Anthelmintic activity of Saba senegalensis(A.DC.)Pichon(Apocynaceae)extract against adult worms and eggs of Haemonchus contortus
Mohamed Bonewende´ Belemlilga,Aristide Traore´,Sylvin Oue´draogo,Adama Kabore´,Hamidou Hamadou Tamboura,Innocent Pierre Guissou1,
1Laboratoire de D´eveloppement du M´edicament,Centre de recherche sur le m´edicament,Ecole Doctorale de la Sant´e,Universit´e de Ouagadougou,Ecole Doctorale de Sant´e,03 BP 7021 Ouagadougou 03,Burkina Faso
2D´epartement M´edecine Pharmacop´ee Traditionnelles-Pharmacie(MEPHATRA-Ph),Institut de Recherche en Sciences de la Sant´e(IRSS/CNRST),03 BP 7192 Ouagadougou 03,Burkina Faso
3D´epartement Productions Animales,Institut de l'Environnement et de Recherches Agricoles(INERA/CNRST),04 BP 8645 Ouagadougou 04,Burkina Faso
Anthelmintic activity of Saba senegalensis(A.DC.)Pichon(Apocynaceae)extract against adult worms and eggs of Haemonchus contortus
Mohamed Bonewende´ Belemlilga1,2*,Aristide Traore´2,Sylvin Oue´draogo2,Adama Kabore´3,Hamidou Hamadou Tamboura3,Innocent Pierre Guissou1,2
1Laboratoire de D´eveloppement du M´edicament,Centre de recherche sur le m´edicament,Ecole Doctorale de la Sant´e,Universit´e de Ouagadougou,Ecole Doctorale de Sant´e,03 BP 7021 Ouagadougou 03,Burkina Faso
2D´epartement M´edecine Pharmacop´ee Traditionnelles-Pharmacie(MEPHATRA-Ph),Institut de Recherche en Sciences de la Sant´e(IRSS/CNRST),03 BP 7192 Ouagadougou 03,Burkina Faso
3D´epartement Productions Animales,Institut de l'Environnement et de Recherches Agricoles(INERA/CNRST),04 BP 8645 Ouagadougou 04,Burkina Faso
ARTICLE INFO
Article history:
revised form 5 Dec 2014,3rd revised
form 3 Jul,4th revised form 27 Aug
2015,5th revised form 29 Jan 2016
Accepted 10 Jul 2016
Available online 14 Sep 2016
Saba senegalensis
Anthelmintic properties
Haemonchus contortus
In vitro
Burkina Faso
Objective:To evaluate the anthelmintic property of Saba senegalensis(A.DC)Pichon(Apocynaceae)(S.senegalensis)on Haemonchus contortus that is traditionally used in Burkina Faso for its gastrointestinal parasites treatment.
Methods:The lyophilized aqueous decoction of leaves of S.senegalensis at concentrations of 0.10,1.00,3.00,10.00 and 15.00 mg/mL was used on eggs and adult worms of Haemonchus contortus collected from gastrointestinal tract of small ruminant.
Results:The LC50on adult worms was 6.79 mg/mL and 3.25 mg/mL for the leaves of S.senegalensis and the levamisole(reference drug),respectively.Inhibition of hatching assay showed a concentration-dependent manner with an inhibition of 93.63%at the concentration of 15.00 mg/mL of S.senegalensis.
Conclusions:These results indicate that the aqueous extract of S.senegalensis possesses an anthelmintic property and may justify its use in traditional medicine for the treatment of gastrointestinal parasites.
1.Introduction
Neglected tropical diseases affect more than one billion people,mostly living in hot climates[1].They represent a major public health and economic importance to both man and animals throughout the tropics and especially in developing countries.Nowadays,the parasitic diseases constitute a growing and frequent human and animal health stress by causing serious economic losses in livestock farming[2].In fact,they are responsible for huge economic losses,cause stunting of individuals and their long-term effects induce debilitating nature of pathology[1].In parasitized hosts,the presence of worms is mostly related by gastrointestinaldisorders(diarrhea,abdominal pain),respiratory disorders(cough),etc.In addition to these ailments,we note most of the time,severe anemia and a deficiency of vitamins A[1].In tropical regions,helminthiases represent the most common parasitic illness[1]. The main strategy of prevention is based on the practice of daily hygiene,nutrition and the use of anthelmintic drugs quite expensive for the rural populations[1].In addition to the importance of economic losses caused by these diseases,the use of anthelmintic drugs led to the development of resistance to available molecules[3].
