免疫因子表达异常促进宫颈癌微环境免疫失衡
赵敏伊,赵 娟,杨 婷,王 丽,裴美丽,田思娟,余 洋,杨筱凤
西安交通大学第一附属医院妇产科,西安 710061
·论 著·
免疫因子表达异常促进宫颈癌微环境免疫失衡
赵敏伊,赵 娟,杨 婷,王 丽,裴美丽,田思娟,余 洋,杨筱凤
西安交通大学第一附属医院妇产科,西安 710061
目的 评估叉头蛋白3(FoxP3)、趋化因子配体22(CCL22)、肿瘤坏死因子受体超家族成员40(OX40)和SMAD家族成员3(Smad3)在宫颈癌免疫微环境中的调节作用和对肿瘤发生的影响。方法 采用qRT-PCR方法检测宫颈癌癌灶、癌旁和正常宫颈组织中FoxP3、CCL22、OX40和Smad3的mRNA表达水平。结果 与正常宫颈组织相比,FoxP3和CCL22 mRNA在癌灶(P=0.000,P=0.002)和癌旁(P=0.048,P=0.040)的表达显著升高,两者在高级别鳞癌癌灶(P=0.019,P=0.020)和癌旁(P=0.023,P=0.031)中的表达明显高于低级别鳞癌。OX40和Smad3的mRNA在癌灶中的表达明显低于正常宫颈(P=0.000,P=0.015),两者在高级别鳞癌癌灶(P=0.018,P=0.030)和癌旁(P=0.027,P=0.014)中的表达明显低于低级别鳞癌。在宫颈癌灶和癌旁中,OX40 mRNA与Smad3 mRNA(r=0.384,P=0.002;r=0.288,P=0.023)、FoxP3 mRNA与CCL22 mRNA均呈正相关(r=0.353,P=0.000;r=0.307,P=0.000),CCL22 mRNA与OX40 mRNA呈负相关(r=-0.288,P=0.031;r=-0.263,P=0.037)。FoxP3和CCL22mRNA在HPV阳性的宫颈癌灶(P=0.024,P=0.039)和癌旁(P=0.032,P=0.034)中的表达明显高于阴性组,Smad3在HPV阳性宫颈癌灶中的表达明显低于HPV阴性组(P=0.017)。结论 在宫颈癌发生的微环境中,存在免疫因子FoxP3、CCL22、OX40和Smad3的转录表达异常,这种表达偏移可能导致宫颈癌局部OX40和Smad3的正性调节被削弱,而FoxP3和CCL22的免疫抑制作用增强的免疫模式,共同参与促成局部免疫失衡和肿瘤的发生。
宫颈癌;肿瘤微环境;叉头蛋白3;趋化因子配体22;肿瘤坏死因子受体超家族成员4;SMAD家族成员3
ActaAcadMedSin,2016,38(5):522-527
