Antiacanthamoebic properties of natural and marketed honey in Pakistan
Farzana Abubakar Yousuf,Malik Hassan Mehmood,Abdul Malik,Ruqaiyyah Siddiqui,Naveed Ahmed Khan*
1Department of Biological and Biomedical Sciences,Aga Khan University,Karachi,Pakistan
2Department of Biological Sciences,Faculty of Science and Technology,Sunway University,Selangor,Malaysia
Antiacanthamoebic properties of natural and marketed honey in Pakistan
Farzana Abubakar Yousuf1,Malik Hassan Mehmood1,Abdul Malik1,Ruqaiyyah Siddiqui2,Naveed Ahmed Khan2*
1Department of Biological and Biomedical Sciences,Aga Khan University,Karachi,Pakistan
2Department of Biological Sciences,Faculty of Science and Technology,Sunway University,Selangor,Malaysia
ARTICLE INFO
Article history:
revised form 19 Mar 2016
Accepted 25 May 2016
Available online 17 Sep 2016
Honey
Acanthamoeba
Amoebicidal
Amoebistatic
Objective:To determine antiacanthamoebic activity of natural and marketed honey samples.
Methods:Natural honey samples were collected directly from the bee hive and marketed honey samples were purchased from the local market in Karachi,Pakistan.Both honey samples were tested for their flavonoid content(quercetin equivalent per gram of the extract)and phenolic content(gallic acid equivalent per gram).Furthermore,their antioxidant activity was determined by measuring 2,2-diphenyl-1-picrylhydrazyl.Using amoebistatic and amoebicidal assays,the effects of honey samples were tested against growth and viability of Acanthamoeba parasites.
Results:Natural honey exhibited potent amoebistatic and amoebicidal effects,in a concentration-dependent manner.Honey-treated Acanthamoeba castellanii showed loss of acanthopodia,following which amoebae detached,rounded up,reduced in size,decreased in cytoplasmic mass and they were observed floating in the culture medium. Importantly,honey-treated amoebae did not revive when inoculated in fresh growth medium,however,glycerol-treated amoebae exhibited viable trophozoite and active growth.In contrast,marketed honey samples varied in their efficacy against Acanthamoeba castellanii.The proportion of flavonoid,as determined by quercetin measurements and the proportion of phenolic,as determined by gallic acid measurements was higher in natural honey compared with marketed honey.Similarly,the antioxidant activity,as determined by 2,2-diphenyl-1-picrylhydrazyl scavenging activity was higher in natural honey vs.marketed honey.
Conclusions:This study shows that natural honey has antiacanthamoebic properties and possesses higher flavonoid,phenolic and antioxidant properties compared with the marketed honey.These findings are of concern to the public,health officials,and to the manufacturers regarding production of honey for medical applications.
1.Introduction
Honeyhasbeenusedasamedicinesinceancienttimesinmany cultures and communities.The major constituent of honey iscarbohydrates,especiallyfructoseandglucose(85%-95%oftotal sugars)[1],while other components present in minor quantities include organic acids,amino acids,proteins,enzymes,lipids,flavonoids and vitamins that are responsible for its multiple biological properties such as,wound healing,antibacterial effects against a wide range of pathogenic bacteria[2,3],antifungal[4,5],antiviral[2,3],antioxidant[6,7],antitumour[8]activities and various skin disorders[2,9].Antioxidants such as polyphenols and flavonoids are effective in reducing the risk of heart disease,cancer,inflammatory processes,asthma,infected wounds,chronic wounds,skin ulcers,and cataracts[2-10].This may explain widespread use of honey resulting in its production commercially,artificially,and through naturalbee hive. However,the composition and antioxidant capacity of honeydepends on various factors,principally the plant source used by the honey bees.Despite its broad-spectrum activities against a range of bacterial pathogens,honey has not been tested against protozoan pathogen,Acanthamoeba.Acanthamoeba castellanii(A.castellanii)is a free-living amoeba that is known to produce cutaneous infections,blinding keratitis and fatal encephalitis[11-13].In the present study,we determined antiacanthamoebic activity of natural honey collected directly from the bee hive and compared its effects with the marketed honey samples,both of them are accessible to the local community.Antioxidant properties(polyphenols and flavonoids)of natural vs.marketed honey were determined further.