Tel:+226 70240809,+226 78081022
E-mail:medilga@yahoo.fr
The tests in this work were performed according to the protocol approved by the ethical statement of the committee of Institute for Research in Health Sciences(IRSS).
Foundation Project:Supported by the Ministry of Health through FARES project(P1/FARES 2013).
Peer review under responsibility of Hainan Medical University.The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright©2016 Hainan Medical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
Thus,the search for more effective and accessible drugs through traditional medicine is necessary to fight against these intestinal parasites.Like other developing countries,plants are widely used in traditional medicine for the treatment of various human and animal diseases such as parasitosis[4].Antibacterial and antiparasitic activities of decoctions of the leaves of Saba senegalensis(A.DC)Pichon(S.senegalensis)were reported in Guinea, Burkina Faso and Ivory Coast following ethnobotanical surveys respectively by Magassouba et al.[5],Traore et al.[6],Kon´e and Atindehou[7],which indicated that these plants would have antiparasitic property against gastrointestinal parasites.
The leaves of Azadirachta indica and stem barks extract of Bridelia ferruginea are used against gastrointestinal nematodes[8,9].The crude aqueous extracts of the leaves of Carissa spinarum,Azadirachta indica,Myrsine africana and Rhus glabrous indicate potential anthelmintic effect on Haemonchus contortus(H.contortus)[10,11].Moreover,in vitro activities of acetonic extracts from leaves of three forage legumes(Calliandra calotyrsus,Gliricidia sepium and Leucaena diversifolia)on H.contortus revealed an anthelmintic activity on the same parasite[12]while the methanolic extract of flower of Mallotus philippensis possessed potent anthelmintic property against the third stage larvae of H.contortus[13].
In Burkina Faso,more than 427plant species were usedby the Mossi populations for the treatment of various diseases including parasites but their scientific results were not all documented[4]. Several studies showed anthelmintic properties of medicinal herbs such as the extracts of Anogeissus leiocarpus and Daniellia oliveri that indicate some effects on the eggs and adult wormsofH.contortus[14].Likely,extractsofCassia sieberiana,Guiera senegalensis and Sapium grahamii were used in Burkina Faso traditional medicine for their anthelmintic effect on adult worms and eggs of H.contortus[15].In this study,we evaluate the anthelmintic property of S.senegalensis(Apocynaceae)on H.contortus that is traditionally used in Burkina Faso for its gastrointestinal parasites treatment.
2.Materials and methods
2.1.Plant material
Leaves of S.senegalensis were collected around Bassinko district about 30 km at the north of Ouagadougou,in July 2012. A sample of the plant was identified by plant taxonomist at the herbarium of the National Center for Scientific and Technological Research and a voucher specimen was deposited under No.00223 HNBU.The leaves of plants were air dried at room temperature,powdered using pestle and mortar and kept in amber colored bottle until use in order to keep all their physicochemical properties.
2.2.Plant extract
Extraction was conducted at the chemical laboratory of the Department of Medicine and Traditional Pharmacopoeia-Pharmacy at the Institute for Research in Health Sciences.A decoction of S.senegalensis was prepared by soaking a weighed amount of the dry powder(50 g)in distilled water(500 mL)and the mixture was boiled for 45 min.After freeze,the decoction obtained was filtered through a nylon cloth and then centrifuged at 2000 r/min for 5 min.The supernatant was collected and a portion was concentrated in an oven at 50°C for 24 h,congealed and then lyophilized.The lyophilized dry powder was then collected in a stoppered sample vial,weighed and kept in a desiccator to avoid absorption of water until use in the assay.
