Anti-nitric oxide production,anti-proliferation and antioxidant effects of the aqueous extract from Tithonia diversifolia
Poonsit Hiransai,Jitbanjong Tangpong,Chuthamat Kumbuar,Namon Hoonheang,Onrunee Rodpech,Padchara Sangsuk,Urairat Kajklangdon,Waraphorn Inkaow
1Molecular Medicine and Cancer Biology Research Unit,School of Allied Health Sciences and Public Health,Walailak University,Nakhon Si Thammarat 80161,Thailand
2Department of Medical Technology,School of Allied Health Sciences and Public Health,Walailak University,Nakhon Si Thammarat 80161,Thailand
3Biomedical Sciences Research Unit,School of Allied Health Sciences and Public Health,Walailak University,Nakhon Si Thammarat 80161,Thailand
Anti-nitric oxide production,anti-proliferation and antioxidant effects of the aqueous extract from Tithonia diversifolia
Poonsit Hiransai1,2*,Jitbanjong Tangpong2,3,Chuthamat Kumbuar2,Namon Hoonheang2,Onrunee Rodpech2,Padchara Sangsuk2,Urairat Kajklangdon2,Waraphorn Inkaow2
1Molecular Medicine and Cancer Biology Research Unit,School of Allied Health Sciences and Public Health,Walailak University,Nakhon Si Thammarat 80161,Thailand
2Department of Medical Technology,School of Allied Health Sciences and Public Health,Walailak University,Nakhon Si Thammarat 80161,Thailand
3Biomedical Sciences Research Unit,School of Allied Health Sciences and Public Health,Walailak University,Nakhon Si Thammarat 80161,Thailand
ARTICLE INFO
Article history:
revisedform12Dec2015,3rdrevised
form 7 Jan 2016
Accepted 23 Feb 2016
Available online 14 Sep 2016
Tithonia diversifolia
Anti-nitric oxide production
Anti-proliferation
Antioxidant
Objective:To determine the cytotoxicity,reduction in nitric oxide production and antioxidative activity of the aqueous leaf extract from Tithonia diversifolia(T.diversifolia)in an in vitro model.
Methods:Leaves of T.diversifolia were collected from natural habitats and extracted with distilled water using the decoction method.The cytotoxic effect of the extract in terms of cell viability was determined using RAW264.7 cells and human peripheral blood mononuclear cells(PBMCs)via the mitochondrial respiration method using the MTT reagent.The effect of the extract on lipopolysaccharide(LPS)-induced nitric oxide production in RAW264.7 cells was measured using the Griess reagent.The chemical antioxidant was evaluated by ABTS-and DPPH-radical scavenging assays.
Results:The half-maximal cytotoxic concentration values were 145.87μg/mL and 73.67μg/mL for human PBMCs and RAW264.7 cells,respectively.In the presence of phytohemagglutinin-M,the IC50on PBMCs proliferation was 4.42μg/mL.The noncytotoxic range of the extracts inhibited LPS-induced nitrite production in RAW264.7 cells with an IC50value of 11.63μg/mL.To determine the anti-oxidative properties,the N-acetyl cysteine equivalent antioxidant capacity of the extract was(32.62±1.87)and(20.99±2.79)mg N-acetyl cysteine/g extract,respectively determined by the ABTS-radical and DPPH-radical assay.However,the extract did not confer death protection in a hydrogen peroxideinduced RAW264.7 co-culturing model.
Conclusions:Our study demonstrated the immunomodulation caused by the aqueous leaf extract of T.diversifolia,resulting from the inhibition of phytohemagglutinin-M-induced PBMCs proliferation and LPS-induced nitric oxide production in RAW264.7 macrophages.Although the anti-oxidative activity was presented in the chemical-based anti-oxidant assay,the extract cannot protect cell death from stress conditions.
Original article http://dx.doi.org/10.1016/j.apjtb.2016.02.002
1.Introduction
Immunomodulation is an alteration of the immunity of the body by immunomodulators that either activate or suppress the immune system.During the activation of immunological processes,free radicals can also highly induce the production of reactive oxygen and reactive nitrogen species[1].Nitric oxide(NO),the reactive nitrogen species molecule synthesized from inducible nitric oxide synthase(iNOS),is highly produced by pyrogen-activated macrophages.Additionally,superoxide(O2-)is also synthesized by phagocytic oxidase in activated immunecells.The interaction between NO and the superoxide anion consequently increases cellular toxicity and oxidative stress[2]. Oxidative stress results from an imbalance between free radical molecules and the anti-oxidative system that may cause more accumulation or the production of free radical molecules in the activation processes of the immune system,especially in inflammation.The presence of free radical molecules at a high concentration during oxidative stress induces structural changes in cellular biomolecules,such as lipids,proteins and DNA,and causes a loss of cellular function and mutagenesis[3]. Recently,oxidative stress has been reported to be associated with the development and progression of chronic diseases,including neurological diseases,pulmonary disease,cardiovascular disease and cancer[3-11].Thus,the immunosuppressant system and increasing activity of the anti-oxidative system are targets for the reduction of the pathogenesis,complication,bad prognosis and incident rate of these chronic diseases.