Tel:+60 03 7491 8622,ext.7176
Fax:+60 03 5635 8630
E-mail:naveed5438@gmail.com
Foundation Project:Support provided by Aga Khan University,Pakistan,and Sunway University through INT-FST-DBS-2015 with grant No.005,Bandar Sunway,Malaysia.
Peer review under responsibility of Hainan Medical University.The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright©2016 Hainan Medical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
2.Materials and methods
2.1.Source of honey samples
For natural honey,two different samples were collected directly from two different bee hives at the Rajanpur District of Southern Punjab,Pakistan.The samples were stored in the laboratory at room temperature until further analysis.For marketed honey,commonly used honey samples were purchased from the local market in Karachi,Pakistan(Table 1).
2.2.Determination of flavonoid in natural and marketed honey
Flavonoid content was determined as previously described[14].Briefly,a 2-mL solution of the test material(1 g/mL)was added to an equal volume of 2%AlCl3·6H2O in methanol.The mixture was vigorously shaken and absorbance was read at 367 nm after 10 min of incubation.Flavonoid content is expressed as mg of quercetin equivalent per gram of the extract.
2.3.Determination of phenolic content
Phenolic content was determined as previously described[15]. Briefly,1 mL of Folin-Ciocalteu reagent was added to the extract solution(1 g/mL)and final volume adjusted to 46 mL by addition of distilled water.After 3 min,3 mL of 2%Na2CO3was added.Subsequently,the mixture was placed on a shaker for 2 h at room temperature and finally absorbance was recorded at 760 nm.Phenolic content is expressed as mg of gallic acid equivalent per gram of the test material.
2.4.Antioxidant activity using 2,2-diphenyl-1-picrylhydrazyl(DPPH)assay
The reducing power and free radical scavenging activity of test samples were determined using DPPH assay as previously described[14].DPPH is a known radical and scavenger for other radicals.Therefore,rate reduction of a chemical reaction upon addition of DPPH is used as an indicator of the radical nature of the reaction.Because of a strong absorption band centred at about 520 nm,the DPPH radical has a deep violet colour in solution,and it becomes colourless or pale yellow when neutralized.This property allows visual monitoring of the reaction.Briefly,test samples of honey(0.5-200.0 mg/mL)and the reference antioxidant,ascorbic acid(0.005-500.000μg/mL)was dissolved in distilled water for free radical scavenging activity.A 0.1-mmol/L solution of DPPH radical in methanol was prepared and 1 mL of this solution was added to 3 mL of test solution in methanol at different concentrations.The absorbance was measured at 517 nm.A decrease in the DPPH solution absorbance indicates an increase of the DPPH radical-scavenging activity.This activity is given as%DPPH radical-scavenging that is calculated in the equation using DPPH solution as control.
%DPPH scavenging activity=[(Control absorbance-Sample absorbance)/Control absorbance]×100
2.5.Acanthamoeba cultures
A.castellanii belonging to the T4 genotype,sourced from keratitis patient,were purchased from the American Type Culture Collection(ATCC 50492).The cultures were grown in 15 mL of peptone glucose yeast(PYG)medium [proteose peptone 0.75%(w/v),yeast extract 0.75% (w/v)and glucose 1.5% (w/v)]in T-75 tissue culture flasks at 37°C without shaking[13].The media were refreshed 15-20 h prior to experiments.A.castellanii adhering to flasks represented the trophozoite form and were collected by placing the flasks on ice for 30 min with gentle agitation and used in all experiments.