2.3.In vitro anthelmintic assays
Adult worms of H.contortus from stomachs or goats or naturally infected sheep from Ouagadougou slaughterhouse have been used for biological tests(method described by Ademola and Eloff)[16].
Briefly,the stomachs were incised longitudinally by using scissors to release the adult worms.These were carefully collected and placed in a Petri dish containing a solution of phosphate buffer saline(PBS,pH 7.2).Worm's perennials were selected,feces were cleaned by PBS and immediately used for biological tests.
2.3.1.Adult worms motility assay
The adult worms were distributed in Petri dishes(3 worms per Petri dish)in the presence of increasing concentrations(0.1,1,3,10 and 15 mg/mL in PBS)of the plant extract. Levamisole was used as the positive reference substance(1% w:v)and PBS(3 mL)was used as negative control.The assay of the effect of the extract and reference substances on adult worms was performed at 37°C for 24 h during which motility and survival worms were observed after 2 h,4 h,6 h and 24 h incubation.
However,6 h after incubation,the adult worms in the presence of the different concentrations of the extract and levamisole were returned to the PBS solution for 30 min to observe the possible resumption of motility.
The number of dead worms versus time was evaluated.The test was performed in triplicate and repeated five times.
The mortality rate for each concentration of the extract was determined using the ratio:

2.3.2.Egg hatch assay
The technique described by Ademola and Eloff[16]was used. Briefly,adult worms were collected and washed in PBS(pH 7.2).They were then lightly crushed in a mortar to release the eggs and the resulting mixture was filtered using two mesh sieve(1 mm and 100μm,respectively).A third sieve of 38μm was used to retain the released eggs.The sieve was then returned,the opposite side has been washed with distilled water and the eggs were collected in a Petri dish and then distributed at a rate of 1000 eggs/mL.
The in vitro anthelmintic activity of the plant extract on the egg hatching of H.contortus was carried out according to a modification of the method described by Ademola and Eloff[17]. After extracted from faeces,eggs suspension(100μL)was distributed in a 24-flat-bottomed microplate so that each well contained 1000 fresh eggs and mixed with 1900μL of lyophilized extract of S.senegalensis at final concentrations of 0.1,1,3,10 and 15 mg/mL.In addition,a negative control(PBS,pH 7.2)and a positive control(levamisole at 1%w:v in PBS)were also included in the assay.
After 48 h of incubation at 25°C,eggs hatching were stopped by adding formaldehyde in each well.The number of dead or living larvae and eggs per well was then counted under a microscope(Olympus BH-2 Optical Co.Ltd,Japan)at 40× magnification.There were three replicates for control and each concentration which were then repeated five times.The percentage of hatched eggs was calculated using the ratio:
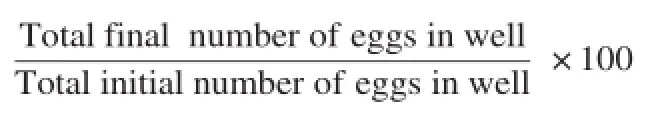
2.4.Statistical analysis
The mean percentages of mortality and inhibition of hatching were subjected to analysis with the Prism 5.0 software.The different figures were drawn and LC50and upper confidence limits and lower reliability were determined using the same Prism 5.0 software.The results of the pharmacological study were expressed as mean±SEM.Changes were considered as significant when the probability of error(P)was less than 0.05(P<0.05).
3.Results
3.1.Adult worms motility assay
The anthelmintic activity of S.senegalensis and levamisole was increased with incubation time and concentration of the extract(Tables1and2).All the concentrations of S.senegalensis showed inhibitory effect on the survival of H.contortus in a dose dependent manner.The results showed that the extract of S.senegalensis caused mortality of adult worms in a dose dependent manner.Concentrations between 0.10 and 3.00 mg/mL had no effect on motility of worms up to4 h of exposure while the concentrations of 10.00 and 15.00 mg/ mL caused a similar mortality(2.22%)on them(Table 1 and Figure 1).It should be noted that the treatment of S.senegalensis extract induced a rigid posture or completely immobilized adult worms beyond 4 h.While the minimum concentration of 0.10 mg/mL killed 29.00%of the adult worms after 24 h,a concentration of 15.00 mg/mL killed 97.77%of the worms by the end of the experiment.However,up to 2 h of incubation in the presence of the extract of S.senegalensis,no effect on motility or mortality of H.contortus adult parasites was shown.