宫颈癌(cervical cancer,CC)是最常见女性生殖道恶性肿瘤,高危型人乳头瘤病毒(high-risk human papillomavirus,HR-HPV)感染是其触发因子[1]。宫颈局部免疫失衡则是HR-HPV持续感染演变至癌的关键环节。许多免疫因子参与和决定了宫颈局部免疫状态,其中,叉头蛋白3(forkhead box p3,FoxP3)+调节性T细胞(regulatory T cells,Treg)是高表达于宫颈癌微环境重要的免疫抑制细胞[2]。FoxP3调控其发育和功能[3- 4],趋化因子配体22[chemokine(C-C motif)ligand 22,CCL22]可诱导其向肿瘤局部浸润[5],肿瘤坏死因子受体超家族成员40(tumor necrosis factor receptor superfamily member 40,OX40)可抑制FoxP3-效应T细胞(FoxP3-effector T cell,FoxP3-Teff)向FoxP3+Treg分化[6],而信号转导蛋白SMAD家族成员3(SMAD family member 3,Smad3)参与介导肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)抑制FoxP3在Treg的表达[7]。本研究初步探索了这些免疫因子表达模式如何影响宫颈微环境免疫趋向,是否促成局部免疫抑制并协同参与癌症的发生。
材料和方法
标本来源 取自2014年1月至2015年3月在西安交通大学第一附属医院妇产科住院的患者,平均年龄(43.6±7.8)岁(27~62岁)。30例为术前病理活检确诊宫颈癌患者,其中,HPV阳性27例,HPV阴性3例;手术时分别取癌组织和癌旁组织;所有患者术前均未行放疗和化疗,术后病理证实均无脉管及淋巴结转移,病理学分级为低级别(Ⅰ/Ⅱ级)22例,高级别(Ⅲ级)8例[8]。20例正常对照标本来自因非宫颈病变行全子宫切除术的宫颈鳞-柱交界处,且术后病理证实为良性病变,其中,HPV阳性6例,HPV阴性14例。标本离体后,迅速置于液氮或-80℃冻存,用于检测mRNA。本研究经西安交通大学伦理委员会审核批准,所有提供标本的患者均已签署《临床标本科学研究知情同意书》。
材料 TRIzol 试剂(life,15596- 026,USA),反转录试剂盒PrimeScriptTM RT Master Mix(TaKaRa,RR037A,Japan),实时定量PCR试剂盒SYBR Prime Ex TaqTMⅡ (TaKaRa,RR820A,Japan),Real-time PCR仪(C1000 Thermal Cycler,BIO-RAD,USA),核酸蛋白定量仪(BioPhotometer Plus,Eppendorf,Germany)。