2.6.Amoebistatic and amoebicidal assays
Amoebistatic and amoebicidal assays were performed as previous described[16].Briefly,A.castellanii were incubated with different concentrations of honey[10%,20%and 30%(v/v)in PYG in 24-well plates(105amoebae per 0.5 mL per well).Plates were incubated at 37°C for 24 h.After this incubation,the number of amoebae was determined by haemocytometer counting.The counts from A.castellanii incubated with PYG alone were taken as 100%and effects of honey were presented as percent relative change.Glycerol(with similar viscosity)was used as control,using same concentrations as for honey i.e.,10%,20%and 30% (v/v),while sodium dodecyl sulphate(0.05%)was used to lyse 100%amoebae trophozoites.
Table 1Natural and marketed honey samples used in the present study.
For amoebicidal assays,A.castellanii were incubated with different concentrations of honey[10%,20%and 30%(v/v)]in phosphate buffer solution in 24-well plates(105amoebae per 0.5 mL per well).Plates were incubated at 37°C for 24 h.After this incubation,the number of amoebae was determined by haemocytometer counting.The counts from A.castellanii incubated with phosphate buffer solution alone were taken as 100%and effects of honey were presented as percent relative change.Glycerol and sodium dodecyl sulphate were used as controls.
Additionally,effects of natural honey and marketed honey on A.castellanii trophozoites were observed periodically under a phase contrast inverted microscope and representative images were recorded.
3.Result
3.1.Antiacanthamoebic activities of natural and marketed honey
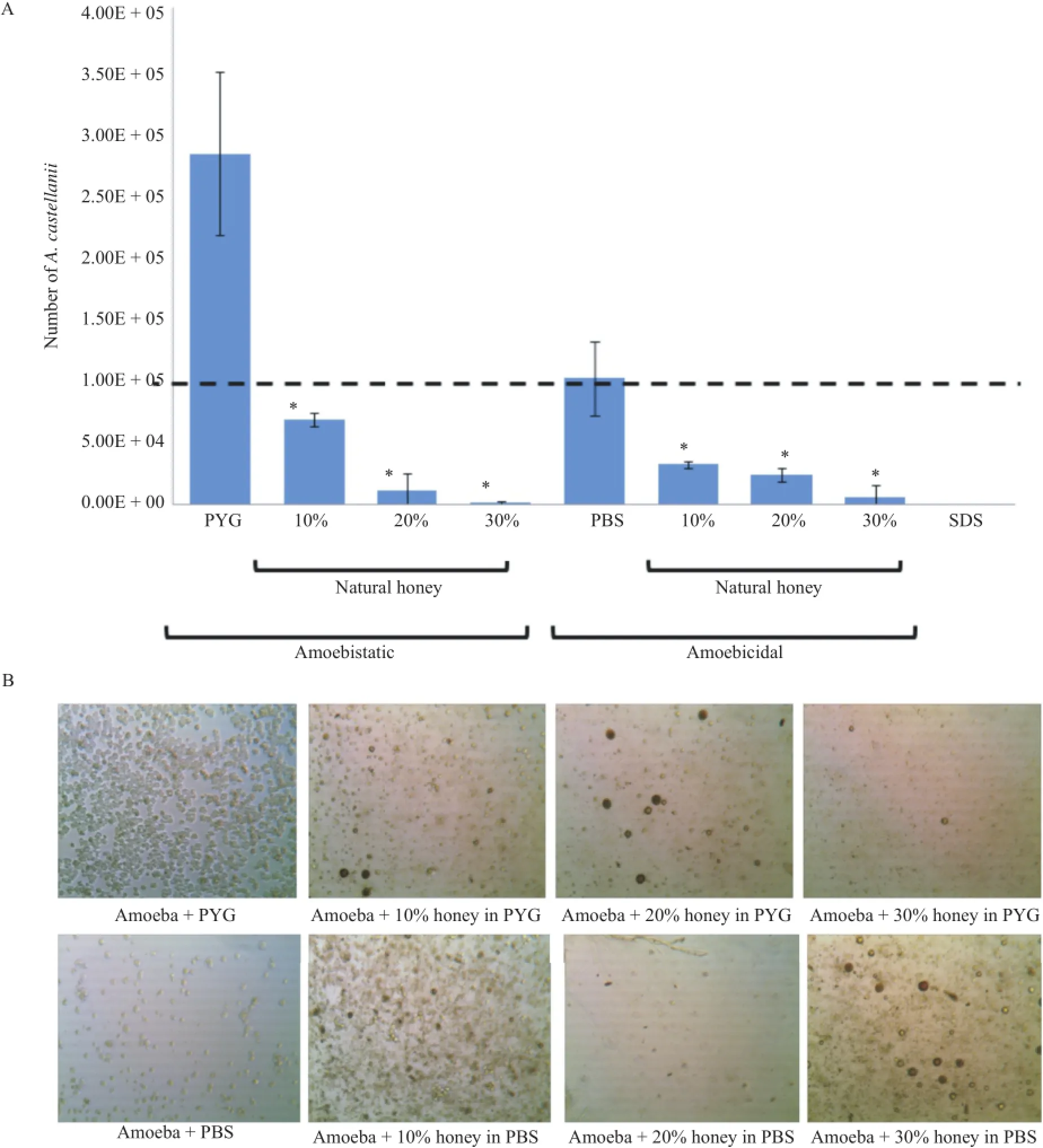
Figure 1.Amoebistatic and amoebicidal properties of natural honey.A:Number of A.castellanii after treatment with natural honey.Both H1 and H2 showed similar effects,however only H1 data is shown.For amoebicidal effects,PYG was replaced with nutrient-free PBS.Again,natural honey exhibited significant amoebicidal effects at all concentrations tested(P<0.01 using two sample t-test;one-tailed distribution);*:Significant difference;Data are presented as mean±SE of three independent experiments performed in duplicate;B:Representative micrograph of A.castellanii incubated with and without natural honey(H1)(×100).

