Cytotoxic activity and phytochemical standardization of Lunasia amara Blanco wood extract
Muhammad Sulaiman Zubair,Syariful Anam,Subehan Lallo
1Department of Pharmacy,Faculty of Science,Tadulako University,Kampus Bumi Tadulako Tondo,94118,Palu,Indonesia
2Department of Pharmacognosy and Phytochemistry,Faculty of Pharmacy,Hasanuddin University,90245,Makassar,Indonesia
Cytotoxic activity and phytochemical standardization of Lunasia amara Blanco wood extract
Muhammad Sulaiman Zubair1*,Syariful Anam1,Subehan Lallo2
1Department of Pharmacy,Faculty of Science,Tadulako University,Kampus Bumi Tadulako Tondo,94118,Palu,Indonesia
2Department of Pharmacognosy and Phytochemistry,Faculty of Pharmacy,Hasanuddin University,90245,Makassar,Indonesia
ARTICLE INFO
Article history:
Accepted 5 Apr 2016
Available online 13 Sep 2016
Lunasia amara
Anticancer
HeLa
T47D
Total alkaloid
Lunacrine
Objective:To evaluate the cytotoxic activity of wood extracts of Lunasia amara Blanco(L.amara)and to perform further phytochemical standardization.
Methods:The wood extracts of L.amara were assessed for cytotoxic activity by in vitro tetrazolium bromide(MTT)method against two human cancer cell lines,cervical cancer cells(HeLa)and breast cancer cells(T47D).Thin layer chromatography,Dragendorf,acetic anhydride-sulfuric acid and ferric chloride were used to detect alkaloids,steroids and polyphenols,respectively.Furthermore,quantitative determination of total alkaloid by ultra fast liquid chromatography-photodiode array detection using lunacrine as a marker compound was performed as well.
Results:The ethyl acetate extract exhibited higher cell-growth inhibition than methanol and n-hexane extracts on HeLa and T47D cancer line cells with the IC50of 71.15 and 79.04μg/mL,respectively.Total alkaloid in ethyl acetate extract was counted as(10.46±0.28)%(w/w),while lunacrine determined by ultra fast liquid chromatographyphotodiode array detection method was found to be(3.55±0.26)%(w/w).
Conclusions:The high total alkaloid and lunacrine concentration on the extract confirm the potential cytotoxic property of ethyl acetate wood extract of L.amara.
1.Introduction
Cancer is one of the most non-communicable diseases in humans.According to the American Cancer Society,there were annually increasing about 2%-3%of deaths caused by cancer reported worldwide.Thus,around 3.5 million people all over the world were killed by cancer.Several chemically synthetic antitumor agents are used to treat cancer,but the wide range of side effects encourages many cancer patients to seek alternative or complementary methods of treatment[1-3].Natural product-based medicine becomes one option to treat cancer.Nowadays,natural product or herbal-based medicine represented about 60%-80%of all drugs in use by 1990.Moreover,70%-95%of world populations applied herbal-based medicine for their primary care,particularly in developing countries[4,5].
Tel:+62 451486221
Fax:+62 451422844
E-mail:sulaiman_zubair80@yahoo.co.id
Foundation Project:Supported by the Ministry of Research,Technology and Higher Education,Republic of Indonesia through Hibah Bersaing grant(Grant No. 2851/UN28/DT/2014).
Peer review under responsibility of Hainan Medical University.The journal implements double-blind peer review practiced by specially invited international editorial board members.
Lunasia amara Blanco(L.amara),belongs to the Rutaceae family,is a small tree widely distributed in tropical forests of the Philippines,Eastern Java,Borneo,Sulawesi,Moluccas,Papua New Guinea and Australia[6].It is a popular traditional medicine,used traditionally in Indonesia,particularly in South Sulawesi Province either as a dried small wood or in a mixture with some herbs called as jamu for enhancing sexual aggressiveness.Moreover,it is also regularly used by local people for antibacterial purpose such as in treatment of swollen limbs,skin diseases,and inflamed or irritated eyes[7].