Real-time PCR 检测癌灶、癌旁和正常宫颈组织中各因子的mRNA水平 用TRIzol法提取组织中的总RNA,在核酸蛋白定量仪上测定RNA样品的浓度。取2 μg总RNA,反转为cDNA。PCR所需引物序列见表1。实时定量PCR扩增,反应体系为20 μl,引物浓度为0.4 μmol/L。扩增条件:95℃预变性30 s,1个循环;95℃变性5 s,60℃退火30 s,共40个循环。每个体系取3个复孔测试取平均值为Ct值,按2-△△Ct计算各因子的相对表达量。
统计学处理 采用SPSS 20.0统计软件,组间数据比较采用Mann-WhitneyU秩和检验,相关性分析采用Spearman相关系数检验,P<0.05为差异有统计学意义。
结 果
FoxP3和CCL22 mRNA在宫颈癌微环境中表达升高 与正常宫颈组织相比,FoxP3和CCL22 mRNA在癌灶(P=0.000,P=0.002)和癌旁(P=0.048,P=0.040)的表达明显升高(图1A),两者在高级别鳞癌癌灶(P=0.019,P=0.020)和癌旁(P=0.023,P=0.031)中的表达明显高于低级别鳞癌(图1B)。
OX40 和Smad3 mRNA在宫颈癌微环境中表达降低 与正常宫颈组织相比,OX40和Smad3的mRNA在癌灶中的相对表达水平均明显降低(P=0.000,P=0.015)(图2A),两者在高级别鳞癌癌灶(P=0.018,P=0.030)和癌旁(P=0.027,P=0.014)中的表达明显低于低级别鳞癌(图2B)。
FoxP3、CCL22、OX40和Smad3之间的相关性 在宫颈癌灶和癌旁中,OX40 mRNA与Smad3 mRNA(r=0.384,P=0.002;r=0.288,P=0.023)、FoxP3 mRNA与CCL22 mRNA均呈正相关(r=0.353,P=0.000;r=0.307,P=0.000),CCL22 mRNA与OX40 mRNA呈负相关(r=-0.288,P=0.031;r=-0.263,P=0.037)。其他因子之间未发现相关性。
FoxP3、CCL22、OX40和Smad3 mRNA在宫颈癌微环境的表达与HPV感染的关系 FoxP3和CCL22 mRNA在HPV阳性的宫颈癌灶(P=0.024,P=0.039)和癌旁(P=0.032,P=0.034)中的表达明显高于阴性组,Smad3在HPV阳性宫颈癌灶中的表达明显低于HPV阴性组(P=0.017) (图3)。与HPV阴性组相比,OX40 mRNA在感染HPV的不同宫颈组织中的表达未见明显变化(P>0.05)。
讨 论