Table 1Evolution of thepercentage of mortality of adult worms H.contortus after 4,6 and 24 h of contact with different concentrations of aqueous decoction of the leaves of S.senegalensis.
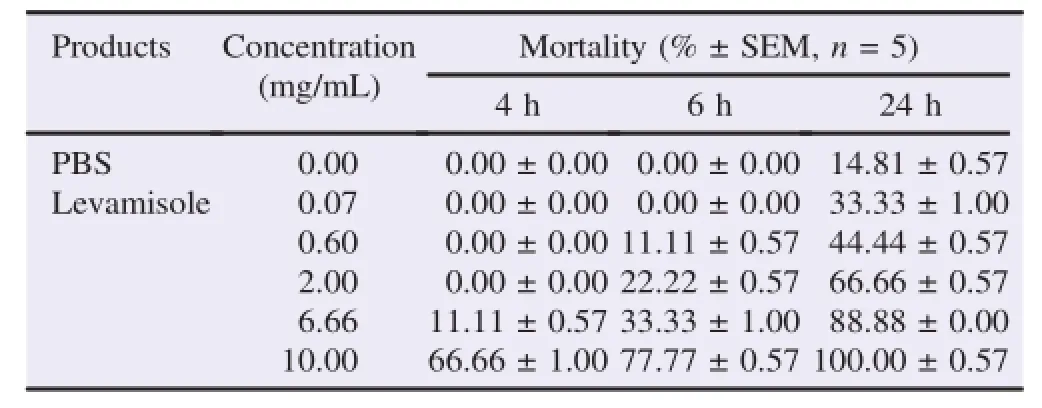
Table 2Evolution of thepercentage of mortality of adult worms H.contortus after 4,6 and 24 h of contact with different concentrations of levamisole.
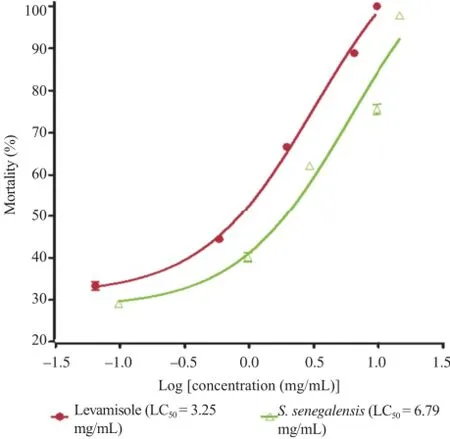
Figure 1.Graph showing the effects of aqueous decoction of the leaves of S.senegalensis and levamisole on the mortality of adult worms of H.contortus in 24 h.
Levamisole induced mortality among treatment according to the time and concentration-dependent with 100%mortality of adults parasites at 10.00 mg/mL(Table 2 and Figure 1).
PBS used as a negative control only induced slight mortality to the order of 14.81%after 24 h of incubation.The concentrations causing the death of 50%(LC50)worms were 6.79 mg/ mL and 3.25 mg/mL for S.senegalensis and levamisole,respectively(Figure 1).
3.2.Egg hatch assay
The results of the percentage of the eggs hatch inhibition of H.contortus based on different concentrations of S.senegalensis and levamisole are shown in Tables 3 and 4.The inhibition of hatching in the presence of S.senegalensis extract was in aconcentration-dependent manner.The concentration of 0.10 mg/ mL of the extract induced inhibition of 31.81%while the maximum concentration 15.00 mg/mL caused inhibition of hatching of 93.63%.PBS had blocked the hatching of parasite eggs for 18.10%.However,levamisole caused a maximal inhibitionof22.72%withthelowconcentrationof0.07mg/mLwhile high concentrations were less efficacy with 12.72%and 0.00%,respectively for the concentrations of 6.66 and 10.00 mg/mL.