Some studies revealed that quinoline alkaloids and sesquiterpenes were the major compounds in this plant.The plant mostly contains quinoline and quinolone type alkaloids such as lunamarine,lunacrine,hidroxy lunacrine,lunacridine,and hydroxyl lunacridine[6].Two new acridone type alkaloids namely 5-hidroxygraveroline and 8-methoxyifflaiamine were also reported[7].Biological activity investigations related to antimicrobial and cytotoxic properties have been associated with the quinoline alkaloids content[6].Quinoline alkaloids from L.amara can inhibit Mycobacterium tuberculosis H37Rv[8].Moreover,one of quinoline alkaloids,namely,lunacridine was reported as DNA topoisomerase II inhibitor compound[9].The type of this mechanism will lead to antimicrobial and cytotoxic activities based on the inhibition on cell proliferation.
2221-1691/Copyright©2016 Hainan Medical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
In our effort to develop a standardized herbal-based medicine from Indonesia medicinal plants,we previously conducted the physicochemical standardization of the wood extract of L.amara collected in South Sulawesi[10].In this recent paper,further phytochemical standardization including the determination of total alkaloid and the concentration of lunacrine as a marker compound were performed to ensure the extract purity and quality which is responsible for affecting biological activity. Cytotoxic activity of wood extract was also performed to prove the potential bioactivity of standardized extract.
2.Materials and methods
2.1.Plant material
The wood of L.amara was collected from Siawung Village,Barru,SouthSulawesiandidentifiedatSulawesiBiodiversityFoundation(Yayasan Keanekaragaman Hayati Sulawesi)Indonesia. Voucher specimen(code:LA071301)was deposited at Laboratory of Pharmacognosy and Phytochemistry,Department of Pharmacy,Tadulako University.
2.2.Preparation of extracts
The wood of L.amara(2.6 kg)in powdered form was initially extracted by reflux method using methanol solvent.The resulted residue was then subjected to solid-liquid extraction with successive solvent of n-hexane and ethyl acetate.The remain solvent of ethyl acetate was then removed in vacuo on rotary evaporator until obtained the viscous extract of n-hexane extract(2.04 g)and ethyl acetate extract(4.06 g).The last traces of solvent were evaporated in freeze dryer.
2.3.Cell cytotoxicity assay
The cytotoxicity of all those extracts on both HeLa and T47D cell lines was determined by MTT colorimetric assay.The cell lines used in this study were obtained from Laboratory of Parasitology,FacultyofMedicine,GajahMadaUniversity,Indonesia. Cells(1×104cells/well)in RPMI-1640 containing 10%fetal bovine serum were seeded into 96-well flat bottom culture plates(Nunc A/S,Denmark).After 24 h of incubation,various concentrations of each extract(final concentrations from 31.25 to 1000.00μg/mL)were added to the culture and incubated for 24 h at 37°C in 5%CO2humidified atmosphere.Then cells were added with 10μL/well of MTT(5 mg/mL)and incubated for 4 h in incubator at 37°C in 5%CO2humidified atmosphere.The reaction was stopped by 100μL sodium dedosyl sulfate(10%in HCl 0.01 mol/L).The plate was then shaken in shaker for 10 min and incubated overnight in the shaded room.The absorbance of eachwellwasreadat550nmwavelengthinElisaReader(Biorad,USA),using wells without cells as blanks.All experiments were performed in triplicate.The effect of all extracts on the proliferation of human breast and cervical cancer cells was expressed as the%cytoviability,using the following formula: %Cytoviability=Absorbance of treated cells/Absorbance of control cells×100%
2.4.Phytochemical analysis
The extract material was subjected to further phytochemical analysisusing thin layerchromatography(TLC)method,following by spraying with Dragendorf,acetic anhydride-sulfuric acid,and ferric chloride reagents.Meanwhile,the total alkaloid was determined using Harborne gravimetric method[11].Further quantitative standardization of lunacrine as a marker compound on ethyl acetate extract after confirmation of its presence by TLC was performed by ultra fast liquid chromatographyphotodiode array detection(UFLC-PDA).A stock solution of both ethyl acetate extract and standard lunacrine in methanol was prepared in concentration of 0.7 mg/mL and 0.2 mg/mL,respectively.UFLC-PDA analysis was carried out using a system with LC-solution software.The separation was carried out on a Shim-Pack-Vd-Ods column VP-ODS size 150 mm×4.6 mm(inside diameter).Optimum efficiency of separation was obtained using methanol:water(90:10,v/v)with a flow rate 1 mL/min.Other parameters adopted were as follows:injection volume 5μL,columntemperature4°C,anddetectionwavelengthat258nm.Linear regressionanalysisusingSPSS17.0(SPSS.Inc,ChicagoIL,USA)wasappliedtocorrelatethepeakarea(Y)againsttheconcentration of lunacrine(X).The quantification of lunacrine in the extract was quantified in reference to the calibration curve.