Figure 2.Amoebistatic and amoebicidal properties of marketed honey.*:Significant difference;Data are presented as mean±SE of three independent experiments performed in duplicate.
Amoebistatic and amoebicidal properties of various concentrations of natural and marketed honey were determined.For amoebistaticassays,A.castellaniiincubatedwithgrowthmedium alone(PYG)for 24 h resulted in increase in numbers,from 105amoebae to 2.8×105±3.7×104amoebae and this was considered as 100%.Natural honey exhibited significant amoebistatic effects in a concentration-dependent manner(P<0.01 using two sample t-test;one-tailed distribution)(Figure 1A).At 10%honey,the number of A.castellanii was reduced to 6.8×104±3.0×103as compared to the control(2.8×105±3.7×104),while 30% honey reduced amoebae number to 8.3×102±8.3×102as compared to the control(2.8×105±3.7×104).Consistent with these findings,natural honey exhibited significant amoebicidal effects in a concentration-dependent manner(P<0.01 using two sample t-test;one-tailed distribution)as observed by reduction in amoebae numbers(Figure 1A).At 10%honey,the number of A.castellanii was reduced to 3.2×104±1.45×103,while 30% honey reduced the number of A.castellanii to 5.8×103± 5.84×103as compared to the control,i.e.,1×105±1.74×104amoebae.When observed under the microscope,honey treated A.castellanii showed loss of acanthopodia initially,following which they detached,rounded up,reduced in size,decrease in cytoplasmic mass and were observed floating in the culture medium(Figure 1B).When treated with glycerol,amoebistatic and amoebicidal effects were observed,however,natural honey produced significantly higher amoebistatic and amoebicidal effects compared with glycerol(P<0.01 using two sample t-test;onetailed distribution).Foramoebistatic effects,30%honey reduced amoebae number to 8.3×102±8.3×102,while 30% glycerol reduced amoebae number to 3.6×104±1.7×103.For amoebicidal effects,30%honey reduced amoebae number to 5.8×103±5.84×103,while 30%glycerol reduced amoebae number to 5.4×104±3.3×103.To determine whether honeyand glycerol-treated amoebae remain viable,A.castellanii were inoculated in the growth medium,PYG,post-treatment with honey and glycerol.In honey-treated samples,no viable amoebae emerged within 24 h of incubation with PYG,however,glyceroltreated amoebae exhibited viable trophozoite and active growth(data not shown).