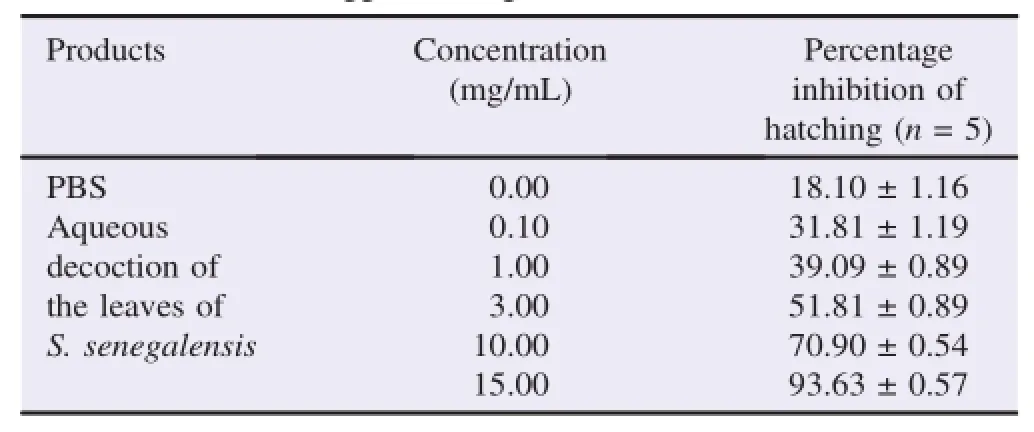
Table 3Effects of aqueous decoction of the leaves of S.senegalensis at different concentrations on the eggs hatching of H.contortus.
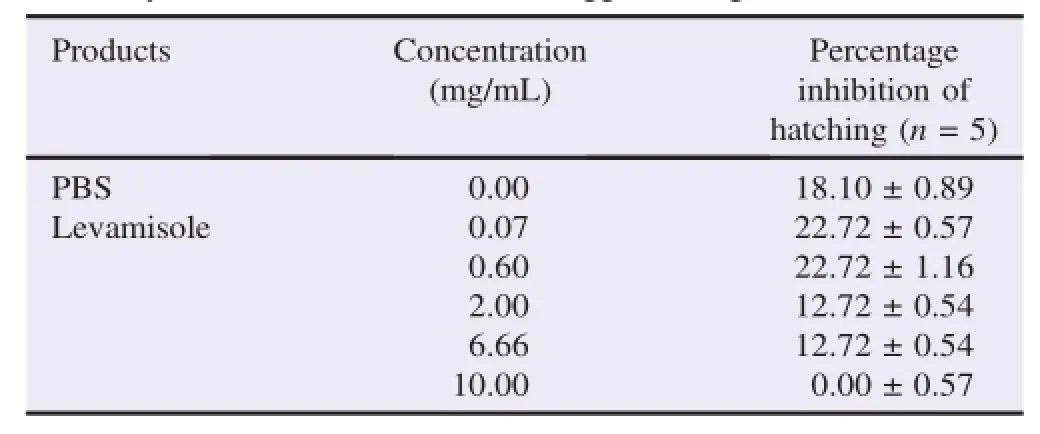
Table 4Inhibitory effects of levamisole on the egg hatching of H.contortus.
4.Discussion
The aqueous decoction of S.senegalensis exhibited anthelmintic activity on in vitro studies against eggs hatching and adult worms of H.contortus.The aqueous decoction of leaves of S.senegalensis on adult worms of H.contortus produced a mortality of 97.77%at a concentration of 15.00 mg/mL and its LC50was 6.79 mg/mL.This anthelmintic effect of the plant extract may attribute to its content of tannins since several studies indicated that these chemical compounds possess the capacity to bind free protein present in the tubes for larval nutrition that can lead to death[18].Other studies indicated that anthelmintic drug may diffuse through the intestinal cells to cause their death[19].Moreover,tannins are well known to possess antiviral[20],antibacterial and anthelmintic activities[21,22].However,at the same concentration of 10.00 mg/mL,the dose-effect curve for levamisole reached 100%mortality ofadultwormsofH.contortuswhiletheextractof S.senegalensis and the negative control PBS were 97.77%and 14.81%after 24 h incubation.It is evident from these results that levamisole(LC50=3.25 mg/mL)used as a positive control had better activity compared with extract of S.senegalensis but with slight difference(Figure 1).