2221-1691/Copyright©2016 Hainan Medical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
Natural compounds from traditional herbal medicines are the major source of anti-oxidative substances and have also been revealed to reduce toxicity caused by free radical molecules duringchronicdiseases.Mexicansunflower[Tithoniadiversifolia(Hemsl.)A.Gray.(T.diversifolia)]in Asteraceae grows in Central and South America,Africa,and Asia[12].This herb has been used in traditional medication for infectious dermatitis,fever,parasitic infection,diarrhea,chronic arthritis,abdominal pain,hepatitis,ascariasis,and malaria[13-16].In Asia,T.diversifolia is mostly used to treat hepatitis,fever,diarrhea,hematomas,dermatic disorders and diabetes associated with polyuria and polydipsia[17].From ethnomedicinal reviews,the chemical constituents and in vitro pharmacological properties of this plant have been identified,and it has been reported to have antimalarial,anti-diabetic and anti-microbial properties[17,18].In addition,the ethanol and methanol extracts of this plant have been found to have in vivo anti-inflammation and anti-oxidative properties[18-20].However,extractions with these organic solvents have been reported to have a toxic effect[20].
Thus,this study evaluated the in vitro effect of the aqueous extract of the dried leaf of T.diversifolia.The cytotoxicity and the anti-oxidative stress-related immunomodulation properties,including the anti-proliferation properties and anti-nitric oxide production effects of the extract,were determined.Peripheral blood mononuclear cells(PBMCs)from normal volunteers were used as a model for cytotoxic investigation.To probe the antinitric oxide production and anti-oxidative damage effects of the extract,lipopolysaccharide(LPS)-and hydrogen peroxideinduced RAW264.7 macrophages were used as a model.Our results may support a previous report of its in vivo activity.
2.Materials and methods
2.1.Plant material preparation and extraction
T.diversifolia leaves were collected during October to December from Narathiwat,Ya-la and Surat Thani provinces,Thailand.The herbarium was identified and documented by the Office of the Forest Herbarium,Department of National Parks,Wildlife and Plant Conservation,Thailand(BKF No.186838 and BKF No.186839).Fresh leaves were washed and dried at 60°C.Then,dried leaves were ground into powders.The aqueous extraction was performed using decoction processes. Briefly,the dried powder was boiled at 100°C with distilled water(1:5 w/v)for 30 min.Then,the aqueous solution was centrifuged at 9 010 r/min(30 min,4°C)and freeze-dried using a lyophilizer(EYELA FDU-2100).The aqueous extract of T.diversifolia was kept at-80°C until use.
2.2.PBMCs collection and isolation
Heparin blood was collected from healthy volunteer donors who had an average age of 20-25 years old.Donors with infection signs,drug intake and abnormal complete blood count profiles were excluded.This project was approved by the Ethical Committee on Human Rights Related to Researches Involving Human Subjects,Walailak University(Protocol number:11/ 055).The PBMCs of each sample were isolated by density gradient-centrifugation using a Ficoll-Hypaque solution(Fresenius Kabi Norge As,Norway).The cells were washed twice with phosphate buffered saline solution(PBS,pH 7.2)and resuspended in RPMI-1640 supplemented with 10%fetal bovine serum and 1%penicillin/streptomycin(Gibco,Grand Island,NY,USA)at a density of 1×106cells/mL.
2.3.Cell culture methods
The mouse macrophage cell line RAW264.7(TIB-71)was obtained from the American Type Culture Collection(Rockville,MD,USA).Both RAW264.7 cells and PBMCs were grown in RPMI supplemented with 10%fetal bovine serum and 100 IU/ mL of penicillin and streptomycin at 37°C with 5%CO2.For RAW264.7 cells,the culture medium was changed and subcultured every 3 days.Cells with a cell passage number less than 30 were used in this experiment.
2.4.Cell viability using a trypan blue exclusion assay
PBMCs(2×105cells/well)were seeded in a 48-well plate for 24 h at 37°C with 5%CO2.The old medium was removed and replaced with fresh medium containing various concentrations of T.diversifolia aqueous extract and then incubated for 24 h.Then,the cells were harvested,and the viable cell numbers were assessed using 0.4%trypan blue staining and counted by a hemocytometer under an inverted microscope.The percentage of viable cells was calculated by the following equation:
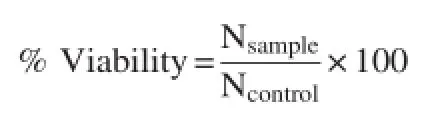
where Nsampleand Ncontrolwere the viable cell numbers from the trypan blue staining of the sample and control well,respectively.
2.5.Cell viability and cell proliferation by an MTT assay
An MTT assay was performed to determine the cell viability. MTT(Sigma,St.Louis,MO)was dissolved in PBS at 5 mg/mL as a stock solution and then sterilized using a 0.2-μm filter.At the end of experimental period,the PBMCs or RAW264.7 cells were incubated with 100μL of the MTT solution(0.5 mg/mL in growth medium)at 37°C with 5%CO2for 1 h.Under light protection,the dark blue crystals of formazan were dissolved with 200μL of dimethyl sulfoxide at room temperature for 30 min,mixed by pipetting and read at 550 and 630 nm on a micro-plate reader.The percentage of viable cells was calculated with the following equation:

where ODsample,ODblank,ODcontrolwere the absorbances at 550/ 630 nm of the sample,blank and control wells,respectively.The phytohemagglutinin(PHA)-M-induced proliferation of PBMCs was expressed as the%of viable cells and was calculated using the same method and equation.
2.6.Measurement of the nitrite concentration in the culture media
The nitrite concentration in the culture supernatant was measured after 24 h of LPS-incubation.Briefly,the sample supernatant(100μL)was incubated with 2%w/v sulfanilamide in 10%v/v o-phosphoric acid(50μL)for 15 min at room temperature.Then,0.2%w/v of N-(1-napthyl)-ethylenediamine dihydrochloride(50μL)was added and left to incubate for an additional 15 min at room temperature.The absorbance at 570 nm was determined by a micro-plate reader.The quantification of nitrite in the sample was standardized with 0-100μmol/L of NaNO2.