宫颈癌是全球第4位女性恶性肿瘤[9]。局部免疫失衡是肿瘤微环境特殊的生理特性,HPV感染后,诱导Th1/Th2偏移,并靶向作用于toll样受体及干扰素(interferon,IFN)-α、IFN-γ和IFN-β等,致使宫颈局部免疫应答异常[10- 12]和肿瘤的发生。
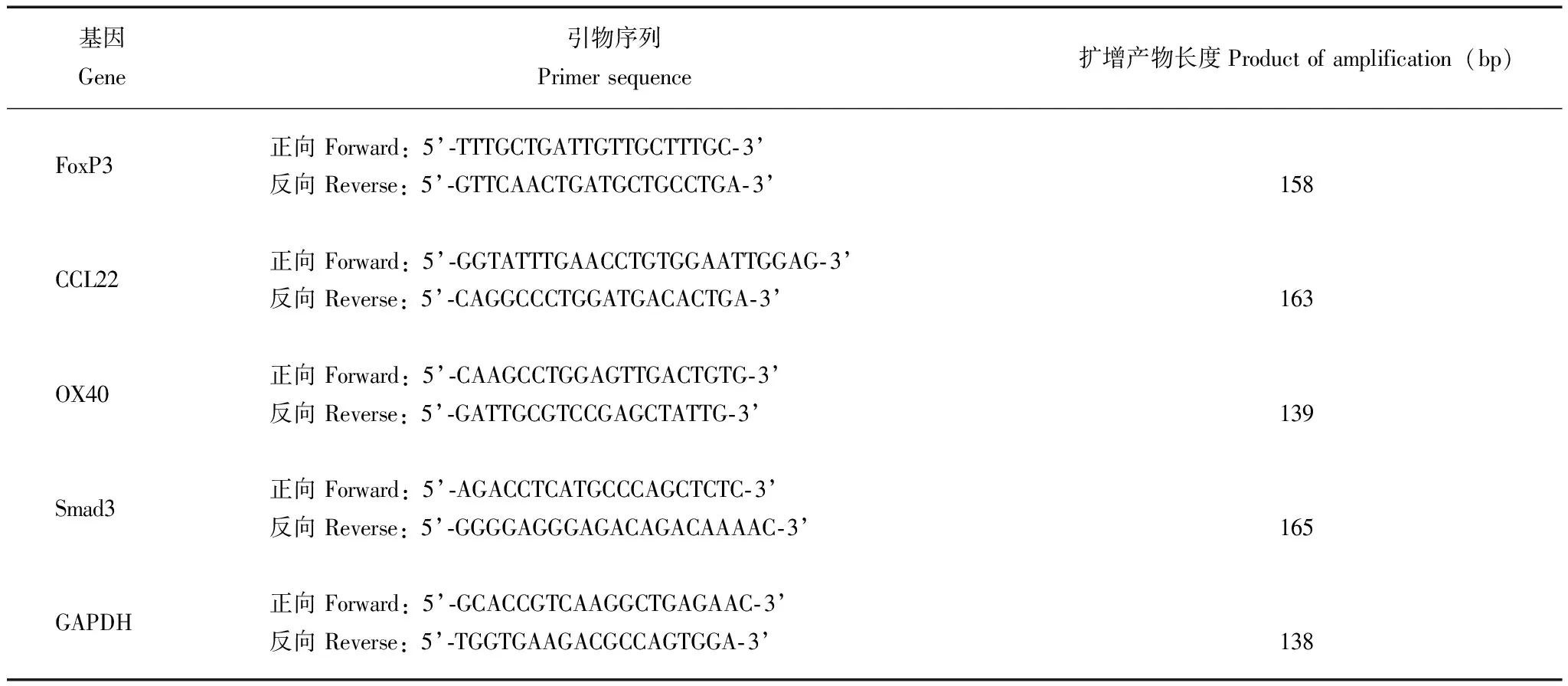
表 1 各免疫因子及管家基因的引物序列
FoxP3:叉头蛋白3;CCL22:趋化因子配体22;OX40:肿瘤坏死因子受体超家族成员4;Smad3:SMAD家族成员3;GAPDH:磷酸甘油醛脱氢酶
FoxP3:forkhead box p3;CCL22:chemokine (C-C motif) ligand 22;OX40:tumor necrosis factor receptor superfamily member 4;Smad3:SMAD family member 3;GAPDH:glyceraldehyde phosphate dehydrogenase
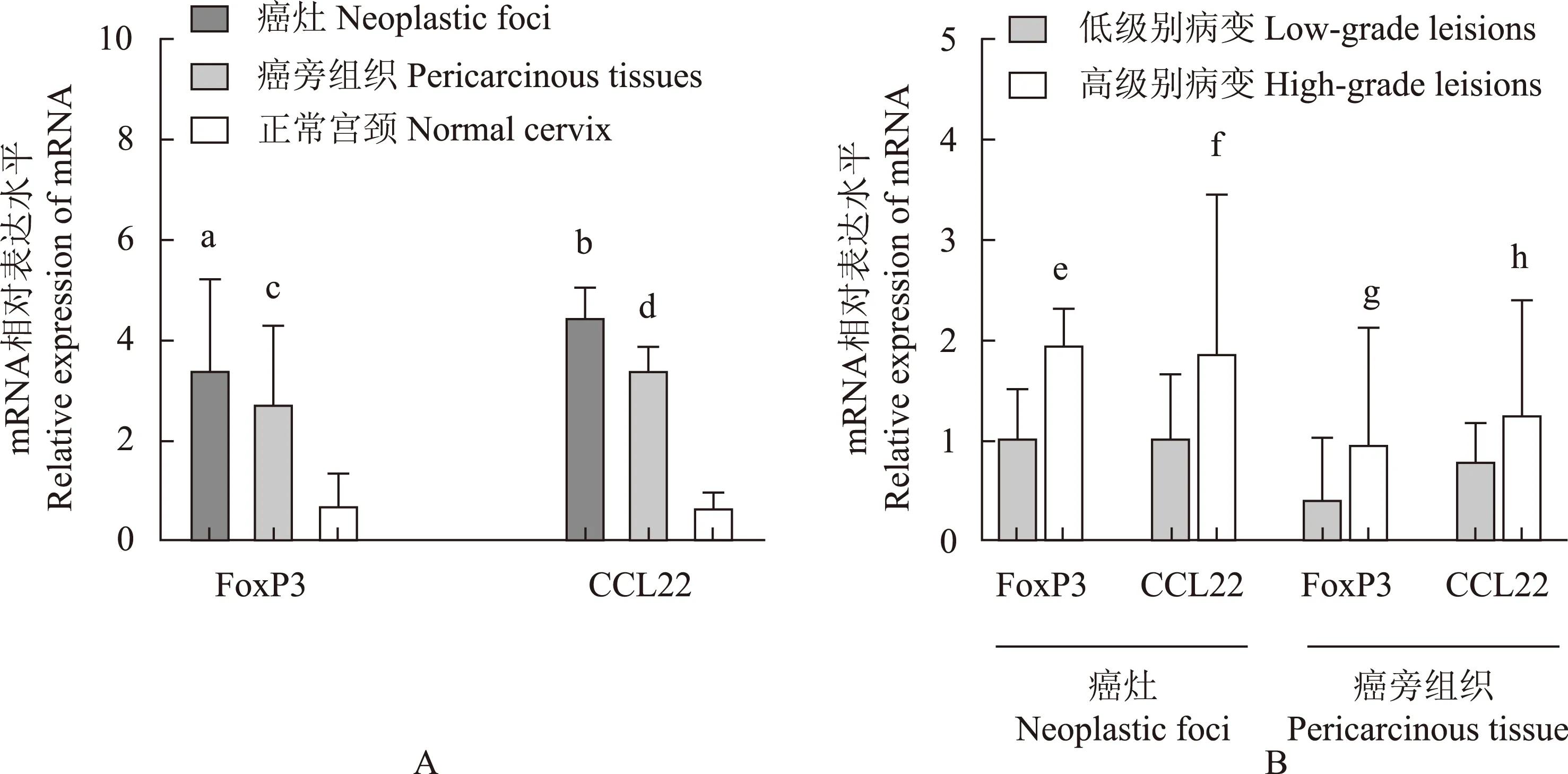
FoxP3:叉头蛋白3;CCL22:趋化因子配体22;与正常宫颈组织相比,aP=0.000,bP=0.002,cP=0.048,dP=0.040;与低级别鳞癌相比,eP=0.019,fP=0.020,gP=0.023,hP=0.031
FoxP3:forkhead box p3;CCL22:chemokine (C-C motif) ligand 22;aP=0.000,bP=0.002,cP=0.048,dP=0.040 compared with normal cervix;eP=0.019,fP=0.020,gP=0.023,hP=0.031 compared with low-grade squamous cell carcinoma
A. FoxP3和CCL22 mRNA在宫颈癌灶、癌旁和正常宫颈组织中的表达水平;B.FoxP3和CCL22 mRNA在不同病理分级的宫颈癌灶和癌旁中的表达水平
A. FoxP3 and CCL22 mRNA expression in neoplastic foci,pericarcinous tissues,and normal cervix; B. FoxP3 and CCL22 mRNA expression in different pathological grades of cervical cancer foci and pericarcinous tissues
图 1 FoxP3 和CCL22 mRNA在宫颈组织中的表达
