame thickness introduced by raising the pressure from 0.1 MPa to 0.3 MPa and from 0.3 MPa to 0.5 MPa are different.Compared with the condition at low pressure levels,an increase in the pressure causes smaller flame thickness variation at higher pressure levels.Therefore,the stability of the flame front is less affected.
However,in contrast to the initial pressure in fluence,the effect of the initial temperature on the Markstein length is unclear.The increase of the initial temperature leads to a slight decrease in Markstein length over the equivalence ratio range of 0.9–1.1,but over the equivalence ratio range of 1.2–1.5,with the initial temperature increasing,the increase in Markstein length is little.
4.3.Laminar combustion speed of RP-3 kerosene
Fig.10 shows the unstretched flame speed and the laminar combustion speed versus the equivalence ratio at various initial pressures and temperatures.The unstretched flame speed and laminar combustion speed of the fuel exhibit the theoretically expected behavior.21With the initial temperature increasing,the unstretched flame speed and the laminar combustion speed increase.The effect of the equivalence ratio on the laminar combustion speed also exhibits the theoretical expectation for fuels consisting ofhydrocarbons only.With the equivalence ratio increasing from 0.7 to 1.5,the unstretched flame speed and the laminar combustion speed increase initially and then decrease gradually.The highest unstretched flame speed and laminar combustion speed are observed under fuel rich condition(the equivalence ratio is 1.2).
Furthermore,as shown in Fig.10,with the initial pressure increasing,the laminar combustion speed decreases over the equivalence ratio range of 0.7–1.5 and at the initial temperature of 390 K.The initial pressure has two types of effects on the flame22:the chemical effect and the thermal effect.With the chemical effect,the flame speed decreases with the initial pressure increasing.However,with the thermal effect,the reaction temperature will increase with the initial pressure increasing,which will accelerate the reaction rate.According to the experimental results in the present study,the gaining from the thermal effect cannot compensate the loss due to the chemical effect.Therefore,the laminar combustion speed will decrease with the increase of the initial pressure.
As discussed by Zeng et al.,23a surrogate fuel was selected for RP-3 kerosene and the chemical reaction mechanism(including 150 species and 591 reactions)of this surrogate fuel was constructed.The simulated auto-ignition delay time of this surrogate fuel using this reaction mechanism excellently agreed with the corresponding experimental data of RP-3 kerosene.In the present work,the laminar combustion speeds of this surrogate fuel are simulated by CHEMKIN PRO release’s flame_speed model under certain conditions using this reaction mechanism as shown in Fig.11 and the comparisons between the simulated and the experimental data are shown in Table 2.The thermodynamics data and transport data of the surrogate fuel used in the simulation were also discussed by Zeng et al.,23and the datas of grid properties and stream properties have no effect on the simulated results.
From Fig.11 and Table 2,we can see that the simulated laminar combustion speeds of this surrogate fuel using this chemical reaction mechanism agree with the corresponding experimental data of RP-3 kerosene within~10%deviation over the equivalence ratio range of 0.8–1.5,and at the initial pressures of 0.1 and 0.5 MPa and the initial temperatures of 390 and 450 K.A surrogate fuel selected must successfully emulate the targeted properties and combustion behavior of the real fuel.24As discussed above,the surrogate fuel for RP-3 kerosene that we selected successfully emulate the auto-ignition and laminar combustion characteristics of RP-3 kerosene.However,we cannot summarize that this surrogate fuel is a good surrogate fuel for RP-3 kerosene because the capabilities of this surrogate fuel to emulate some other physical and chemical properties of RP-3 kerosene are unclear.
The laminarcombustion speedsofRP-3 kerosene measured in the present work are compared with those of Jet A1,9and Jet A-1 kerosene10under similar conditions as shown in Fig.12.

Table 2 Experimental and simulated values of laminar combustion speed ul.
There exists a little discrepancy between these experimental values although under similar conditions.The existing discrepancy could possibly be due to the different chemical properties ofthesefuelsandthedifferent experimentalmethodsemployed.