The effect of T.diversifolia aqueous extract on the iNOS activity was screened using the method of Booke et al.with minor modifications[21].RAW264.7 cells were treated with 100 ng/mL of LPS for 24 h to induce the expression of iNOS,and the LPS was washed out twice with PBS.Then,the LPS-pretreated RAW264.7 cells were incubated with T.diversifolia aqueous extract at concentrations ranging from 0.94 to 30.00μg/ mL(2-fold serial dilution)or a competitive iNOS inhibitor 2-ethyl-2-thiopseudourea hydrobromide(ETU).
2.7.2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulphonic acid)(ABTS)-radical scavenging assay
An ABTS-radical scavenging assay was used with minor modifications[22].Briefly,the ABTS solution(7 mmol/L)was mixed with an equal amount of potassium persulfate(4.90 mmol/L)and incubated for 24 h.The resulting ABTS radical solution was diluted in distilled water,and the optical density was determined at 415 nm until the absorbance was 0.700±0.100.Then,fifteen microliters of the samples(250-2000μg/mL)or the standards[31.25-250.00μmol/L Trolox and 3.13-25.00μg/mL N-acetyl cysteine(NAC)]were reacted with the ABTS radical solution(150μL)for 15 min at room temperature and protected from light.The absorbances of the reaction mixtures were determined at 415 nm,and the data were calculated as the inhibitory percentage.
2.8.2,2-Diphenyl-1-picrylhydrazyl(DPPH)-radical scavenging assay
A DPPH-radical scavenging assay was used with minor modifications[23].Briefly,the DPPH solution(100 mmol/L in 80%methanol)was diluted in 80%methanol,and the optical density at 570 nm was determined until the absorbance was equal to 0.700±0.100.Then,fifteen microliters of the samples(1250-5000μg/mL)or the standards(125-1000μmol/L Trolox and 25-200μg/mL NAC)was reacted with the DPPH radical solution(150μL)for 60 min at room temperature and protected from light.Then,the optical density of the reaction mixtures was determined at 570 nm,and the data were calculated as the inhibitory percentage.
2.9.Statistical analysis
All assays were carried out in triplicate with a minimum of five independent experiments.The data were expressed as mean±SEM.The half-maximal cytotoxic concentration(CC50)and the IC50were obtained by linear regression analysis of the concentration-response curve by plotting the sample concentrations and the percentage of inhibition or the percentage of viable cells.The data of control group and treatment group were compared using One-way ANOVA,and a P value less than 0.05 was considered significant.
3.Results
3.1.The chemical-based anti-oxidative properties of T.diversifolia aqueous extract
The ABTS-radical and DPPH-radical assays were used to probe the chemical-based antioxidant activity of the extracts(Figure 1).In these assays,well-known antioxidants,including Trolox and NAC were used as control.Under these conditions,Trolox showed a half-maximal percentage of its radical scavenging properties(IC50)of(193.34±12.21)μmol/L(R2>0.99)and(381.62±3.85)μmol/L(R2>0.99)according to the ABTS assayandDPPHassay,respectively.Inaddition,NACshowedan IC50for its radical scavenging properties of(26.11±2.11)μg/mL(R2>0.97)and(84.30±4.14)μg/mL(R2>0.98)accordingtothe ABTS and DPPH assays,respectively.In the T.diversifolia aqueous extract determination,the IC50values of the radical scavenging activities of the extract according to the ABTS and DPPH assays were(801.06±13.61)μg/mL(R2>0.99)and(4099.12±339.20)μg/mL(R2>0.97),respectively.In comparison with the reference antioxidants used in each assay,the equivalent ABTS-radical scavenging capacities of T.diversifolia aqueous extract were(241.04±11.93)μmol Trolox and(32.62±1.87)mg NAC per gram of dry extraction weight. Furthermore,the equivalent DPPH-radical scavenging capacities ofT.diversifoliaaqueousextractwere(94.89±2.69)μmolTrolox and(20.99±2.79)mg NAC per gram of dry extraction weight.
3.2.The cytotoxicity of T.diversifolia aqueous extract on PBMCs and RAW264.7 cells
PBMCs were exposed to T.diversifolia aqueous extract ranging from 31.3 to 500.0μg/mL(2-fold serial dilution)for 24 h.The viable cell number was determined by the trypan blue exclusion assay.The results showed that the viability of PBMCs in the vehicle control group(0.2%PBS)was(97.47±10.61)%. In the presence of T.diversifolia aqueous extract,the viability of the PBMCs decreased dose-dependently from(97.62±8.08)% to(17.91±2.73)%.The cytotoxicity of T.diversifolia aqueous extract was significantly observed in the T.diversifolia aqueous extract-treated cells at 250 and 500μg/mL of T.diversifolia aqueous extract(P<0.01),which reduced cell viabilities at(31.33±9.35)%and(17.91±2.73)%,respectively(Figure 2A). The CC50of T.diversifolia aqueous extract was 145.87μg/mL(Figure 2A).An MTT assay was used to confirm the cytotoxic effect of T.diversifolia aqueous extracts in both PBMCs andRAW264.7 cells(Figure 2B,C),PBMCs were exposed to T.diversifolia aqueous extract in a range of non-cytotoxic concentrations as determined by a trypan blue exclusion assay(6.3-25.0μg/mL using 2-fold serial dilutions)for 24 h.The result showed that 25.0μg/mL T.diversifolia aqueous extract also reduced the PBMCs viability to(73.52±5.30)%with significance(P<0.01)in the comparison with the vehicle control.For the cytotoxic effect on RAW264.7 macrophages,T.diversifolia aqueous extract concentrations ranging from 6.25 to 100.00μg/mL were tested,and the results showed that T.diversifolia aqueous extract reduced cell viability from(104.16±2.96)%to(21.57±2.02)%.Significant cytotoxicity was observed at 50 and 100μg/mL(P<0.01),and the CC50of T.diversifolia aqueous extract for RAW264.7 cells was 73.67μg/mL(Figure 2C).