For amoebistatic assays,amoebae(105)were incubated with marketed honey samples(H3,H4,H5)for 24 h and enumerated. In growth medium (PYG)alone,amoebae number increased from original inoculum(dotted line)to 2.8×105±3.7×104. Among marketed honey samples tested,H3 showed higher amoebistatic properties as compared to H4 and H5(Figure 2). For amoebicidal effects,PYG was replaced with nutrient-free PBS.Consistently,amoebicidal effects of H3 sample(i.e.,2.4×104±7.3×103)were more pronounced compared with the amoebicidal effects of H4(4.9×104±7.1×103)and H5(7.3×104±1.3×104)(P<0.01 using two sample t-test;onetailed distribution).However,the amoebicidal effects of H4 and H5 were similarto theamoebicidaleffectsof glycerol(5.4×104±3.3×103).When inoculated in the growth medium,H3-,H4-,and H5-treated amoebae exhibited viable trophozoite and active growth(data not shown).Overall,the natural bee hive honey was more effective in inhibiting A.castellanii as compared to marketed honey.
3.2.Phenolic and flavonoid contents and antioxidant activities of natural and marketed honey
With potent antiamoebic effects of natural honey,we next determined phenolic and flavonoid contents and antioxidant activities of natural honey vs.marketed honey.The results(Figure 3A,B)revealed that among honey samples tested,the proportion of flavonoid and phenolic contents was found in the following order;H1>H2>H5>H4>H3,with an exception of slightly higher proportion of phenolic contents in H3 compared to its levels in H4.Natural honey showed higher flavonoid and phenolic contents compared with the marketed honey samples.
Natural honey showed higher antioxidant activities compared with the marketed honey samples(Figure 4A,B).As for flavonoid and phenolic contents,antioxidant activities were higher in natural honey samples compared with marketed honey samples.Notably,similar pattern of antioxidant activity was observed in both natural honey samples tested.Honey samplesexhibited concentration-dependent%free radical scavenging activity with maximum effect at highest tested concentrations in the following order:H1(65.66% ±2.89%,n=3)≥ H2(57.33% ±2.51%)>H5(45% ±5%)>H4(34.33% ± 8.14%)>H3(24.00%±3.46%).
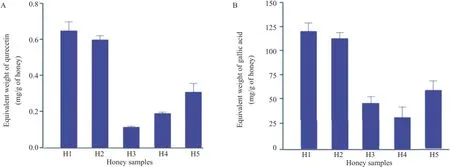
Figure 3.Flavonoid and phenolic contents of natural honey and market honey samples.A:Flavonoid contents;B:Phenolic contents.Data are presented as mean±SE of three independent experiments performed in duplicate.