Fig 1 The expression patterns of FoxP3 mRNA and CCL22 mRNA in different cervical tissues
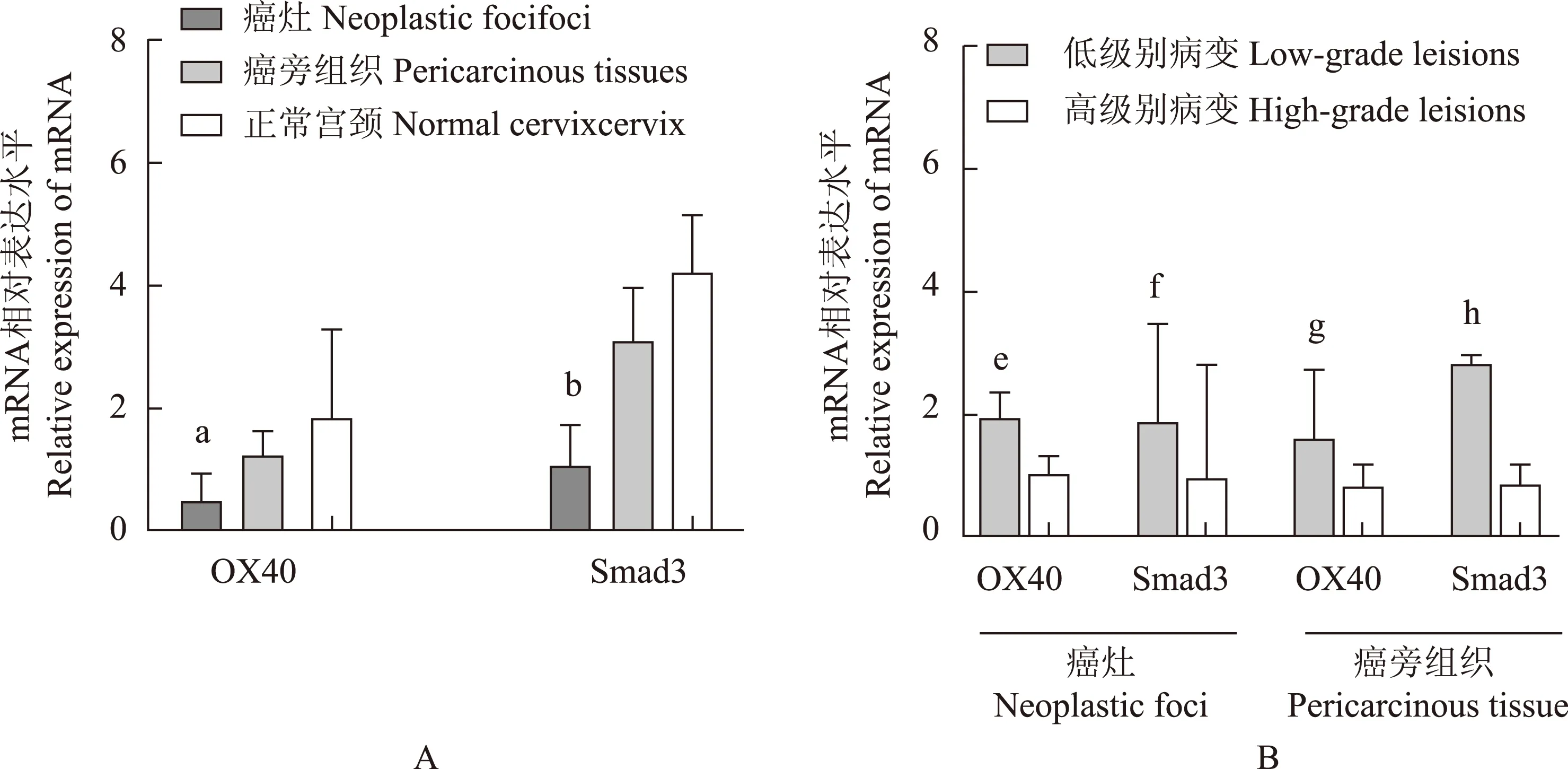
OX40:肿瘤坏死因子受体超家族成员4;Smad3:SMAD家族成员3;与正常宫颈组织相比,aP=0.000,bP=0.015;与低级别鳞癌相比,cP=0.018,dP=0.030,eP=0.027,fP=0.014
OX40:tumor necrosis factor receptor superfamily member 4;Smad3:SMAD family member 3;aP=0.000,bP=0.015 compared with normal cervix;cP=0.018,dP=0.030,eP=0.027,fP=0.014 compared with low-grade squamous cell carcinoma
A. OX40和Smad3 mRNA在宫颈癌灶、癌旁和正常宫颈组织中的表达水平;B.OX40和Smad3 mRNA在不同病理分级的宫颈癌灶和癌旁中的表达水平
A. OX40 and Smad3 mRNA expression in neoplastic foci,pericarcinous tissues,and normal cervix;B. OX40 and Smad3 mRNA expression in different pathological grades of cervical cancer foci and pericarcinous tissues
图 2 OX40 和Smad3 mRNA在宫颈组织中的表达
Fig 2 The expression patterns of OX40 mRNA and Smad3 mRNA in different cervical tissues
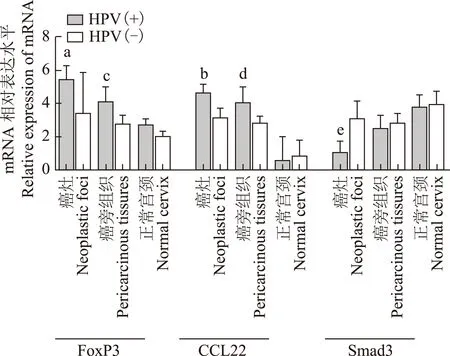
与HPV阴性组比较,aP=0.024,bP=0.039,cP=0.032,dP=0.034,eP=0.017
aP=0.024,bP=0.039,cP=0.032,dP=0.034,eP=0.017 compared with HPV negative group
图 3 各免疫因子mRNA在不同HPV感染状态下的表达
Fig 3 The mRNA expression patterns of immune factors in cervical tissues with different HPV infection status