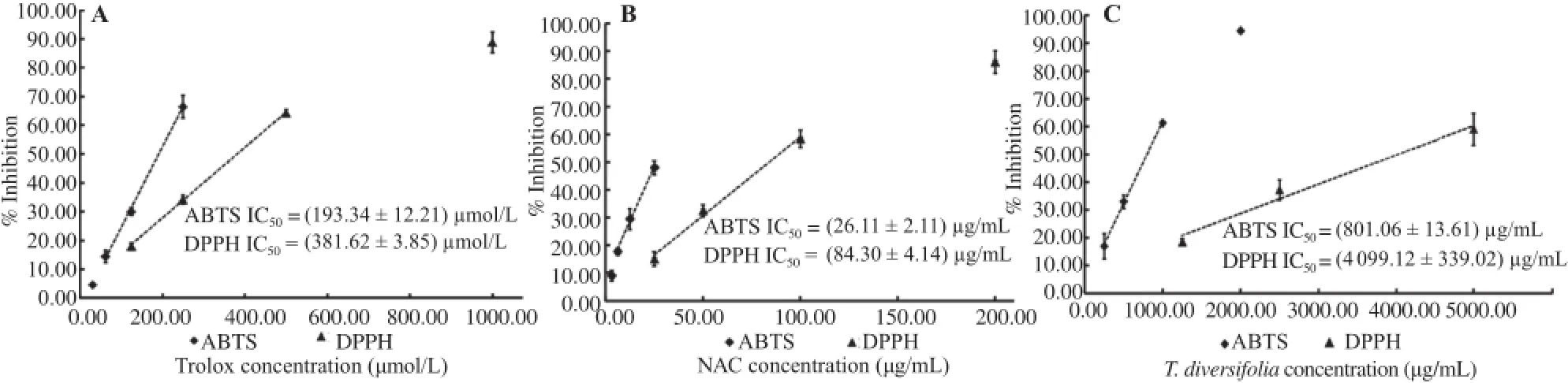
Figure 1.The chemical-based anti-oxidative activity.A:Trolox;B:NAC;C:T.diversifolia aqueous extract.The anti-oxidative activity was determined using ABTS-radical and DPPH-radical scavenging assay. The values were expressed as mean±SEM from five independent experiments(n=5)performed in triplicate wells.
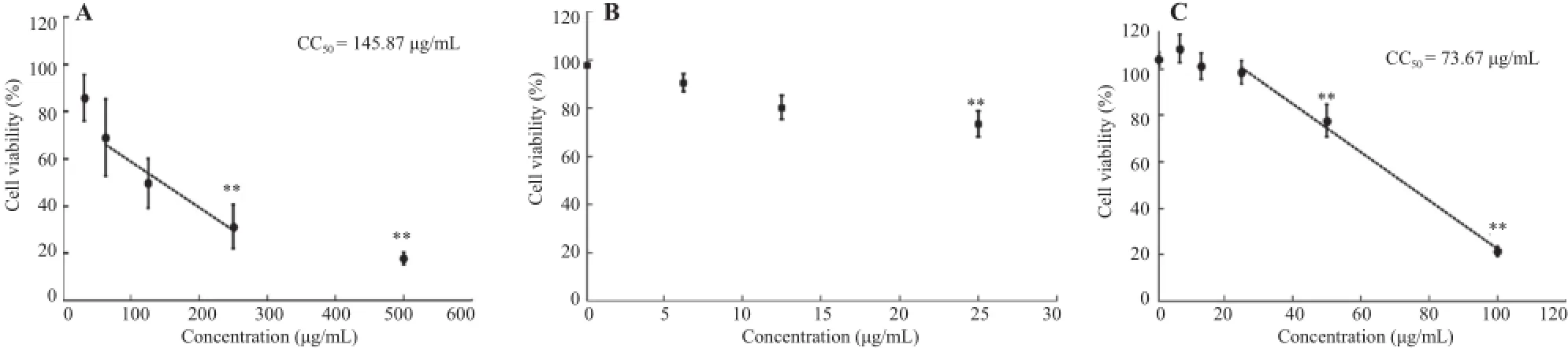
Figure 2.Cytotoxicity of T.diversifolia aqueous extract on PBMCs and RAW264.7 cells.A:PBMCs(1×106cells/mL in 48-well plate)were treated with various concentrations of T.diversifolia aqueous extract for 24 h.The cell viability was determined by trypan blue exclusion assay.The CC50was calculated by linear regression model(y=-0.193 1x+78.167,R2=0.959);B:PBMCs(1×106cells/mL in 96-well plate)and C:RAW264.7 cells(1×106cells/mL in 96-well plate)were treated with various concentrations of T.diversifolia aqueous extract for 24 h.The cell viability was determined by MTT assay.The CC50of RAW264.7 cells was calculated by linear regression model(y=-1.040 4x+126.65,R2=0.995).The values were expressed as mean±SEM from five independent experiments(n=5)performed in triplicate wells.**:Significantly different(P<0.01)compared with vehicle control group(0μg/mL,0.2%PBS).