3.Results
3.1.Cytotoxic activity
TheresultofcytotoxicactivityofL.amarawoodextractagainst HeLaandT47DcancercelllinesarepresentedinFigures1and2.It was observed that both cancer cells treated with L.amara extract showed a decrease in cell proliferation with the increase of concentration.Ethyl acetate extract showed higher inhibition to both cancercelllinesthantheothertwoextracts.Attheconcentrationof 62.50μg/mL,ethyl acetate extract inhibited cell proliferation on HeLa and T47D cells at 56.16%and 69.97%,respectively. Meanwhile,at the same concentration,that of n-hexane was95.59%and 100.43%,and methanol extract was 82.25%and 84.43%,respectively.Only ethyl acetate extract was able to inhibit the cell proliferation of both cancer cell lines as revealed by the value of IC50of 71.15 and 79.04μg/mL,respectively(Table 1).

Figure 1.Cytotoxic activity of L.amara extract against HeLa cancer cells.
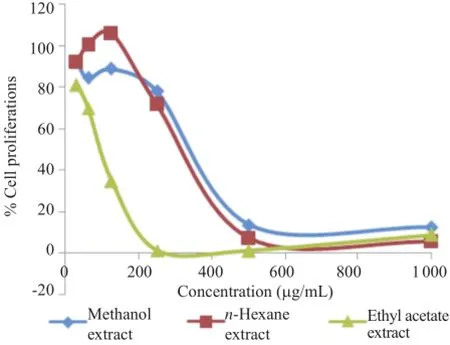
Figure 2.Cytotoxic activity of L.amara extract against T47D cancer cells.
3.2.Phytochemical analysis
Identification of chemical constituents on the n-hexane extract was performed by TLC using stationary phase silica gel 60 F254 and mobile phase n-hexane-chloroform-methanol(5:9:0.5)showed that the extract contains alkaloids(two spots with Rf0.752 and 0.811 after spraying Dragendorf reagent)and steroids(four spots with Rf0.423,0.764,0.917 and 0.929 after acetic anhydride-sulfuric acid spraying).Meanwhile,the TLC analysis of ethyl acetate extract revealed that the extract contains alkaloids(four spot with Rf0.305,0.741,0.858,and 0.941),polyphenols(one spot with Rf0.705)and steroids(four spots with Rf0.458,0.776,0.929,and 0.941).Quantitative total alkaloid content determined by Harborne gravimetric method showed that methanol extract contains(19.44±1.72)%,nhexane extract contains(8.55±1.08)%and ethyl acetate extract contains(10.46±0.28)%.Further quantitative standardization by UFLC-PDA using lunacrine as a marker compound,chromatogram of the ethyl acetate extract showed a sharp peak for lunacrine which can be comparable with the standard lunacrine(RT 2.48)(Figure 3).Figure 3B represents good separation of lunacrine with adequate peak resolution that indicates that the used method is selective for identification of lunacrine.The calibration curve was based on the analysis of working solutions at five concentration levels for lunacrine standard as shown in Figure 4.The calibration curve has good linearity relationship in the specified concentration range(5-25μg/mL)with a correlation coefficient(R2)greater than 0.98.The quantity of lunacrine in the extract was found to be(3.55±0.26)%(w/w).

Figure 3.UFLC chromatogram profile of lunacrine in ethyl acetate extract from L.amara Blanco wood extract. In figure A:standard peak of lunacrine and B:peak of lunacrine present in ethyl acetate extract.

Figure 4.Calibration curve of standard lunacrine.
4.Discussion
It is well established that plants based compounds have been playinganimportantsourceofseveralclinicallyanticanceragents. Thepredominantgoalsoftheassessmentofcytotoxicactivityfrom thecrudeplantextractsareeithertoisolatebioactiveagentsthatcan beasaleadmoleculeforanticancerdrugstherapyortodevelopthe crude plant extract itself to become standardized herbal medicine that can be used as co-chemopreventive agents[2,12].In the other hand,to ensure the therapeutic importance of herbal medicine,correct identification and quantification of active principal,proper purity assessment and quality measurement of the starting materials are an essential prerequisite to obtain the safety and efficacy of herbal medicine[4,13-16].