研究人员先后采用免疫组织化学染色方法在结肠癌、胰腺导管腺癌、肝细胞性肝癌和卵巢癌中发现FoxP3+Treg表达升高,提示FoxP3在多种实体肿瘤微环境的免疫抑制状态调节中扮演重要角色[13- 16]。我们前期研究发现FoxP3仅定位于宫颈癌巢周围的肿瘤浸润性淋巴细胞,本研究结果显示FoxP3 mRNA在宫颈癌组织中表达高于正常宫颈组织,且在高级别鳞癌中的表达较低级别鳞癌高,提示在宫颈癌局部微环境中,负性因子FoxP3的免疫抑制作用可能增强,且随着肿瘤恶性分化程度加重,负性作用更加显著。
本研究还发现,CCL22 mRNA在宫颈癌局部的表达有所升高,并随着宫颈细胞恶性程度增加表达进一步增强,提示CCL22在宫颈癌微环境中可能抑制着局部免疫应答,且这种抑制功能随着肿瘤恶性分化程度加重而加剧,与在鼻自然杀伤/T细胞淋巴瘤、肺癌和乳腺癌中发现CCL22表达增强的研究结果相似[17- 19]。相关性分析所得CCL22 mRNA与FoxP3 mRNA表达正相关的结果表明,在宫颈癌微环境中,免疫负性因子CCL22与FoxP3可能协同放大彼此的免疫抑制作用,加剧局部免疫失衡。
依据正性免疫因子OX40和Smad3 mRNA表达正相关和在宫颈癌局部表达减弱的趋势,以及它们与肿瘤分化负相关的关系,我们猜测在宫颈癌微环境中,两者上调免疫应答、抑制宫颈癌发生的作用被削弱,且被削弱的程度随着肿瘤恶性分化程度的增加而增强。同时,两者之间的协同作用被削弱。相关性分析结果显示,CCL22 mRNA与OX40 mRNA呈负相关,提示OX40上调免疫的效应可能被CCL22拮抗,导致免疫抑制效应的叠加。此外,在HPV感染阳性的宫颈癌免疫微环境中,负性免疫因子FoxP3 mRNA和CCL22 mRNA表达增加,而正性因子Smad3 mRNA表达减低,我们猜测HPV感染后,宫颈局部免疫因子表达谱发生改变,负性因子的负性调节作用逐渐倾斜,最终促成局部免疫抑制和癌症的发生。
综上,本研究结果表明,在宫颈癌微环境中存在免疫因子FoxP3、CCL22、OX40和Smad3的转录表达异常,提示这种表达偏移可能造成宫颈癌局部OX40和Smad3的正性调节被削弱,而负性因子FoxP3和CCL22免疫抑制作用增强的免疫模式,这种模式导致局部免疫失衡,或许也参与了HPV感染的结局和病毒致癌的发生。我们期待后续研究进一步解释这些相关性后面的具体机制。
[1]Guan P,Howell-Jones R,Li N,et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer[J]. Int J Cancer,2012,131(10):2349- 2359.