3.3.The effect of T.diversifolia aqueous extract on PHA-M-induced PBMCs proliferation
PBMCs were exposed to PHA-M for 24 h in the presence of 0.2%PBS(vehicle)and various safe concentrations of T.diversifoliaaqueousextract(0.66-25.00μg/mLwiththe2-fold serial dilution method).Then,the viability of the PBMCs was assessed using an MTT assay,and the data were expressed as% viable cells.The results indicated that PHA-M induced the increase in the%viable cells from(97.79±0.62)%in the vehicle group to(169.98±17.14)%in the PHA-M-stimulated groups(Figure 3).In the presence of T.diversifolia aqueous extract,the proliferation of PBMCs decreased in a concentrationdependent manner to(120.50±8.44)%,(100.82±9.41)%and(83.54±8.56)%at the concentrations of 0.66,12.50 and 25.00μg/mL,respectively.Significant differences were observed at every tested concentration(P<0.05).The calculated IC50was 4.42μg/mL.However,the anti-proliferative effect of 25.00μg/ mL T.diversifolia aqueous extract might be affected by cytotoxicity,as indicated by the MTT assay in Figure 2B.

Figure 3.Effect of T.diversifolia aqueous extract on PHA-M-induced PBMCs proliferation.PBMCs(1×106cells/mL in 96-well plate)were treated with various concentrations of T.diversifolia aqueous extract prior to stimulation with PHA-M for 24 h.The cell viability was determined by MTT assay.The values were expressed as mean±SEM from five independent experiments(n=5)performed in triplicate wells.**:Significantly different(P<0.01)compared with vehicle control group(0μg/mL,0.2%PBS).TDA: T.diversifolia aqueous extract.
3.4.The effect of T.diversifolia aqueous extract on LPS-induced nitric oxide production by RAW264.7 cells
The incubation of RAW264.7 cells with T.diversifolia aqueous extract at concentrations ranging from 0.94 to 30μg/mL(2-fold serial dilution)prior to their activation with 100 ng/mL of LPS significantly suppressed the production of NO compared to the LPS activation only group,with IC50values of 11.63μg/mL. As shown in Figure 4A,the basal nitrite levels were(0.08±0.25)and(0.21±0.22)μmol/L in the absence(basal)and presence(vehicle)of 0.2%PBS,respectively.Upon stimulation with 100 ng/mL of LPS,the production of NO rose significantly to levels of(14.18±1.16)and(13.53±1.05)μmol/L in the absence(basal)and presence(vehicle)of 0.2%PBS,respectively.In the absence of LPS,T.diversifolia aqueous extract treatment did not cause a significant change in the nitrite concentration.The nitrite levels of T.diversifolia aqueous extract-treated RAW264.7 cell showed a minimum level of(0.01±0.10)μmol/L and a maximal level of(0.37±0.09)μmol/L at 0.94 and 15μg/mL of T.diversifolia aqueous extract,respectively.In addition,T.diversifolia aqueous extract exhibited LPS-induced NO suppression in a concentration-dependent manner(Figure 4A).The significant difference of NO levels were(8.25±0.73),(5.09±0.56)and(1.83±0.34)μmol/L at 7.5,15.0 and 30.0μg/ mL of T.diversifolia aqueous extraction,respectively(P<0.01). This suppression was not due to chemically induced cytotoxicity at any dosage below 30μg/mL of T.diversifolia aqueous extract,which was determined by the MTT assay(Figure 4B).
Using the screening method for iNOS activity,the nitrite concentration in the culture supernatants are shown in Figure 4. The data indicated that the nitrite levels were(0.31±0.22)and(0.35±0.18)μmol/L in the absence(basal)and presence(vehicle)of 0.2%PBS without LPS stimulation,respectively. After pre-stimulation with 100 ng/mL of LPS for 24 h,NO production reached significance at levels of(6.99±0.58)and(6.50±0.54)μmol/L in the absence(basal)and presence(vehicle)of 0.2%PBS without LPS stimulation,respectively.In the presence of ETU,LPS-induced NO production was suppressed to(2.48±0.33)μmol/L[68.85%of inhibitory effect with significance(P<0.01)].However,T.diversifolia aqueousextract at any tested concentration did not inhibit NO production.
3.5.The effect of T.diversifolia aqueous extract on hydrogen peroxide-induced RAW264.7 cells
In principle,oxidative stress could induce cell death caused by the presence of ROS,and anti-oxidative molecules can protect against this cell death.In this study,hydrogen peroxide(H2O2)at 150μmol/L induced a 50%cytotoxic effect on RAW264.7 cells,which was determined by a MTT assay.The potent water-soluble antioxidant,NAC,was used as a positive control to protect cells from death due to H2O2.In the experimental design,RAW264.7 cells were treated with 150μmol/L of H2O2for 30 min prior to exposure with various concentration of T.diversifolia aqueous extract(3.75-30.00μg/mL,2-fold serial dilution).After 24 h of incubation,the percentage of viable cellswas measured by a MTT assay.The results showed that the H2O2-treated group had(45.11±8.50)%cell viability,which was significant(P<0.05)compared to the H2O2-untreated vehicle groups,whereas the cell viability of the NAC-treated group significantly rose to(81.67±5.22)%cell viability in comparison with the H2O2-treated group(P<0.05).In the presence of T.diversifolia aqueous extract,a protective effect against H2O2-induced cell death was not observed at all of the tested concentrations(Figure 5).