Phytochemical screening of S.senegalensis plant extract showed the presence of saponins,triterpene glycoside and steroid which might contribute to the anthelmintic activity[23-25]with an independent or synergistic effect[26,27].
The observation of adult worms after treatment shows a reducing in their motility which could have been prevented by the terpenoid compounds present in the extract[19].
In the current study,aqueous extract of S.senegalensis possessed an inhibitory effect on eggs hatching of H.contortus(Table 3).Indeed,the extract exhibited inhibition 31.81±1.19 and 93.63±0.57 of eggs hatchability at 0.10 mg/mL and 15.00 mg/mL,respectively.
In the PBS solution,18.10%of the eggs did not hatch. However,eggs hatching need to undergo maturation and therefore require an optimum temperature(20-30°C)and nonhatching may be due to poor eggs.The average value of inhibition of eggs hatching obtained at 10.00 mg/mL of levamisole is 12.72%while no larva was observed in the solution.This value contrasts with the properties of this drug which is recognized as an anthelmintic reference.This suggests that levamisole has no inhibitory effect on H.contortus eggs or it induces a lysis of eggs by diffusion through the shell egg,hence the decrease in the number recorded based on concentrations.Thus,egg hatch activity of S.senegalensis or levamisole may be explained by its possible capacity directly binding to the lipoproteins of the eggshell membrane which induces better permeability leading to their hatching.Likewise,this activity could be due to the binding of plant extract on egg hatching enzymes which raises the rate of hatching[19,28,29].
In the literature,few previous studies have been done on the anthelmintic activity of S.senegalensis.Indeed,an ethnobotanical survey conducted in the Ferkessedougou region(Northern Cˆote d'Ivoire)showed that S.senegalensis was one of the most used in traditional veterinary medicine and demonstrated its activity against nematodes[7].Further results showed that the roots,leaves and fruits of Tabernaemontana citrifolia,species belonging to the same family as S.senegalensis possess similar results on eggs and adult worms of H.contortus[30].
The present in vitro study of the effects of aqueous decoction of leaves of S.senegalensis showed anthelmintic activity on adult worms and possessed an inhibitory effect on eggs hatching of H.contortus.Taking together,S.senegalensis would have anthelmintic properties that may justify its use in traditional medicine to treat gastrointestinal parasites.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors wish to express their sincere gratitude to Department of Medicine and Traditional Pharmacopoeia-Pharmacy of Institute for Research in Health Sciences where the tests were performed.We also thank the Ministry of Health for financial support for the achievement of our work through FARES project(P1/FARES 2013).
References
[1]World HealthOrganization.Workingto overcometheglobal impact ofneglectedtropicaldiseases:firstWHOreportonneglectedtropical diseases.Geneva:World Health Organization;2010.[Online]Available from:http://apps.who.int/iris/bitstream/10665/44440/1/ 9789241564090_eng.pdf[Accessed on 20th January,2016]
[2]Stepek G,Buttle DJ,Duce IR,Lowe A,Behnke JM.Assessment of the anthelmintic effect of natural plant cysteine proteinases against the gastrointestinal nematode, Heligmosomoides polygyrus,in vitro.Parasitology 2005;130(2):203-11.
[3]Chandra S,Prasad A,Sankar M,Yadav N,Dalal S.Molecular diagnosis of benzimidazole resistance in Haemonchus contortus in sheep from different geographic regions of North India.Vet World 2014;7:337-41.
[4]Nacoulma-Ou´edraogo OG.Medicinal plants and traditional medical practices in Burkina Faso[dissertation].Ouagadougou:University of Ouagadougou;1996.