[2]丁爱萍,张瑶,魏恒,等. HPV16型存在状态与Treg/Th17细胞因子的相关性研究[J].中华医学杂志,2013,93(37):2957- 2960.
[3]Hori S,Nomura T,Sakaguchi S. Control of regulatory T cell development by the transcription factor FoxP3[J]. Science,2003,299 (5609): 1057- 1061.
[4]Fontenot JD,Gavin MA,Rudensky AY. FoxP3 programs the development and function of CD4+CD25+regulatory T cells[J]. Nat Immunol,2003,4 (4): 330- 336.
[5]Li YQ,Liu FF,Zhang XM,et al. Tumor secretion of CCL22 activates intratumoral treg infiltration and is independent prognostic predictor of breast cancer[J]. PLoS One,2013,8(10):e76379.doi:10.1371/journal.pone.0076379.eCollection 2013.
[6]Vu MD,Xiao X,Gao W,et al. OX40 costimulation turns off FoxP3+Tregs[J]. Blood,2007,110 (7): 2501- 2510.
[7]Zhang Q,Cui F,Fang L,et al. TNF-alpha impairs differentiation and function of TGF-beta-induced Treg cells in autoimmune diseases through Akt and Smad3 signaling pathway[J]. J Mol Cell Biol,2013,5 (2): 85- 98.
[8]Kurman RJ,Carcangiu ML,Herrington CS,et al. WHO classification of tumors of female reproductive organs[M]. 4th ed. Lyon:IARC Press,2014:179.
[9]Wu H,Zhang J. miR- 124 rs531564 polymorphism influences genetic susceptibility to cervical cancer[J]. Int J Clin Exp Med,2014,7 (12): 5847- 5851.
[10]Woo YL,van den Hende M,Sterling JC,et al. A prospective study on the natural course of low-grade squamous intraepithelial lesions and the presence of HPV16 E2- ,E6 and E7-specific T-cell responses[J]. Int J Cancer,2010,126(1): 133- 141.
[11]Ghosh A,Dasgupta A,Bandyopadhyay A,et al. A study of the expression and localization of toll-like receptors 2 and 9 in different grades of cervical intraepithelial neoplasia and squamous cell carcinoma[J]. Exp Mol Pathol,2015,99 (3): 720- 724.
[12]Chiantore MV,Mangino G,Iuliano M,et al. IFN-β antiproliferative effect and miRNA regulation in Human Papilloma Virus E6-and E7-transfected keratinocyte[J]. Cytokine,2015,pii:S1043- 4666(15)30127- 7.doi:10.1016/j.cyto.2015.12.014.
[13]Kim M,Grimmig T,Grimm M,et al. Expression of FoxP3 in colorectal cancer but not in Treg cells correlates with disease progression in patients with colorectal cancer[J]. PLoS One,2013,8 (1): e53630. doi:10.1371/journal.pone.0053630.
[14]de Reuver PR,Mehta S,Gill P,et al. Immunoregulatory forkhead box protein p3-positive lymphocytes are associated with overall survival in patients with pancreatic neuroendocrine tumors[J]. J Am Coll Surg,2016,223 (3): 281- 287.
[15]Lin SZ,Chen KJ,Xu ZY,et al. Prediction of recurrence and survial in hepatocellular carcinoma based on two Cox models mainly determined by FoxP3+regulatory T cells[J]. Cancer Prev Res(Phila),2013,6(6): 594- 602.
[16]Winkler I,Wilczynska B,Bojarska-Junak A,et al. Regulatory T lymphocytes and transforming growth factor beta in epithelial ovarian tumors-prognostic significance[J].J Ovarian Res,2015,8:39.doi:10.1186/s13048- 015- 0164- 0.