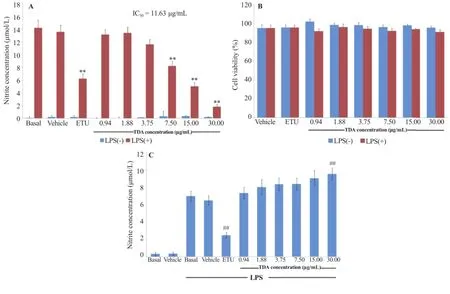
Figure 4.Effect of T.diversifolia aqueous extract on LPS-induced nitric oxide production from RAW264.7 cells.A:RAW264.7 cells(1×106cells/mL)were treated with various concentrations of T.diversifolia aqueous extract prior to stimulation with 100 ng/mL LPS for 24 h;B:The viability of the remaining cells after removal of supernatants was determined by MTT assay;C:RAW264.7 cells(1×106cells/mL)were treated with 100 ng/mL LPS for 24 h and then exposed to various concentrations of T.diversifolia aqueous extract for 24 h.The values were expressed as mean±SEM from five independent experiments(n=5)performed in triplicate wells.**:P<0.01 compared with vehicle control group(Vehicle±LPS)and##:P<0.01 compared with LPS-pre-treated vehicle control group.TDA:T.diversifolia aqueous extract.

Figure5.TheeffectofTDA on theviabilityofH2O2-induced RAW264.7 cells.The values were expressed as the mean±SEM from five independent experiments(n=5)performed in triplicate wells.##:Significantly different(P<0.01)compared with the H2O2-untreated vehicle control group;**:Significantly different(P< 0.01)compared with the H2O2-treated group.
4.Discussion
It has been previously reported that T.diversifolia extracted withether,dichloromethane,methanolorethanolhasanti-malarial,anti-hyperglycemic,anti-cancer and anti-inflammation properties[15,17,18,24-34].However,other research groups revealed the toxic effects of the extracts using a brine shrimp lethality test,mice and rat models[20,24,35,36].In the present study,the CC50in PBMCs was 145.87μg/mL,as shown by a trypan blue exclusion assay,and the minimal significant cytotoxicity was exhibited by the concentration of 25μg/mL,as determined by an MTT assay. In contrast,the cytotoxicity in RAW264.7 cells was lower than that for human PBMCs,in which the CC50value was found to be 73.67μg/mL.The maximum non-cytotoxic concentration in PBMCs was found to be 12.5μg/mL.These results agreed with a previous report,which revealed the non-toxic dose of the infusion ofdriedT.diversifolialeaf(withboilingdistilledwaterat1:10w/v)was 10 mg/kg body weight[36].In that report,an alteration in the hematological parameters was found in the 90-day-repeated dose,including a decrease in the total white blood cells count and neutrophils and an increase in the total number of mononuclear cellsat10and100mg/kg.Additionally,ourreportshowedtheantiproliferative activity of PHA-M-induced human PBMCs.However,the aqueousextractionmethod of Passoni et al.was different in our study,which had a longer period of extraction time[36].
Next,non-cytotoxic concentrations were used to study the effect ofT.diversifolia aqueousextract on the productionof nitric oxide in RAW264.7 macrophages.Nitric oxide is continuously produced in the presence of LPS,inducing the expression of iNOS,which synthesizes NO molecules[11].In this study,T.diversifolia aqueous extract significantly inhibited NO production in a concentration-dependent manner,with IC50values of 11.63μg/mL.Our study revealed the anti-inflammatory activity of T.diversifolia aqueous extract,which had been reported in in vivo models[15,30-34].The inhibitory effect of T.diversifolia aqueous extract on nitric oxide production was proposed to inhibit either iNOS activity or iNOS transcription signaling processes.Thus,the inhibition of iNOS activity was screened using the method reported by Booke et al.[21].The screening results showed that T.diversifolia aqueous extract did not inhibit nitric oxide production in 24-h-LPS-pretreated RAW264.7 cells,whereas competitive inhibition by ETU was observed(Figure 4C).These data indicated that T.diversifolia aqueous extract did not inhibit NO production through the inactivation of iNOS activity.Therefore,the inhibitory effect of T.diversifolia aqueous extract may be at the transcriptional level,which is supported by a previous study of Bork et al.[32].
Finally,hydrogen peroxide has recently been used to induce cell death under stress conditions through apoptosis,and this process could be protected by anti-oxidant compounds[37,38].In this study,200μg/mL of NAC could protect H2O2-induced cell death,which restored the viability of RAW264.7 cells from(48.82±7.03)%to(86.23±3.98)%.Based on the results of the ABTS and DPPH assays,T.diversifolia aqueous extract showed an anti-oxidative capacity equivalent of(241.04±11.93)and(94.89±2.69)μmol Trolox/g,respectively.WhenNAC was used as the standard compound,the anti-oxidative capacity of T.diversifolia aqueous extract was equivalent to(32.62±1.87)and(20.99±2.79)mg NAC/g in the ABTS and DPPH assays,respectively.Using this information,the amount of T.diversifolia aqueousextractthatwouldbeequivalentto200μgofNACshould be6.13and9.53mg,respectively,ascalculatedfromtheresultsof the ABTS and DPPH assays.However,the calculated concentration was out of the non-cytotoxic range,which shouldbe lower than 30μg/mL.Thus,this calculated concentration based on the antioxidant capacity could not be used in practice in the hydrogen peroxide-induced cell death model.In Figure 5,this hypothesis wasproventhattheanti-oxidativeeffectofT.diversifoliaaqueous extractatnon-cytotoxicconcentrationsdidnotprotectfromH2O2-induced cell death in our cell culture model.