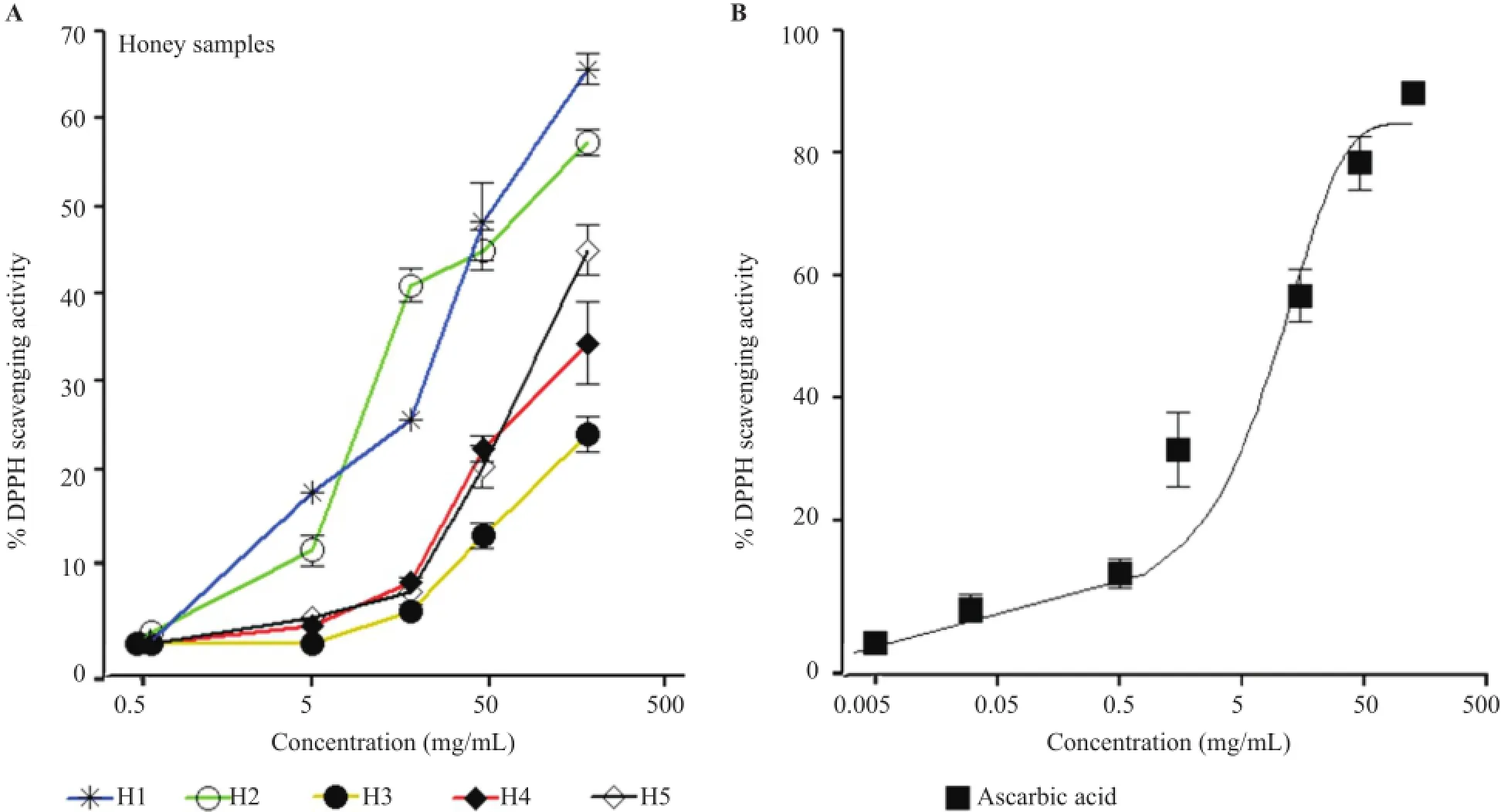
Figure 4.The antioxidant activities of natural honey(H1,H2)and marketed honey samples(H3,H4,H5)determined by measuring%DPPH scavenging activity.Data are presented as the mean±SE of three independent experiments performed in duplicate.
4.Discussion
It is well established that honey is a natural product of medicinal value and widely used in communities for its wound healing,anti-inflammatory,and antibacterial properties[17,18]. The broad spectrum antibacterialpropertiesofhoney is multifactorial in nature,partly attributing to hydrogen peroxide,high osmolarity,antibacterial compound methylglyoxal[17],however,its source,production,manufacturing,and storage is likely to affect its contents and therapeutic properties.For example,Kwakman et al.[19],showed that Revamil medicalgrade honey,produced under standardized conditions in greenhouses,has potent reproducible bactericidal activity suggesting that natural honey possess potent medicinal properties.Later,Kwakman et al.[17],identifieddefensin-1 as apotent antibacterial agent from honey,which is part of the honey bee immune system and is added by bees to honey.