5.Conclusions
The experimental datas of the combustion characteristics of RP-3 kerosene are scarce.So,in the present study,the determinations of the flame structure,laminar combustion speed and Markstein length of RP-3 kerosene are successfully accomplished by employing the combustion chamber method.Furthermore,the influences of initialtemperature,initial pressure and mixture composition on the flame structure,laminar combustion speed and Markstein length are also investigated.The main results are summarized as follows:
(1)With the initial pressure increasing,the laminar combustion speed and Markstein length of RP-3 kerosene decrease.Increasing the initial pressure leads to a decrease in the flame thickness,and this makes the flame front more sensitive to disturbances.
(2)With the equivalence ratio increasing from 0.6 to 1.6,the laminar combustion speed of RP-3 kerosene increases initially and then decreases gradually.The highest laminar combustion speed is obtained under fuel rich condition.However,with the equivalence ratio increasing,the Markstein length decreases.
(3)An increase in the initial temperature from 390 K to 450 K causes an increase in the laminar combustion speed.However,the effect of initial temperature on the Markstein length is unclear.The Markstein length gives a decreasing trend at the equivalence ratios of 0.9,1.0 and 1.1,and an increasing trend at other equivalence ratios.
Acknowledgments
The authors appreciate the financial supports from the NationalNaturalScience Foundation ofChina (No.51376133 and No.51506132).
1.Kumar K,Sung CJ,Hui X.Laminar flame speeds and extinction limits of conventional and alternative jet fuels.Fuel2011;90(3):1004–11.
2.Bosschaart KJ,Lph DG.The laminar burning velocity of flames propagating in mixtures of hydrocarbons and air measured with the heat flux method.Combust Flame2004;136(3):261–9.
3.Weiss M,Zarzalis N,Suntz R.Experimental study of Markstein length effects on laminar flamelet velocity in turbulent premixed flames.Combust Flame2008;154(4):671–91.
4.Gu XJ,Haq MZ,Lawes M,Woolley R.Laminar burning velocity and Markstein lengths of methane–air mixtures.Combust Flame2000;121(2):41–58.
5.Eisazadeh-Far K,Parsinejad F,Metghalchi H.Flame structure and laminar burning speeds of JP-8/air premixed mixtures at high temperatures and pressures.Fuel2010;89(5):1041–9.
6.Eisazadeh-Far K,Moghaddas A,Metghalchi H,Keck JC.The effect of diluent on flame structure and laminar burning speeds of JP-8/oxidizer/diluent premixed flames.Fuel2011;90(4):1476–86.Y,Bloomer D,et al.Effects of vitiation and pressure on laminar lf ame speeds of n-decane.Reston:AIAA;2012.Report No.:AIAA-2012-0167.
9.Singh D,Nishiie TI,Qiao L.Laminar burning speeds and Markstein lengths of n-decane/air,n-decane/O2/He,jet-A/air and S-8/air flames.Reston:AIAA;2010.Report No.:AIAA-2010-0951.
10.Vukadinovic V,Habisreuther P,Zarzalis N.Influence of pressure and temperature on laminar burning velocity and Markstein length of kerosene Jet A-1:Experimental and numerical study.Fuel2013;111(3):401–10.
11.Hu EJ,Huang ZH,He JJ,Jin C,Zheng JJ.Experimental and numerical study on laminar burning characteristics of premixed methane-hydrogen-air flames.IntJHydrogenEnergy2009;34:4876–88.
12.Bradley D,Hicks RA,Lawes M,Sheppard CGW,Woolley R.The measurement of laminar burning velocities and Markstein lengths for iso-octane-air and iso-octane-n-heptane-air mixtures at elevated temperatures and pressures in an explosion chamber.Combust Flame1998;115(1–2):126–44.
13.Liang YT,Zeng W,Hu E.Experimental study of the effect of nitrogen addition on gas explosion.J Loss Prev Process Ind2013;26(1):1–9.
14.Bradley D,Gaskell PH,Gu XJ.Burning velocities,Markstein lengths,and flame quenching for spherical methane-air flames:a computational study.Combust Flame1996;104(1–2):176–98.
15.Huang ZH,Wang Q,Yu JR,Zhang Y,Zeng K,Miao HY.Measurement of laminar burning velocity of dimethyl ether-air premixed mixtures.Fuel2007;86(15):2360–6.