In conclusion,our study indicated the effects of the immunomodulation caused by the aqueous extract from T.diversifolia,including anti-PHA-M-induced PBMCs proliferation and anti-LPS-induced nitric oxide production from macrophages in the range of non-cytotoxic concentrations,which were studied. Although an anti-oxidative capacity of the T.diversifolia extract was found in the ABTS and DPPH assays,it could not be shown in our cell culture model.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This project was supported by the Institute of Research and Development,Walailak University,Thailand(GrantNo. WU55304).
References
[1]Schaue D,Kachikwu EL,McBride WH.Cytokines in radiobiological responses:a review.Radiat Res 2012;178(6):505-23.
[2]Lundberg JO,Carlstr¨om M,Larsen FJ,Weitzberg E.Roles of dietary inorganic nitrate in cardiovascular health and disease.Cardiovasc Res 2011;89(3):525-32.
[3]Jones DP,Sies H.The redox code.Antioxid Redox Signal 2015;23(9):734-46.
[4]Penticuff JC,Kyprianou N.Therapeutic challenges in renal cell carcinoma.Am J Clin Exp Urol 2015;3(2):77-90.
[5]Duarte JM.Metabolic alterations associated to brain dysfunction in diabetes.Aging Dis 2015;6(5):304-21.
[6]Casas-Grajales S,Muriel P.Antioxidants in liver health.World J Gastrointest Pharmacol Ther 2015;6(3):59-72.
[7]Barjaktarevic IZ,Arredondo AF,Cooper CB.Positioning new pharmacotherapies for COPD.Int J Chron Obstruct Pulmon Dis 2015;10:1427-42.
[8]Maehre HK,Jensen IJ,Elvevoll EO,Eilertsen KE.ω-3 Fatty acids and cardiovascular diseases:effects,mechanisms and dietary relevance.Int J Mol Sci 2015;16(9):22636-61.
[9]Hermann A,Sitdikova GF,Weiger TM.Oxidative stress and maxi calcium-activated potassium(BK)channels.Biomolecules 2015;5(3):1870-911.
[10]Go YM,Jones DP.Cysteine/cystine redox signaling in cardiovascular disease.Free Radic Biol Med 2011;50(4):495-509.
[11]Radi R.Peroxynitrite,a stealthy biological oxidant.J Biol Chem 2013;288(37):26464-72.
[12]Sharrock RA,Sinclair FL,Gliddon C,Rao IM,Barrios E,Mustonen PJ,et al.A global assessment using PCR techniques of mycorrhizal fungal populations colonising Tithonia diversifolia. Mycorrhiza 2004;14(2):103-9.
[13]Maregesi SM,Ngassapa OD,Pieters L,Vlietinck AJ.Ethnopharmacological survey of the Bunda district,Tanzania:plants used totreatinfectiousdiseases.JEthnopharmacol2007;113(3):457-70.
[14]Wambugu SN,Mathiu PM,Gakuya DW,Kanui TI,Kabasa JD,KiamaSG.Medicinalplantsusedinthemanagementofchronicjoint painsinMachakosandMakuenicounties,Kenya.JEthnopharmacol 2011;137(2):945-55.
[15]Owoyele VB,Wuraola CO,Soladoye AO,Olaleye SB.Studies on the anti-inflammatory and analgesic properties of Tithonia diversifolia leaf extract.J Ethnopharmacol 2004;90(2-3):317-21.
[16]Stangeland T,Alele PE,Katuura E,Lye KA.Plants used to treat malaria in Nyakayojo sub-county,western Uganda.J Ethnopharmacol 2011;137(1):154-66.
[17]Miura T,Nosaka K,Ishii H,Ishida T.Antidiabetic effect of Nitobegiku,the herb Tithonia diversifolia,in KK-Ay diabetic mice. Biol Pharm Bull 2005;28(11):2152-4.
[18]Goffin E,Ziemons E,De Mol P,de Madureira Mdo C,Martins AP,da Cunha AP,et al.In vitro antiplasmodial activity of Tithonia diversifolia and identification of its main active constituent:tagitinin C.Planta Med 2002;68(6):543-5.
[19]Oyewole IO,Magaji ZJ,Awoyinka OA.Biochemical and toxicological studies of aqueous extract of Tithonia diversifolia(Hemsl.)leaves in Wistar albino rats.J Med Plants Res 2007;1(2):30-3.
[20]ElufioyeTO,Alatise OI,FakoyaFA,AgbedahunsiJM,Houghton PJ.Toxicity studies of Tithonia diversifolia A.Gray(Asteraceae)in rats.J Ethnopharmacol 2009;122(2):410-5.
[21]Booke M,Hinder F,McGuire R,Traber LD,Traber DL.Selective inhibition of inducible nitric oxide synthase:effects on hemodynamics and regional blood flow in healthy and septic sheep.Crit Care Med 1999;27(1):162-7.
[22]ReR,PellegriniN,ProteggenteA,PannalaA,YangM,Rice-EvansC. Antioxidant activity applying an improved ABTS radical cation decolorization assay.Free Radic Biol Med 1999;26(9-10):1231-7.
[23]Kim DO,Lee KW,Lee HJ,Lee CY.Vitamin C equivalent antioxidant capacity(VCEAC)of phenolic phytochemicals.J Agric Food Chem 2002;50(13):3713-7.