[5]Magassouba FB,Diallo A,Kouyat´e M,Mara F,Mara O,Bangoura O,et al.Ethnobotanical survey and antibacterial activity of some plants used in Guinean traditional medicine. J Ethnopharmacol 2007;114:44-53.
[6]Traore A,Ouedraogo S,Lompo M,Traore S,Some N,Guissou IP. Ethnobotanicalsurveyofmedicinalplantsusedtotreatgastrointestinalparasites in human and livestock in four geographic areas of Burkina Faso(West Africa).Arch Appl Sci Res 2013;5(6):172-7.
[7]Kon´e WM,Atindehou KK.Ethnobotanical inventory of medicinal plants used in traditional veterinary medicine in Northern Cˆote d'Ivoire(West Africa).S Afr J Bot 2008;74:76-84.
[8]Sawleha Q,Dixit AK,Dixit P.Use of medicinal plants to control Haemonchus contortus infection in small ruminants.Vet World 2010;3(11):515-8.
[9]Lasisi AA,Kareem SO.Evaluation of anthelmintic activity of the stem bark extract and chemical constituents of Bridelia ferruginae(Benth)Euphorbiaceae.Afr J Plant Sci 2011;5(8):469-74.
[10]Getachew S,Ibrahim N,Abebe B,Eguale T.In vitro evaluation of anthelmintic activities of crude extracts of selected medicinal plants against Haemonchus contortus in Alemgena Wereda,Ethiopia. Acta Parasitol Globalis 2012;3(2):20-7.
[11]Abdi M,Abebe W,Mirutse G,Getachew T,Nigatu K.In vitro anthelminthic activities of four medicinal plants against Haemonchus contortus.Afr J Plant Sci 2013;7(8):369-73.
[12]Wabo Pon´e J,Kenne Tameli F,Mpoame M,Pamo Tedonkeng E,Bilong Bilong CF.In vitro activities of acetonic extracts from leaves of three forage legumes(Calliandra calotyrsus,Gliricidia sepium and Leucaena diversifolia)on Haemonchus contortus. Asian Pac J Trop Med 2011;4:125-8.
[13]Deepa CK,Darsana U,Sujith S,Priya MN,Juliet S.Effect of Mallotus phillipensis flower extracts against the third stage larvae of Haemonchus contortus.Int J Appl Pure Sci Agric 2015;1(3): 19-23.
[14]Kabor´e A.[Anthelmintic activity of two tropical plants tested in vitro and in vivo in gastro-intestinal strongly in sheep race Mossi of Burkina Faso][dissertation].Bobo-dioulasso:University Polytechnique of Bobo-dioulasso;2009.French.
[15]Traor´e A,Ouedraogo S,Kabor´e A,Tamboura HH,Guissou IP.The acute toxicity in mice and the in vitro anthelminthic effects on Haemonchus contortus of the extracts from three plants(Cassia sieberiana,Guiera senegalensis and Sapium grahamii)used in traditional medicine in Burkina Faso.Ann Biol Res 2014;5(2):41-6.
[16]Ademola IO,Eloff JN.In vitro anthelmintic effect of Anogeissus leiocarpus(DC.)Guill.&Perr.leaf extracts and fractions on developmental stages of Haemonchus contortus.Afr J Tradit Complement Altern Med 2011;8(2):134-9.
[17]Ademola IO,Eloff JN.In vitro anthelmintic activity of Combretum molle(R.Br.ex G.Don)(Combretaceae)against Haemonchus contortus ova and larvae.Vet Parasitol 2010;169:198-203.
[18]Martinez-Ortiz-de-Montellano C,Vargas-Magana JJ,Canul-Ku HL,Miranda-Soberanis R,Capetillo-Leal C,Sandoval-Castro CA,et al. Effect of a tropical tannin-rich Lysiloma latisiliquum on adult populations of Haemonchus contortus in sheep.Vet Parasitol 2010;172:283-90.
[19]Ahmed MA,Basha NA,Nsahlai IV.Wattle tannins as control strategy for gastrointestinal nematodes in sheep.Afr J Agric Res 2014;9(28):2185-9.