Although antibacterial properties of natural honey have been well documented,there are no reports of effects of honey against pathogenicAcanthamoebaspp.Forthefirsttime,thepresentstudy showed that natural honey has antiacanthamoebic properties and possesses higher flavonoid,phenolic and antioxidant properties compared with the marketed honey.Phenolics and flavonoids are a group of bioactive low molecular weight compounds derived from plants and known for their antioxidant and anticancer properties.They occur as flavanones,flavones,flavonols,isoflavonoids,anthocyanins,and flavans.Flavonoids and phenolics exhibit health promoting effects such as reducing the risk of cancer,heart disease,asthma,stroke and brain tonic in relation to the antioxidant activity[20,21].In the present study,natural honey exhibited potentamoebistatic and amoebicidalproperties,compared with the marketed honey,albeit the molecular events of amoebae cytotoxicity require further studies.A comparison clearly indicates that the naturally sourced honey samples possess higher concentrations of flavonoids and phenolic with greater antioxidant potential than honey samples obtained from the local market.Thus the observed differences in antiamoebic properties may be attributed to variations in constituents of flavonoids and phenolic,or possibly a combination of other factors,however,the precise mechanisms are yet to be explored. It is also unclear whether the antiamoebic property of natural honey is due to an individual ingredient or a combination of antimicrobial components.By selectively neutralizing individual components present in natural honey,future studies willdeterminethe underlying molecularmechanismsof antiamoebic properties of natural honey to identify novel antiamoebic factor(s).Such honeys,or isolated components thereof,could serve as novel agents to prevent or treat infections,in particular those caused by antibiotic-resistant bacteria,and serve as novel molecules to prevent or treat amoebic infections.A careful selection of honey,containing factors with antiamoebic and antibacterial properties would be of therapeutic value,in particular for topical use and/or may provide added benefit when supplemented with known chemical remedies for such infections.Additionally,the isolation of ingredients from natural honey should identify novel factors that could be of value against infections due to other pathogen free-living amoebae.
Overall,these findings suggest remarkable differences in antiamoebic of marketed vs.natural honey,and these differences are likely attributed to variations in constituents or properties of honey,including flavonoids,phenolic,defensing-1,osmolarity,pH,or possibly a combination of factors,however,the precise mechanisms are yet to be explored.These findings are of concern to the general public,health officials and to the local andmarketed honey manufacturers regarding the production and storage for standardization of honey for medical applications.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors are grateful for the kind support provided by Aga Khan University,Pakistan,and Sunway University through INT-FST-DBS-2015 with grant No.005,Bandar Sunway,Malaysia.
References
[1]de Rodríguez GO,de Ferrer BS,Ferrer A,Rodriíguez B.Characterization of honey produced in Venezuela.Food Chem 2004;84: 499-502.
[2]VandammeL,Heyneman A,HoeksemaH,Verbelen J,Monstrey S.Honey in modern wound care:a systematic review. Burns 2013;39:1514-25.
[3]Oryan A,Alemzadeh E,Moshiri A.Biological properties and therapeutic activities of honey in wound healing:a narrative review and meta-analysis.J Tissue Viability 2016;25:98-118.
[4]Estevinho ML,Afonso SE,Fe´as X.Antifungal effect of lavender honey against Candida albicans,Candida krusei and Cryptococcus neoformans.J Food Sci Technol 2011;48:640-3.
[5]Moussa A,Noureddine D,Saad A,Abdelmelek M,Abdelkader B. Antifungal activity of four honeys of different types from Algeria against pathogenic yeast:Candida albicans and Rhodotorula sp. Asian Pac J Trop Biomed 2012;2:554-7.
[6]Alvarez-Suarez JM,Tulipani S,Díaz D,Estevez Y,Romandini S,Giampieri F,et al.Antioxidant and antimicrobial capacity of several monofloral Cuban honeys and their correlation with color,polyphenol content and other chemical compounds.Food Chem Toxicol 2010;48:2490-9.
[7]Erejuwa OO,Sulaiman SA,Ab Wahab MS.Honey:a novel antioxidant.Molecules 2012;17:4400-23.