[24]Elufioye TO,Agbedahunsi JM.Antimalarial activities of Tithonia diversifolia(Asteraceae)and Crossopteryx febrifuga(Rubiaceae)on mice in vivo.J Ethnopharmacol 2004;93(2-3):167-71.
[25]Zhao G,Li X,Chen W,Xi Z,Sun L.Three new sesquiterpenes from Tithonia diversifolia and their anti-hyperglycemic activity. Fitoterapia 2012;83(8):1590-7.
[26]Lin HR.Sesquiterpene lactones from Tithonia diversifolia act as peroxisome proliferator-activated receptor agonists.Bioorg Med Chem Lett 2012;22(8):2954-8.
[27]Liao MH,Lin WC,Wen HC,Pu HF.Tithonia diversifolia and its main active component tagitinin C induce survivin inhibition and G2/M arrest in human malignant glioblastoma cells.Fitoterapia 2011;82(3):331-41.
[28]Lee MY,Liao MH,Tsai YN,Chiu KH,Wen HC.Identification and anti-human glioblastoma activity of tagitinin C from Tithonia diversifolia methanolic extract.J Agric Food Chem 2011;59(6):2347-55.
[29]Liao MH,Tsai YN,Yang CY,Juang CL,Lee MY,Chang LH,et al.Anti-human hepatoma Hep-G2 proliferative,apoptotic,and antimutagenic activity of tagitinin C from Tithonia diversifolia leaves.J Nat Med 2013;67(1):98-106.
[30]Herrera J,Troncone G,S´anchez MR,Miguel V,Lopez SE.The effect of furanoheliangolides from Tithonia diversifolia on superoxide anion generation in human neutrophils.Fitoterapia 2007;78(7-8):465-9.
[31]Chagas-Paula DA,Oliveira RB,da Silva VC,Gobbo-Neto L,Gasparoto TH,Campanelli AP,et al.Chlorogenic acids from Tithonia diversifolia demonstrate better anti-inflammatory effect than indomethacin and its sesquiterpene lactones.J Ethnopharmacol 2011;136(2):355-62.
[32]Bork PM,Schmitz ML,Weimann C,Kist M,Heinrich M.Nahua indian medicinal plants(Mexico):inhibitory activity on NF-κB as an anti-inflammatory model and antibacterial effects.Phytomedicine 1996;3(3):263-9.
[33]R¨ungeler P,Castro V,Mora G,G¨oren N,Vichnewski W,Pahl HL,et al.Inhibition of transcription factor NF-κB by sesquiterpene lactones:a proposed molecular mechanism of action.Bioorg Med Chem 1999;7(11):2343-52.
[34]R¨ungeler P,Lyss G,Castro V,Mora G,Pahl HL,Merfort I.Study of three sesquiterpene lactones from Tithonia diversifolia on their anti-inflammatory activity using the transcription factor NF-κB and enzymes of the arachidonic acid pathway as targets.Planta Med 1998;64(7):588-93.
[35]Ajaiyeoba EO,Abiodun OO,Falade MO,Ogbole NO,Ashidi JS,Happi CT,et al.In vitro cytotoxicity studies of 20 plants used in Nigerian antimalarial ethnomedicine.Phytomedicine 2006;13(4): 295-8.
[36]Passoni FD,Oliveira RB,Chagas-Paula DA,Gobbo-Neto L,Da Costa FB.Repeated-dose toxicological studies of Tithonia diversifolia(Hemsl.)A.gray and identification of the toxic compounds. J Ethnopharmacol 2013;147(2):389-94.
[37]Piao S,Cha YN,Kim C.Taurine chloramine protects RAW264.7 macrophages against hydrogen peroxide-induced apoptosis by increasing antioxidants.J Clin Biochem Nutr 2011;49(1):50-6.
[38]Chen YW,Hwang KC,Yen CC,Lai YL.Fullerene derivatives protect against oxidative stress in RAW264.7 cells and ischemiareperfused lungs.Am J Physiol Regul Integr Comp Physiol 2004;287(1):R21-6.
19 Oct 2015
in revised form 6 Nov,2nd
Poonsit Hiransai,Molecular Medicine and Cancer Biology Research Unit,School of Allied Health Sciences and Public Health,Walailak University,Nakhon Si Thammarat 80161,Thailand.
Tel:+66 75672104
Fax:+66 75672105
E-mails:poonsit.hi@wu.ac.th,poonsit.hi@gmail.com
The human peripheral blood mononuclear cells were collected from healthy volunteers under the project approval by the Ethical Committee on Human Rights Related to Researches Involving Human Subjects,Walailak University(Protocol number:11/055).
Foundation Project:Supported by the Institute of Research and Development,Walailak University,Thailand(Grant No.WU55304).
Peer review under responsibility of Hainan Medical University.The journal implements double-blind peer review practiced by specially invited international editorial board members.
 Asian Pacific Journal of Tropical Biomedicine2016年11期
Asian Pacific Journal of Tropical Biomedicine2016年11期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Human and animal sarcocystosis in Malaysia∶A review
- Therapeutic applications of collagenase(metalloproteases)∶A review
- Cytotoxic activity and phytochemical standardization of Lunasia amara Blanco wood extract
- Antiacanthamoebic properties of natural and marketed honey in Pakistan
- GC-MS analysis of bioactive compounds present in different extracts of an endemic plant Broussonetia luzonica(Blanco)(Moraceae)leaves
- Anthelmintic activity of Saba senegalensis(A.DC.)Pichon(Apocynaceae)extract against adult worms and eggs of Haemonchus contortus