[20]Cheng HY,Lin CC,Lin TC.Antiherpes simplex virus type 2 activity of casuarinin from the bark of Terminalia arjuna Linn. Antiviral Res 2002;55:447-55.
[21]Perumal Samy R,Ignacimuthu S,Sen A.Screening of 34 Indian medicinal plants for antibacterial properties.J Ethanopharmacol 1998;62:173-82.
[22]Molan AL.Effect of purified condensed tannins from pine bark on larval motility,egg hatching and larval development of Teladorsagia circumcincta and Trichostrongylus colubriformis(Nematoda:Trichostrongylidae).Folia Parasitol(Praha)2014;61(4): 371-6.
[23]Katiki LM,Ferreira JF,Gonzalez JM,Zajac AM,Lindsay DS,Chagas AC,et al.Anthelmintic effect of plant extracts containing condensed and hydrolyzable tannins on Caenorhabditis elegans,and their antioxidant capacity.Vet Parasitol 2013;192:218-27.
[24]Mondal H,Hossain H,Awang K,Saha S,Rashid SMU,Islam MK,et al.Anthelmintic activity of ellagic acid,a major constituent of Alternanthera sessilis against Haemonchus contortus.Pak Vet J 2015;35(1):58-62.
[25]Sirama V,Kokwaro J,Owuor B,Yusuf A,Kodhiambo M.In vitro anthelmintic activity of Vernonia amygdalina Del.(Asteraceae)roots using adult Haemonchus contortus worms.Int J Pharmacol Res 2015;5:1-7.
[26]Quijada J,Fryganas C,Ropiak HM,Ramsay A,Mueller-Harvey I,Hoste H.Anthelmintic activities against Haemonchus contortus or Trichostrongylus colubriformis from small ruminants are influenced by structural features of condensed tannins.J Agric Food Chem 2015;63(28):6346-54.
[27]Molefe NI,Tsotetsi AM,Ashafa AOT,Thekisoe OMM.In vitro anthelmintic activity of Cotyledon orbiculata,Hermannia depressa and Nicotiana glauca extracts against parasitic gastrointestinal nematodes of livestock.J Med Plants Res 2013;7(9):536-42.
[28]Tibe O,Sutherland IA,Lesperance L,Harding DR.The effect of purified condensed tannins of forage plants from Botswana on the free-living stages of gastrointestinal nematode parasites of livestock.Vet Parasitol 2013;197(1-2):160-7.
[29]Macedo IT,Bevilaqua CM,de Oliveira LM,Camurça-Vasconcelos AL,Morais SM,Machado LK,et al.In vitro activity of Lantana camara,Alpinia zerumbet,Mentha villosa and Tagetes minuta decoctions on Haemonchus contortus eggs and larvae.Vet Parasitol 2012;190(3-4):504-9.
[30]Marie-MagdeleineC,MahieuM,D'AlexisS,PhilibertL,Archimede H.In vitro effects of Tabernaemontana citrifolia extracts on Haemonchus contortus.Res Vet Sci 2010;89:88-92.
15 Oct 2014
in revised form 29 Oct,2nd
Original article http://dx.doi.org/10.1016/j.apjtb.2016.07.015
Mohamed Bonewend´e Belemlilga,Laboratoire de D´eveloppement du M´edicament,Centre de recherche sur le m´edicament,Universit´e de Ouagadougou,Ecole Doctorale de Sant´e,03 BP 7021 Ouagadougou 03,Burkina Faso.
 Asian Pacific Journal of Tropical Biomedicine2016年11期
Asian Pacific Journal of Tropical Biomedicine2016年11期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Human and animal sarcocystosis in Malaysia∶A review
- Therapeutic applications of collagenase(metalloproteases)∶A review
- Cytotoxic activity and phytochemical standardization of Lunasia amara Blanco wood extract
- Antiacanthamoebic properties of natural and marketed honey in Pakistan
- GC-MS analysis of bioactive compounds present in different extracts of an endemic plant Broussonetia luzonica(Blanco)(Moraceae)leaves
- Anti-nitric oxide production,anti-proliferation and antioxidant effects of the aqueous extract from Tithonia diversifolia