16.Burke MP,Chen Z,Ju Y,Dryer FL.Effect of cylindrical confinement on the determination of laminar flame speeds using outwardly propagating flames.Combust Flame2009;156(4):771–9.
17.Oran ES,Gardner JH.Chemical-acoustic interactions in combustion systems.Prog Energy Combust Sci1985;11(4):253–76.
18.Jomaas G,Law CK,Bechtold JK.On transition to cellularity in expanding spherical flames.J Fluid Mech2007;583:1–26.
19.Gu X,Huang ZH,Wu S,Li Q.Laminar burning velocities and flame instabilities of butanol isomers-air mixtures.Combust Flame2010;157(2):2318–25.
7.Kelley AP,Smallbone AJ,Zhu D,Law CK.Laminar flame speeds of C5 to C8 n-alkanes at elevated pressures and temperatures.Reston:AIAA;2010.Report No.:AIAA-2010-0774.
8.Fuller CC,Gokulakrishnan P,Klassen MS,Adusumilli S,Kochar
20.Law CK,Jomaas G,Bechtold JK.Cellular instabilities of expanding hydrogen/propane spherical flames at elevated pressures:theory and experiment.ProcCombustInst2005;30(1):159–67.
21.Warnatz J,Maas U,Dibble RW.Combustion.Berlin:Springer;2000.
22.Jiang D,Chen C,Yang J,Yang Z.Advanced internal combustion engine fundamental.Xi’an:Xi’an Jiaotong University Press;2006.p.249–61.
23.Zeng W,Li HX,Chen BD,Ma HA.Experimental and kinetic modeling study of ignition characteristics of Chinese RP-3 kerosene.Combust Sci Technol2015;187(3):396–409.
24.Kim D,Martz J,Angela Violi.A surrogate for emulating the physical and chemical properties of conventional jet fuel.Combust Flame2014;161(6):1489–98.
14 May 2015;revised 4 December 2015;accepted 11 January 2016
Available online 24 February 2016
*Corresponding author.Tel.:+86 2489723722.
E-mail addresses:mahongan_sy@163.com(H.Ma),xmz@dlut.edu.cn(M.Xie),zengwen928@sohu.com(W.Zeng),baodong_chen@sina.com(B.Chen).
Peer review under responsibility of Editorial Committee of CJA.
Ma Honganreceived the M.S.degree in Aerospace Propulsion Theory and Engineering from Shenyang Aerospace University in 2006,and then became a teacher there.Now he will get the Ph.D.degree at School of Energy and Power Engineering,Dalian University of Technology.His main research interests are the experimental and simulated study on the combustion process in the aero-engine combustor.
Xie Maozhaois a professor at School of Energy and Power Engineering,Dalian University of Technology.His area of research includes simulations of the combustion process in the internal combustion engine and the combustion characteristics of fuel.
Zeng Wenis a professor at Liaoning Key Laboratory of Advanced Test Technology for Aerospace Propulsion System,Shenyang Aerospace University.He received the Ph.D.degree from the School of Energy and Power Engineering,Dalian University of Technology in 2003.His current research interests are the combustion process in the aero-engine combustor and the combustion characteristics of kerosene.
Chen Baodongis a professor at Liaoning Key Laboratory of Advanced Test Technology for Aerospace Propulsion System,Shenyang Aerospace University.His area of research includes the new surrogate fuel for kerosene and the advanced combustion technology in the aeroengine.
 CHINESE JOURNAL OF AERONAUTICS2016年2期
CHINESE JOURNAL OF AERONAUTICS2016年2期
- CHINESE JOURNAL OF AERONAUTICS的其它文章
- Hypersonic starting flow at high angle of attack
- Advances and trends in plastic forming technologies for welded tubes
- Instability and sensitivity analysis of flows using OpenFOAM®
- Numerical simulations of high enthalpy flows around entry bodies
- Modeling and simulation of a time-varying inertia aircraft in aerial refueling
- Experimental investigations for parametric effects of dual synthetic jets on delaying stall of a thick airfoil