In the present study,L.amara wood extract was prepared using methanol as the first solvent to dissolve any types of organic compounds in the wood,and then continued by solidliquid extraction using successive solvent of n-hexane and ethyl acetate to differentiate the extract into less polar compounds fraction in n-hexane extract and more polar compounds fraction in ethyl acetate extract.We found that there is a significant difference of cytotoxic activity of each extract in inhibiting the cancer cells growth of HeLa and T47D cell lines as we can see in Table 1.Ethyl acetate extract exhibited higher inhibition to both cancer cell lines(IC50of 71.15 and 79.04μg/mL,respectively)than n-hexane and methanol extracts(both of IC50>100μg/mL). This results indicated that ethyl acetate extract was potential to be a starting material for anticancer herbal-based medicine.
The biological activity of crude extracts are usually attributed by their chemical constituents.TLC identification using several spraying reagents revealed the presence of alkaloids,steroids and polyphenolscompounds.Wesuggestedalkaloidsasamainsource for cytotoxic activity.Alkaloids have been reported to exhibit broad pharmaceutical action ranging from cytotoxic,antimalaria,pain killer,antihypertension,antiparkinson,central nervous system disorders to anti-inflammation activities[15,17].Moreover,one ofreported alkaloids,namely lunacridine,which is converted to 2′-O-trifluoroacetyl lunacridine based on stability reason,posses intercalation mechanism to DNA topoisomerase II enzyme leading to apoptotic cancer cells death via activating caspase 3/7.The cytotoxic activity of lunacridine against P388 murine leukemic cell lines was also reported[9,18].The potent cytotoxic activity in ethyl acetate extract can be related to the high levels of total alkaloid number.This result was justified by the higher total alkaloid number in more polar extract than less polar extract.This study also further measured the UFLC-PDA quantification of lunacrine on the ethyl acetate extract exhibiting potential cytotoxic activity.UFLC-PDA method was chosen based on its reproducible and accurate analysis in organic acid extraction from plants and edible mushrooms[19,20].Lunacrine is a quinoline alkaloid found in L.amara reported to posses central nervous system stimulator and low CYP3A4 inhibitory activities[6,21].Therefore,lunacrine can be used as a chemical marker for standardization of wood from L.amara.This investigation is the first report of cytotoxic activity of L.amara wood extract that confirm the potential cytotoxic activity from alkaloid based extract.Also the first report dealing with the quantitative estimation of lunacrine from this plant extract.
Ethyl acetate wood extract of L.amara showed potential cellgrowth inhibition to both HeLa and T47D cancer cell lines that can be correlated to the high concentration of total alkaloid content and lunacrine concentration.This study can be used also as a referential source of valuable information for purity and quality of extract as starting material for herbal based medicine.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
We are greatly thankful to the Ministry of Research,Technology and Higher Education,Republic of Indonesia,for supporting Hibah Bersaing grant(Grant No.2851/UN28/DT/2014)for this investigation.
References
[1]Shah U,Shah R,Acharya S,Acharya N.Novel anticancer agents from plant sources.Chin J Nat Med 2013;11(1):16-23.
[2]Rupacandra S,Sarada DVL.Anticancer activity of methanol extract of the seeds of Borreria hispida and Momordica dioica. J Pharm Res 2013;6:565-8.
[3]Hu Y,Zhou J,Ye F,Xiong H,Peng L,Zheng Z,et al.BRD4 inhibitor inhibits colorectal cancer growth and metastasis.Int J Mol Sci 2015;16(1):1928-48.
[4]Lee KH.Discovery and development of natural product-derived chemotherapeutic agents based on a medicinal chemistry approach. J Nat Prod 2010;73:500-16.
[5]Chawla R,Thakur P,Chowdhry A,Jaiswal S,Sharma A,Goel R,et al.Evidence based herbal drug standardization approach in coping with challenges of holistic management of diabetes:a dreadful lifestyle disorder of 21st century.J Diabetes Metab Disord 2013;12(1):35.
[6]Macabeo APG,Aguinaldo AM.Chemical and phytomedicinal investigations in Lunasia amara.Pharmacogn Rev 2008;2(4): 317-25.