[8]Nik Man NM,Hassan R,Ang CY,Abdullah AD,Mohd Radzi MA,Sulaiman SA.Antileukemic effect of Tualang honey on acute and chronic leukemia cell lines.Biomed Res Int 2015;2015: e307094.
[9]Stephen Haynes J.Achieving clinical outcomes:the use of honey. Wound Essentials 2011;6:14-9.
[10]Kozłowska A,Szostak-Wegierek D.Flavonoids-food sources and health benefits.Rocz Panstw Zakl Hig 2014;65:79-85.
[11]Marciano-Cabral F,Cabral G.Acanthamoeba spp.as agents of disease in humans.Clin Microbiol Rev 2003;16:273-307.
[12]Visvesvara GS,Moura H,Schuster FL.Pathogenic and opportunistic free-living amoebae:Acanthamoeba spp.,Balamuthia mandrillaris,Naegleria fowleri,and Sappinia diploidea.FEMS Immunol Med Microbiol 2007;50:1-26.
[13]Siddiqui R,Khan NA.Biology and pathogenesis of Acanthamoeba.Parasit Vectors 2012;5:6.
[14]Huang DJ,Lin CD,Chen HJ,Lin YH.Antioxidant and antiproliferative activities of sweet potato(Ipomoea batatas[L.]Lam‘Tainong 57')constituents.Bot Bull Acad Sin 2004;45:179-86.
[15]Singleton VL,Orthofer R,Lamuela-Raventos RM.Analysis of total phenols and other oxidation substrates and oxidants by means of Folin-Ciocalteu reagent.Methods Enzymol 1999;299:152-78.
[16]Lakhundi S,Khan NA,Siddiqui R.Inefficacy of marketed contact lens disinfection solutions against keratitis-causing Acanthamoeba castellanii belonging to the T4 genotype.Exp Parasitol 2014;141: 122-8.
[17]Kwakman PH,te Velde AA,de Boer L,Speijer D,Vandenbroucke-Grauls CM,Zaat SA.How honey kills bacteria.FASEB J 2010;24:2576-82.
[18]Mandal MD,Mandal S.Honey:its medicinal property and antibacterial activity.Asian Pac J Trop Biomed 2011;1:154-60.
[19]KwakmanPH,VandenAkkerJP,GucluA,AslamiH,Binnekade JM,De Boer L,et al.Medical-grade honey kills antibiotic-resistant bacteria in vitro and eradicates skin colonization.Clin Infect Dis 2008;46:1677-82.
[20]Roleira FM,Tavares-da-Silva EJ,Varela CL,Costa SC,Silva T,Garrido J,et al.Plant derived and dietary phenolic antioxidants: anticancer properties.Food Chem 2015;183:235-58.
[21]Liao CY,Lee CC,Tsai CC,Hsueh CW,Wang CC,Chen IH,et al. Novel investigations of flavonoids as chemopreventive agents for hepatocellular carcinoma.Biomed Res Int 2015;2015:e840542.
19 Jan 2016
inrevisedform14Mar,2nd
Original article http://dx.doi.org/10.1016/j.apjtb.2016.05.010
Naveed Ahmed Khan,Department of Biological Sciences,Faculty of Science and Technology,Sunway University,Selangor,47500,Malaysia.
 Asian Pacific Journal of Tropical Biomedicine2016年11期
Asian Pacific Journal of Tropical Biomedicine2016年11期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Human and animal sarcocystosis in Malaysia∶A review
- Therapeutic applications of collagenase(metalloproteases)∶A review
- Cytotoxic activity and phytochemical standardization of Lunasia amara Blanco wood extract
- GC-MS analysis of bioactive compounds present in different extracts of an endemic plant Broussonetia luzonica(Blanco)(Moraceae)leaves
- Anti-nitric oxide production,anti-proliferation and antioxidant effects of the aqueous extract from Tithonia diversifolia
- Anthelmintic activity of Saba senegalensis(A.DC.)Pichon(Apocynaceae)extract against adult worms and eggs of Haemonchus contortus