[17]Kumai T,Nagato T,Kobayashi H,et al. CCL17 and CCL22/CCR4 signaling is a strong candidate for novel targeted therapy against nasal natural killer/T-cell lymphoma[J]. Cancer Immunol Immunother,2015,64(6):697- 705.
[18]Nakamura ES,Koizumi K,Kobayashi M,et al. RANKL-induced CCL22/macrophage-derived chemokine produced from osteoclasts potentially promotes the bone meta stasis of lung cancer expressing its receptor CCR4[J]. Clin Exp Metastasis,2006,23 (1):9- 18.
[19]Gobert M,Treilleux I,Bendriss-Vermare N,et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome[J]. Cancer Res,2009,69 (5): 2000- 2009.
Aberrant Expressions of Immune Factors Facilitate the Disequilibrium ofImmune Status in Cervical Cancer
ZHAO Min-yi,ZHAO Juan,YANG Ting,WANG Li,PEI Mei-li,TIAN Si-juan,YU Yang,YANG Xiao-feng
Department of Obstetrics and Gynecology,the First Affiliated Hospital,Xi’an Jiaotong University,Xi’an 710061,China
YANG Xiao-feng Tel/Fax:029- 85323844,E-mail:yxf73@163.com
Objective To explore the expressions and co-relationship of immune factors forkhead box p3 (FoxP3),chemokine (C-C motif) ligand 22 (CCL22),tumor necrosis factor receptor superfamily member 40(OX40),and SMAD family member 3 (Smad3) in cervical carcinoma and investigate their immunomodulatory roles in cervical carcinogenesis.Methods Totally 30 cases of cervical carcinoma with adjacent tissues and 20 cases of normal cervix were collected in this study. FoxP3,CCL22,OX40,and Smad3 mRNA expressions were detected by real-time polymerase chain reaction (RT-PCR). Results Compared to normal cervix,the expression levels of FoxP3 and CCL22 mRNA were elevated in neoplastic foci(P=0.000,P=0.002) and tumor periphery (P=0.048,P=0.040).The mRNAs increased modestly in high-grade squamous cell carcinoma focal(P=0.019,P=0.020) and periphery tissue (P=0.023,P=0.031) in comparison with low-grade squamous cell carcinoma. The expression levels of OX40 and Smad3 mRNA were significantly lower in neoplastic foci(P=0.000,P=0.015) than normal cervix. Compared to low-grade squamous cell carcinoma focal and periphery tissue,the mRNAs decreased moderately in high-grade squamous cell carcinoma(P=0.018,P=0.030;P=0.027,P=0.014). In both neoplastic foci and tumor periphery,the mRNA expression level of CCL22 was positively correlated with FoxP3 (r=0.353,P=0.000;r=0.307,P=0.000) but negatively correlated with OX40 (r=-0.288,P=0.031;r=-0.263,P=0.037),while OX40 was positively correlated with Smad3 (r=0.384,P=0.002;r=0.288,P=0.023). The mRNA expressions of FoxP3 and CCL22 were increased in foci and pericarcinous tissues (P=0.024,P=0.039;P=0.032,P=0.034) while Smad3 was decreased in neoplastic foci (P=0.017) in contrast to HPV negative corresponding group. Conclusion FoxP3 and CCL22 expressions increase while OX40 and Smad3 expression decrease at mRNA level in the microenvironment of cervical cancer,which may be associated with such immunological model that the immunosuppressive roles of FoxP3 and CCL22 enhance while the immunity-boosting roles of OX40 and Smad3 are impeded,contributing to the deterioration of immune disequilibrium in local site and cervical cancer carcinogenesis.
cervical cancer; tumor microenvironment; forkhead box p3; chemokine (C-C motif) ligand 22; tumor necrosis factor receptor superfamily member 4;SMAD family member 3
国家自然科学基金面上项目(81472428)、新世纪优秀人才支持计划(NCET- 12- 0441)和西安交通大学基本科研经费Supported by the National Natural Sciences Foundation of China (81472428),the Program for New Century Excellent Talents in University (NCET- 12- 0441),and the Basic Research Foundation of Xi’an Jiaotong University
杨筱凤 电话/传真:029- 85323844,电子邮件:yxf73@163.com
R73;R71
A
1000- 503X(2016)05- 0522- 06
10.3881/j.issn.1000- 503X.2016.05.005
2016- 05- 13)