[7]Takahashi N,Subehan,Kadota S,Tezuka Y.Mechanism-based CYP2D6 inactivation by acridone alkaloids of Indonesian medicinal plant Lunasia amara.Fitoterapia 2012;83:774-9.
[8]Aguinaldo AM,Dalangin-Mallari VM,Macabeo AP,Byrne LT,Abe F,Yamauchi T,et al.Quinoline alkaloids from Lunasia amara inhibit Mycobacterium tuberculosis H37Rv in vitro.Int J Antimicrob Agents 2007;29(6):744-6.
[9]Prescott TA,Sadler IH,Kiapranis R,Maciver SK.Lunacridine from Lunasia amara is a DNA intercalating topoisomerase II inhibitor.J Ethnopharmacol 2007;109:289-94.
[10]Anam S,Yusran M,Trisakti A,Ibrahim N,Khumaidi A,Ramadanil R,et al.[Standardization of Lunasia amara Blanco ethyl acetate extract].Online J Nat Sci 2013;2(3):1-8.Indonesian.
[11]Harborne JB.Phytochemical methods:a guide to modern techniques of plant analysis.London:Chapman and Hill Ltd;1998.
[12]Singh S,Ishar MPS,Saxena AK,Kaur A.Cytotoxic effect of Anthocephalus cadamba Miq.leaves on human cancer cell lines. Pharmacogn J 2013;5:127-9.
[13]Prasad SK,Sahu AN,Hemalatha S.Cytomorphological and physicochemical evaluations of Cryptocoryne spiralis(Retzius)Wydler.J Herbs Spices Med Plants 2012;18:304-7.
[14]Nagani K,Kaneria M,Chanda S.Pharmacognostic studies on the leaves of Manilkara zapota L.(Sapotaceae).Pharmacogn J 2012;4:38-41.
[15]Prasad SK,Laloo D,Kumar M,Hemalatha S.Quality control standardization and antioxidant activity of roots from Eriosema chinense.Pharmacogn J 2013;5:149-55.
[16]Murdifin M,Wahyudin E,Lawrence GS,Subehan,Manggau MA,Alam G.Phytochemical analysis and antioxidant activity of Mezzetia parvifloraBeccwoodbarkextract.PharmacognJ2012;4(34):18-21.
[17]Rathbone DA,Bruce NC.Microbial transformation of alkaloids. Curr Opin Microbiol 2002;5:274-81.
[18]Sulaiman Z,Subehan.Molecular docking of lunacridine from Lunasia amara to DNA:its inhibition and interaction study correlated with the cytotoxic activity on P388 murine leukemic cells.Indo J Cancer Chemoprev 2010;1(2):108-17.
[19]Pereira C,Barros L,Carvalho AM,Ferreira ICFR.Use of UFLCPDA for the analysis of organic acids in thirty-five species of food and medicinal plants.Food Anal Methods 2013;6:1337-44.
[20]Barros L,Pereira C,Ferreira ICFR.Optimized analysis of organic acids in edible mushrooms from Portugal by ultra fast liquid chromatography and photodiode array detection.Food Anal Methods 2013;6(1):309-16.
[21]Subehan,Takahashi N,Kadota S,Tezuka Y.Cytochrome P450 2D6 inhibitory constituents of Lunasia amara.Phytochem Lett 2011;4:30-3.
29 Jan 2016
inrevisedform26Feb2016
Original article http://dx.doi.org/10.1016/j.apjtb.2016.04.014
Muhammad Sulaiman Zubair,Department of Pharmacy,Faculty of Science,Tadulako University,Kampus Bumi Tadulako Tondo,94118,Palu,Indonesia.
 Asian Pacific Journal of Tropical Biomedicine2016年11期
Asian Pacific Journal of Tropical Biomedicine2016年11期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Human and animal sarcocystosis in Malaysia∶A review
- Therapeutic applications of collagenase(metalloproteases)∶A review
- Antiacanthamoebic properties of natural and marketed honey in Pakistan
- GC-MS analysis of bioactive compounds present in different extracts of an endemic plant Broussonetia luzonica(Blanco)(Moraceae)leaves
- Anti-nitric oxide production,anti-proliferation and antioxidant effects of the aqueous extract from Tithonia diversifolia
- Anthelmintic activity of Saba senegalensis(A.DC.)Pichon(Apocynaceae)extract against adult worms and eggs of Haemonchus contortus
